Molecular Targets of Natural Products for Chondroprotection in Destructive Joint Diseases
Abstract
1. Introduction
2. MMPs and Osteoarthritis
3. Inflammatory Cytokines in Osteoarthritic Chondrocytes
4. Phorbol 12-myristate 13-acetate (PMA) in Osteoarthritis
5. Animal Models for Osteoarthritis
Dosage of MIA in Osteoarthritis Model
6. Role of Nuclear Factor Kappa-B (NF-κB) in Osteoarthritis
7. Molecular Targets of Natural Products and Their Therapeutic Intervention for Osteoarthritis
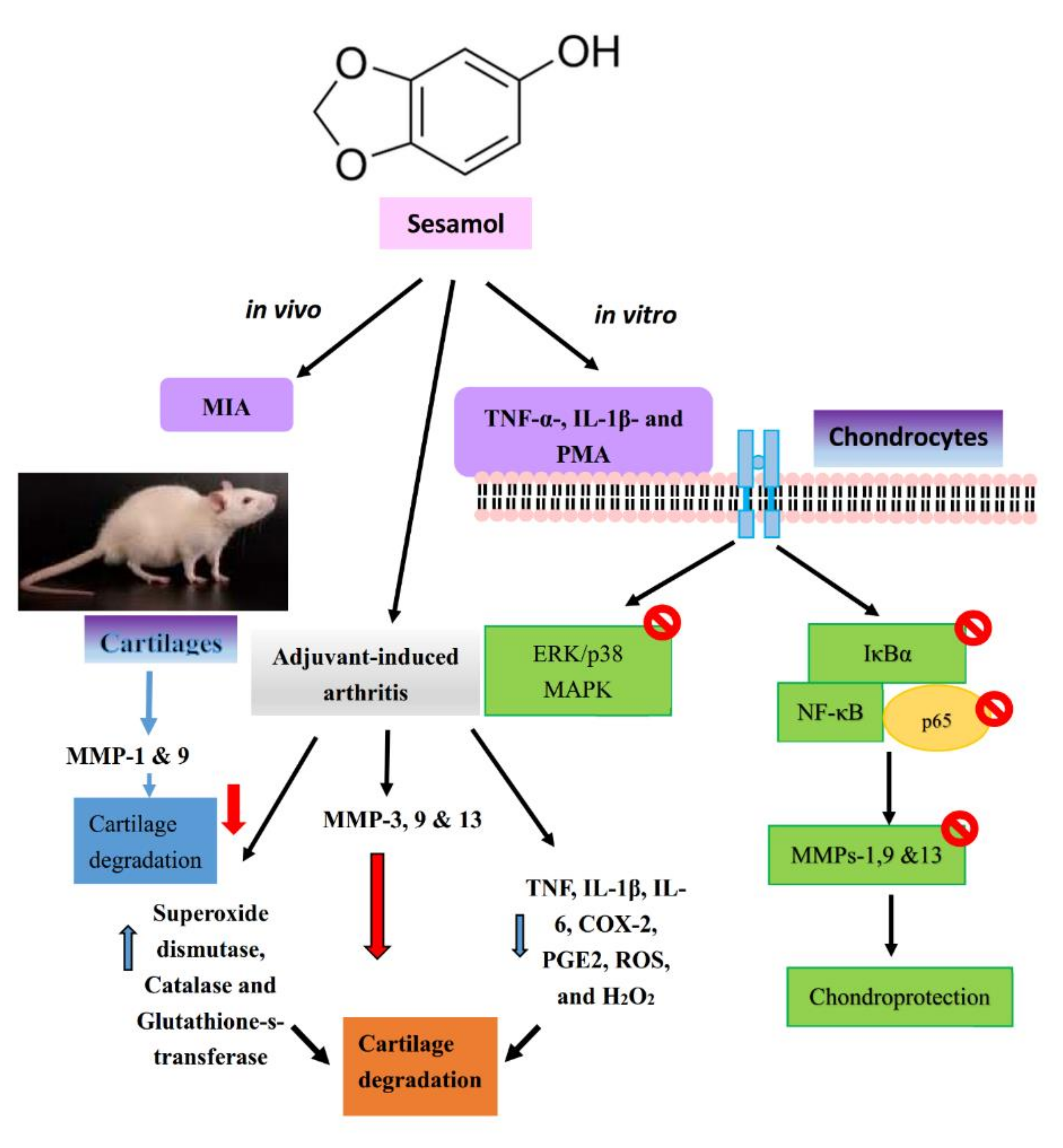
7.1. Sesamol
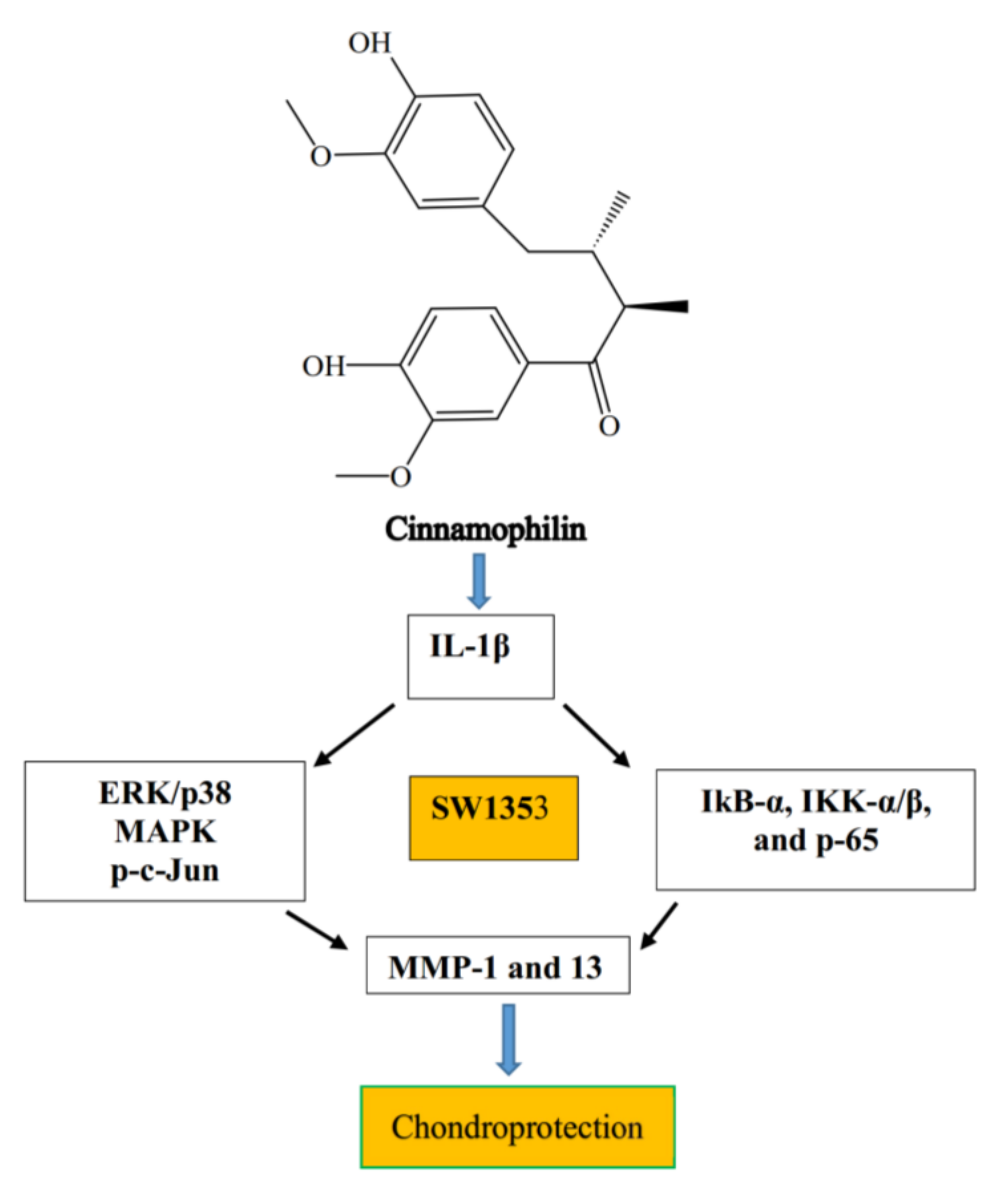
7.2. Cinnamophilin
7.3. Apigenin
7.4. Aucubin
7.5. Baicalein
7.6. Berberine
7.7. Betulin and Biochanin
7.8. Catechin
7.9. Celastrol, Crocin, and Ginsenosides
7.10. Honokiol, Icariin, and Luteolin
7.11. Monotropein
7.12. Morin
7.13. Oleanolic Acid
7.14. Curcumin and Shogaol
| S. No | Compounds Name | OA/RA Stimulators | Molecular Targets | References |
|---|---|---|---|---|
| 1 | Sesamol | TNF-α-, IL-1β- or PMA in SW1353 cells | Reduced MMPs-1, -9, and -13 expression, MAPKs expression and NF-κB signaling pathway | [37] |
| MIA in rats | Reduced MMPs-3, -9, and -13 and bone joint exoglycosidases, cathepsin D and tartarate-resistant acid phosphatases | |||
| Adjuvant-induced arthritis | Reduced TNF, IL-1β, IL-6, COX-2, PGE2, ROS, and H2O2 | |||
| 2 | Cinnamophilin | IL-1β in SW1353 cells | Reduced MMPs-1 and 13 expression | [78] |
| Decreased IKK-α/β and degradation of IκB-α and p-p65 expression | ||||
| 3 | Apigenin | IL-1β in rabbit chondrocytes and rat knee | Decreased MMP-1, -3, and -13 expression | [80] |
| Adjuvant-induced arthritis rats | Reduced MMP-3 expression | [81] | ||
| Reduced NF-κB p65, IKK-α, IKK-β and IκB-α expression | ||||
| Alleviated pain and paw swelling | ||||
| 4 | Aucubin | IL-1β in rat chondrocytes | Recovered NF-κB p65 and IκB-α | [84] |
| Reduced NO production and iNOS, COX-2 and MMPs expression | ||||
| Increased ROS scavenging | ||||
| Mechanical stimulus | Decreased apoptosis and necrosis | [85] | ||
| H2O2 | Reduced caspase-3 expression | |||
| 5 | Baicalein | IL-1β in chondrocytes | Decreased caspase-3, COX-2, MMPs-3 and -9 expression | [88] |
| Increased Bcl-2 activation | ||||
| Arthritis and colitis | Regulates JAK-STAT pathway | [89] | ||
| 6 | Berberine | IL-1β in chondrocytes | Decreased MMPs via the Akt pathway | [91] |
| Decreased IL-1β and cartilage degradation | ||||
| CCN2 | Increased CDK inhibitors Cip1/p21 and Kip1/p27; Decreased CDK2, CDK4, and CDK6, and cyclins D1, D2 and E; | [92] | ||
| Reduced caspase-3 and -9 | [94] | |||
| 7 | Betulin | IL-1β in chondrocytes | Decreased MMPs-1, -3, and -13 expression | [95] |
| Rat knee joint | Increased type-II collagen gene expression | |||
| Decreased MMP-3 expression | ||||
| 8 | Biochanin | IL-1β in chondrocytes | Decreased mRNA and protein of MMPs-1, -3, and -13 | [98] |
| Increased TIMP-1 mRNA and its protein | ||||
| Decreased IκB-α degradation and NF-κB activation | ||||
| 9 | Green tea | IL-1β and TNF-α | Decreased proteoglycan breakdown and release from OA and RA cartilage | [104] |
| 10 | EGCG | IL-1β in chondrocytes | Decreased cartilage proteoglycan degradation, and MMPs-1 and -13 release and expression | [105] |
| Decreased the activation and promoter binding activity of NF-κB and AP-1 | ||||
| Decreased MMP-13, NF-κB, AP-1/c-Jun, and p38 | ||||
| IL-1β in chondrocytes | Decreased MMPs-1, -3, -8, -13, ADAMTS5, IL-1β, and TNF-α mRNAs | [106,107,108] | ||
| Articular cartilages | Increased CITED2 and decreased OA pain | [110] | ||
| 11 | Catechin | IL-1β in chondrocytes | Decreased IL-8, PGE2, and COX-2 | [109] |
| 12 | Celastrol | IL-1β in chondrocytes | Decreased protein and mRNA expression of MMPs-1, -3, -13, COX-2, and iNOS-2 | [111] |
| Decreased NF-κB pathways | ||||
| 13 | Crocin | Type II collagen-induced arthritis in rats | Decreased arthritis scores, paw swelling, and weight loss | [112] |
| Decreased chondrocyte death, cartilage surface erosion, and bone erosion | ||||
| Decreased MMPs-1, -3, and -13 expression | ||||
| IL-1β | Decreased TNF-α, IL-6, IL-17, and CXCL8 | [113] | ||
| Rabbit cartilages | Decreased NF-κB pathways | |||
| Decreased degeneration of cartilage | ||||
| 14 | Ferulic acid | H2O2 | Decreased mRNA expression of MMPs-1, -13, TNF-α, and IL-1β | [114] |
| 15 | Ginsenosides | H2O2 and | Decreased MMPs-1, -13, NO, iNOS, IL-1β, and TNF-α | [115,116] |
| IL-1β | Increased type II collagen expression | |||
| 16 | Honokial | IL-1β in chondrocytes | Decreased MMP-13, IL-6, iNOS, NO, COX-2, and PGE2 | [119] |
| Decreased NF-κB signaling pathway | ||||
| Type II collagen-induced arthritis in rats | Decreased MDA, IL-1β, and TNF-α | [120] | ||
| Increased GSH, CAT and SOD | ||||
| 18 | Icarin | IL-1β in chondrocytes | Decreased MMP-13 expression | [122] |
| Increased extracellular matrix synthesis | ||||
| 19 | Luteolin | IL-1β in chondrocytes | Increased gene expression, secretion, and enzyme activity of MMP-3 | [123] |
| Increased gene expression of MMP-13 and ADAMTS-5 | ||||
| Rat knee joint | Decreased MMP-3 expression | |||
| 20 | Monotropein | IL-1β in chondrocytes | Decreased MMPs-3 and 13 | [124] |
| TNF-α in chondrocytes | Decreased iNOS, COX-2, MMP-1, -3, and -13 | [125] | ||
| Decreased MAPK/NF-κB | ||||
| 21 | Morin | IL-1β in chondrocytes | Decreased NO, PGE2, iNOS, and COX-2 | [130] |
| Inhibited degradation of bone and cartilage via regulation of the activities/levels of lysosomal acid hydrolases, glycoproteins, bone collagen, and urinary constituents | [132] | |||
| Decreased MMPs-3 and 13, and TIMP-1 | ||||
| ERK1/2 and p38 | ||||
| 22 | Oleanolic acid | Type II collagen-induced arthritis in rats | Decreased Th1/Th17 phenotype CD4+ T lymphocyte expansions | [134] |
| Decreased expression and production of cytokines and MMPs-1 and 3 | ||||
| Decreased Akt, MAPKs, and NF-κB | ||||
| Inhibited ADAMTS-5, MMPs-1, -13, and ADAMTS-4 gene expression | ||||
| Type II collagen-induced arthritis in rats | Decreased MMP-3 protein expression | [135] | ||
| Inhibited in vitro enzyme activity and in vivo MMP-3 production | ||||
| 23 | Curcumin | DMM induced OA in mice | Decreased proteoglycan loss, cartilage erosion, synovitis and subchondral plate thickness | [136] |
| Decreased IL-1β and TNF-α, MMPs -1, 3, and 13, and aggrecanase ADAMTS5 | [137] | |||
| MIF induced synovial fibroblasts of RA patients | Decreased MMPs-1 and -3 mRNAs | [138] | ||
| IL-1β-induced chondrocytes | Recovered cellular and morphological changes | |||
| IL-1β and TNF-α induced chondrocytes | Decreased caspase-3 via AP-1 and NF-κB | [139] | ||
| Decreased COX-2, MMP-9 | ||||
| Decreased NF-κB, IκB-α phosphorylation, IκB-α degradation, p65 phosphorylation, and p65 nuclear translocation | ||||
| 24 | 6-Shogaols | CFA-induced monoarthritis in rats | Decreased paw edema via VCAM-1 | [144] |
| LPS-stimulated chondrocytes | Decreased MMPs- 2 and 9 induction | [145] |
8. Conclusions and Future Direction on Therapy for Osteoarthritis
Author Contributions
Funding
Conflicts of Interest
References
- Brosseau, L.; Wells, G.A.; Kenny, G.P.; Reid, R.; Maetzel, A.; Tugwell, P.; Huijbregts, M.; McCullough, C.; De Angelis, G.; Chen, L. The implementation of a community-based aerobic walking program for mild to moderate knee osteoarthritis (OA): A knowledge translation (KT) randomized controlled trial (RCT): Part II: Clinical outcomes. BMC Public Health 2012, 12, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Lippiello, L. Biochemical and Metabolic Abnormalities in Articular Cartilage from Osteo-Arthritic Human Hips. J. Bone Jt. Surg. Am. 1970, 52, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Jubb, R.W.; Fell, H.B. The breakdown of collagen by chondrocytes. J. Pathol. 1980, 130, 59–167. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Rezzonico, R.; Li, J.M.; Modoux, C.; Pierce, R.A.; Welgus, H.G.; Dayer, J.M. Imbalance between interstitial collagenase and tissue inhibitor of metalloproteinases 1 in synoviocytes and fibroblasts upon direct contact with stimulated T lymphocytes: Involvement of membrane-associated cytokines. Arthritis Rheum. 2004, 41, 1748–1759. [Google Scholar] [CrossRef]
- Lin, N.; Liu, C.; Xiao, C.; Jia, H.; Imada, K.; Wu, H.; Ito, A. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem. Pharmacol. 2007, 73, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Brinckerhoff, C.E. Joint destruction in arthritis: Metalloproteinases in the spotlight. Arthritis Rheum. 1991, 34, 1073–1075. [Google Scholar] [CrossRef]
- Abeles, A.M.; Pillinger, M.H. The role of the synovial fibroblast in rheumatoid arthritis-cartilage destruction and the regulation of matrix metalloproteinases. Bull. NYU Hosp. Jt. Dis. 2006, 64, 20–24. [Google Scholar]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef]
- Shlopov, B.V.; Lie, W.R.; Mainardi, C.L.; Cole, A.A.; Chubinskaya, S.; Hasty, K.A. Osteoarthritic lesions: Involvement of three different collagenases. Arthritis Rheum. 1997, 40, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Knauper, V.; Cowell, S.; Smith, B.; Lopez-Otin, C.; O’Shea, M.; Morris, H.; Zardi, L.; Murphy, G. The role of the C-terminal domain of human collagenase- 3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J. Biol. Chem. 1997, 272, 7608–7616. [Google Scholar] [CrossRef]
- Cawston, T.E.; Wilson, A.J. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract. Res. Clin. Rheumatol. 2006, 20, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.G.; Magna, H.A.; Reeves, L.M.; Lopresti-Morrow, L.L.; Yocum, S.A.; Rosner, P.J.; Geoghegan, K.F.; Hambor, J.E. Cloning, expression and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996, 97, 761–768. [Google Scholar] [CrossRef]
- Teslow, L.C.; Adlam, D.J.; Woolley, D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage. Arthritis Rheum. 2001, 44, 585–594. [Google Scholar]
- Wu, W.; Billinghurst, R.C.; Pidour, I.; Antoniou, J.; Zukor, D.; Tanzer, M.; Poole, A.R. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002, 46, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Ceponis, A.; Takagi, M.; Ainola, M.; Sorsa, T.; Sutinen, M.E.; Salo, T.; Ma, J.; Santavirta, S.; Seiki, M. New collagenolytic enzymes cascade identified at the pannus–hard tissue junction in rheumatoid arthritis: Destruction from above. Matrix Biol. 1998, 17, 585–601. [Google Scholar] [CrossRef]
- Ohuchi, E.; Imai, K.; Fujii, Y.; Sato, H.; Seiki, M.; Okada, Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997, 272, 2446–2451. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Ainola, M.; Valleala, H.; Ma, J.; Ida, H.; Mandelin, J.; Kinne, R.W.; Santavirta, S.; Sorsa, T.; López-Otín, C.; et al. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: Different profiles in trauma and rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58, 691–697. [Google Scholar] [CrossRef]
- Mengshol, J.A.; Vincenti, M.P.; Coon, C.I.; Barchowsky, A.; Brinckerhoff, C.E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000, 43, 801–811. [Google Scholar] [CrossRef]
- Shi, J.; Schmitt-Talbot, E.; Dimattia, D.A.; Dullea, R.G. The differential effects of IL-1 and TNF-alpha on proinflammatory cytokine and matrix metalloproteinase expression in human chondrosarcoma cells. Inflamm. Res. 2004, 53, 377–389. [Google Scholar] [CrossRef]
- Malemud, C.J.; Islam, N.; Haqqi, T.M. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies. Cells Tissues Organs 2003, 174, 34–48. [Google Scholar] [CrossRef]
- Jimi, E.; Aoki, K.; Saito, H.; D’Acquisto, F.; May, M.J.; Nakamura, I.; Suda, T.; Kojima, T.; Okamoto, F.; Fukushima, H.; et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 2004, 10, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Héraud, F.; Héraud, A.; Harmand, M.F. Apoptosis in normal and osteoarthritic articular cartilage. Ann. Rheum. Dis. 2000, 59, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Grunke, M.; Schulze-Koops, H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann. Rheum. Dis. 2006, 65, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Dray, A.; Read, S.J. Arthritis and Pain. Future Targets to Control Osteoarthritis Pain. Arthritis Res. Ther. 2007, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B. Osteoarthritis and cartilage: The role of cytokines. Curr. Rheumatol. Rep. 2000, 2, 459–465. [Google Scholar] [CrossRef]
- Westacott, C.I.; Sharif, M. Cytokines in osteoarthritis: Mediators or markers of joint destruction? Semin. Arthritis Rheum. 1996, 25, 254–272. [Google Scholar] [CrossRef]
- Ku, G.; Faust, T.; Lauffer, L.L.; Livingston, D.J.; Harding, M.W. Interleukin-1_ converting enzyme inhibition blocks progression of type II collageninduced arthritis in mice. Cytokine 1996, 8, 377–386. [Google Scholar] [CrossRef]
- Murata, M.; Trahan, C.; Hirahashi, J.; Mankin, H.J.; Towle, C.A. Intracellular interleukin-1 receptor antagonist in osteoarthritis chondrocytes. Clin. Orthop. Relat. Res. 2003, 409, 285–295. [Google Scholar] [CrossRef]
- Richardson, D.W.; Dodge, G.R. Effects of interleukin-1β and tumor necrosis factor-α on expression of matrix-related genes by cultured equine articular chondrocytes. Am. J. Vet. Res. 2000, 61, 624–630. [Google Scholar] [CrossRef]
- Bau, B.; Gebhard, P.M.; Haag, J.; Knorr, T.; Bartnik, E.; Aigner, T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002, 46, 2648–2657. [Google Scholar] [CrossRef]
- Brenn, D.; Richter, F.; Schaible, H. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: An inflammatory mechanism of joint pain. Arthritis Rheum. 2007, 56, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Silacci, P.; Dayer, J.M.; Desgeorges, A.; Peter, R.; Manueddu, C.; Guerne, P.A. Interleukin (IL)-6 and its soluble receptor induce TIMP-1 expression in synoviocytes and chondrocytes, and block IL-1-induced collagenolytic activity. J. Biol. Chem. 1998, 273, 13625–13629. [Google Scholar] [CrossRef] [PubMed]
- Obreja, O.; Biasio, W.; Andratsch, M.; Lips, K.S.; Rathee, P.K.; Ludwig, A.; Rose-John, S.; Kress, M. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain 2005, 128, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Sakao, K.; Takahashi, K.A.; Arai, Y.; Saito, M.; Honjo, K.; Hiraoka, N.; Asada, H.; Shin-Ya, M.; Imanishi, J.; Mazda, O.; et al. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J. Bone Miner. Metab. 2009, 27, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Makkar, H.P.S.; Francis, G.; Becker, K. Phorbol esters: Structure, biological activity, and toxicity in animals. Int. J. Toxicol. 2007, 26, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Iwamura, H.; Larbre, J.P.; Scott, D.L.; Willoughby, D.A. Cartilage degradation by polymorphonuclear leucocytes: In vitro assessment of the pathogenic mechanisms. Ann. Rheum. Dis. 1993, 52, 27–31. [Google Scholar] [CrossRef]
- Lu, Y.C.; Jayakumar, T.; Duann, Y.F.; Chou, Y.C.; Hsieh, C.Y.; Yu, S.Y.; Sheu, J.R.; Hsiao, G. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-KB signaling in activated SW1353 cells. J. Agric. Food Chem. 2011, 59, 4969–4978. [Google Scholar] [CrossRef]
- Aigner, T.; Cook, J.L.; Gerwin, N.; Glasson, S.S.; Laverty, S.; Little, C.B.; McIlwraith, W.; Kraus, V.B. Histopathology atlas of animal model systems—Overview of guiding principles. Osteoarthr. Car. 2010, 18, S2–S6. [Google Scholar] [CrossRef]
- Takahashi, I.; Matsuzaki, T.; Hoso, M. Long-term histopathological developments in knee-joint components in a rat model of osteoarthritis induced by monosodium iodoacetate. J. Phys. Ther. Sci. 2017, 27, 590–597. [Google Scholar] [CrossRef]
- Bove, S.E.; Calcaterra, S.L.; Brooker, R.M.; Huber, C.M.; Guzman, R.E.; Juneau, P.L.; Schrier, D.J.; Kilgore, K.S. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarth. Cart. 2003, 11, 821–830. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imaizumi, R.; Sumichika, H.; Tanaka, H.; Goda, M.; Fukunari, A.; Komatsu, H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J. Veter. Med. Sci. 2003, 65, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Janusz, M.J.; Hookfin, E.B.; Heitmeyer, S.A.; Woessner, J.F.; Freemont, A.J.; Hoyland, J.A.; Brown, K.K.; Hsieh, L.C.; Almstead, N.G.; De, B.; et al. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarth. Cartil. 2001, 9, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Udo, M.; Muneta, T.; Tsuji, K.; Ozeki, N.; Nakagawa, Y.; Ohara, T.; Saito, R.; Yanagisawa, K.; Koga, H.; Sekiya, I. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: Proposed model-specific scoring systems. Osteoarth. Cartil. 2016, 24, 1284–1291. [Google Scholar] [CrossRef]
- Lampropoulou-Adamidou, K.; Lelovas, P.; Karadimas, E.V.; Liakou, C.; Triantafillopoulos, I.K.; Dontas, I.; Papaioannou, N.A. Useful animal models for the research of osteoarthritis. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 263–271. [Google Scholar] [CrossRef]
- Nolan, G.P.; Baltimore, D. The inhibitory ankyrin and activator Rel proteins. Curr. Opin. Genet. Dev. 1992, 2, 211–220. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Siebenlist, U.; Franzo, G.; Brown, K. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell Biol. 1994, 10, 405–455. [Google Scholar] [CrossRef]
- Grilli, M.; Chiu, J.J.S.; Lenardo, M.J. NF-kappa B and Rel: Participants in a multiform transcriptional regulatory system. Int. Rev. Cytol. 1993, 143, 1–62. [Google Scholar]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Jimenez, S.A. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarth. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Calao, M.; Burny, A.; Quivy, V.; Dekoninck, A.; Van Lint, C. A pervasive role of histone acetyltransferases and deacetylases in an NF-kappaB-signaling code. Trends Biochem. Sci. 2008, 33, 339–349. [Google Scholar] [CrossRef]
- Spange, S.; Wagner, T.; Heinzel, T.; Kramer, O.H. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell. Biol. 2009, 41, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappa B activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Matsui, T.; Saisho, K.; Tohma, S. Current treatments of rheumatoid arthritis: From the “NinJa” registry. Exp. Rev. Clin. Immunol. 2012, 8, 455–465. [Google Scholar] [CrossRef]
- Lichtenstein, D.R.; Syngal, S.; Wolfe, M.M. Nonsteroidal antiinflammatory drugs and the gastrointestinal tract. The double-edged sword. Arthritis Rheum. 1995, 38, 5–18. [Google Scholar] [CrossRef]
- Boileau, C.; Martel-Pelletier, J.; Caron, J.; Msika, P.; Guillou, G.B. Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: Inhibition of nitric oxide synthase and matrix metalloproteinase-13. Arthritis Res. Ther. 2009, 11, R41. [Google Scholar] [CrossRef]
- Jacobs, J.W.G.; Kraaimaat, F.W.; Bijlsma, J.W. Why do patients with rheumatoid arthritis use alternative treatments? Clin. Rheumatol. 2001, 20, 192–196. [Google Scholar] [CrossRef]
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef]
- Sankar, D.; Sambandam, G.; Ramakrishna, R.; Pugalendi, K.V. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin. Chim. Acta 2005, 355, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Osawa, T.; Namiki, M. Chemistry of lignan antioxidants in sesame seed and oil. In Food Phytochemicals for Cancer Prevention II: Teas, Spices and Herbs; Ho, C.T., Osawa, T., Huang, M.T., Rosen, R.T., Eds.; ACS Symposium Series: Washington, DC, USA, 1994; Volume 547, pp. 264–274. [Google Scholar]
- White, J.P. Fatty acids in oil seeds. In Fatty Acids in Foods and Their Health Applications; Dekker M. Incorporated: New York, NY, USA, 1992; pp. 237–262. [Google Scholar]
- Parihar, V.K.; Prabhakar, K.R.; Veerapur, V.P.; Kumar, M.S.; Reddy, Y.R.; Joshi, R.; Unnikrishnan, M.K.; Rao, C.M. Effect of sesamol on radiation-induced cytotoxicity in Swiss albino mice. Mutat. Res. 2006, 611, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Mo, F.E.; Chen, S.Y.; Chang, C.C.; Liu, M.Y. Sesamol attenuates isoproterenol-induced acute myocardial infarction via inhibition of matrix metalloproteinase-2 and -9 expression in rats. Cell. Physiol. Biochem. 2011, 27, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Hsu, D.Z.; Hsu, P.; Liu, M.Y. Sesamol down-regulates the lipopolysaccharide-induced inflammatory response by inhibiting nuclear factor-kappa B activation. Innate Immun. 2010, 16, 333–339. [Google Scholar] [CrossRef]
- Geetha, T.; Rohit, B.; Pal, K.I. Sesamol: An efficient antioxidant with potential therapeutic benefits. Med. Chem. 2009, 5, 367–371. [Google Scholar] [CrossRef]
- Shenoy, R.R.; Sudheendra, A.T.; Nayak, P.G.; Paul, P.; Kutty, N.G.; Rao, C.M. Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamum indicum (L.) in albino rats. J. Ethnopharmacol. 2011, 133, 608–612. [Google Scholar] [CrossRef]
- Campo, G.; Avenoso, M.; Campo, A.; D’Ascola, S.; Traina, A.; Calatroni, P. Differential effect of molecular size HA in mouse chondrocytes stimulated with PMA. Biochim. Biophys. Acta 2009, 1790, 1353–1367. [Google Scholar] [CrossRef]
- Chockalingam, P.S.; Varadarajan, U.; Sheldon, R.; Fortier, E.; LaVallie, E.R.; Morris, E.A.; Yaworsky, P.J.; Majumdar, M.K. Involvement of protein kinase Czeta in interleukin-1β induction of ADAMTS-4 and type 2 nitric oxide synthase via NF-κB signaling in primary human osteoarthritic chondrocytes. Arthritis Rheum. 2007, 56, 4074–4083. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Jnaneshwari, S.; Devaraja, S.; Kemparaju, K.; Girish, K.S. Attenuation of adjuvant-induced arthritis by dietary sesamol via modulation of inflammatory mediators, extracellular matrix degrading enzymes and antioxidant status. Eur. J. Nutr. 2013, 52, 1787–1799. [Google Scholar] [CrossRef]
- Hsiao, G.; Teng, C.M.; Sheu, J.R.; Cheng, Y.W.; Lam, K.K.; Lee, Y.M.; Wu, T.S.; Yen, M.H. Cinnamophilin as a novel antiperoxidative cytoprotectant and free radical scavenger. Biochim. Biophys. Acta 2001, 1525, 77–88. [Google Scholar] [CrossRef]
- Yu, S.M.; Ko, F.N.; Wu, T.S.; Lee, J.Y.; Teng, C.M. Cinnamophilin, a novel thromboxane A2 receptor antagonist, isolated from Cinnamomum philippinense. Eur. J. Pharmacol. 1994, 256, 85–91. [Google Scholar] [CrossRef]
- Su, M.J.; Chen, W.P.; Lo, T.Y.; Wu, T.S. Ionic mechanisms for the antiarrhythmic action of cinnamophilin in rat heart. J. Biomed. Sci. 1999, 6, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.T.; Chang, H. Reduction of reperfusion injury in rat skeletal muscle following administration of cinnamophilin, a novel dual inhibitor of thromboxane synthase and thromboxane A2 receptor. Thorac. Cardiovasc. Surg. 1995, 43, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Chen, H.Y.; Lee, M.Y.; Chen, T.Y.; Hsu, Y.S.; Hu, Y.L.; Chang, G.L.; Wu, T.S. Cinnamophilin reduces oxidative damage and protects against transient focal cerebral ischemia in mice. Free Radic. Biol. Med. 2005, 39, 495–510. [Google Scholar] [CrossRef]
- Lee, E.J.; Chen, H.; Hung, Y.C.; Chen, T.Y.; Lee, M.Y.; Yu, S.C.; Chen, Y.H.; Chuang, I.C.; Wu, T.S. Therapeutic window for cinnamophilin following oxygen-glucose deprivation and transient focal cerebral ischemia. Exp. Neurol. 2009, 217, 74–83. [Google Scholar] [CrossRef]
- Lu, Y.C.; Hsiao, G.; Lin, K.H.; Hsieh, M.S.; Jayakumar, T.; Wu, T.S.; Sheu, J.R. Cinnamophilin isolated from Cinnamomum Philippinense protects against collagen degradation in human chondrocytes. Phytother. Res. 2013, 27, 892–899. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.K.; Shin, H.D.; Lee, H.J.; Jo, H.S.; Jeong, J.H.; Choi, Y.L.; Lee, C.J.; Hwang, S.C. Apigenin regulates interleukin-1β-induced production of matrix metalloproteinase both in the knee joint of rat and in primary cultured articular chondrocytes. Biomol. Ther. 2016, 24, 163–170. [Google Scholar] [CrossRef]
- Chang, X.; He, H.; Zhu, L.; Gao, J.; Wei, T.; Ma, Z.; Ya, T. Protective effect of apigenin on Freund’s complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-κB pathway. Chem. Biol. Interact. 2015, 236, 41–46. [Google Scholar] [CrossRef]
- Li, Y.; Sato, T.; Metori, K.; Koike, K.; Che, Q.M.; Takahashi, S. The promoting effects of geniposidic acid and aucubin in Eucommia ulmoides oliver leaves on collagen synthesis. Biol. Pharm. Bull. 1998, 21, 1306–1310. [Google Scholar] [CrossRef]
- Jin, L.; Xue, H.Y.; Jin, L.J.; Li, S.Y.; Xu, Y.P. Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin-induced diabetes. Eur. J. Pharmacol. 2008, 582, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Xie, G.P.; Qin, C.H.; Chen, Y.R.; Zhang, K.R.; Li, X.; Wu, Q.; Dong, W.Q.; Yang, J.; Yu, B. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matri degradation via inhibition of NF-κB signaling pathway in rat articular chondrocytes. Int. Immunopharmacol. 2015, 24, 408–415. [Google Scholar] [CrossRef]
- Chi, Y.I.; Chuang, S.T.; Hsu, C.H.; Sun, Y.J.; Liu, H.C.; Chen, Y.S.; Lin, F.H. Protective effects of aucubin on osteoarthritic chondrocyte model induced by hydrogen peroxide and mechanical stimulus. BMC Comp. Altern. Med. 2017, 17, 1–11. [Google Scholar]
- Wei, Z.F.; Wang, X.Q.; Peng, X.; Wang, W.; Zhao, C.J.; Zu, Y.G.; Fu, Y.J. Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind. Crops Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Chen, X.; Zhang, Y.; Zhang, Y.; Jia, Y.; Wang, H.; Liu, Y.; Xiao, L. Baicalein ameliorates inflammatory-related apoptotic and catabolic phenotypes in human chondrocytes. Int. Immunopharmacol. 2014, 21, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Song, X.; Bai, H.; Ma, T.; Zhang, Z.; Li, X.; Jiang, R.; Wang, G.; Fan, X.; et al. Effects of baicalein on IL-1β-induced inflammation and apoptosis in rat articular chondrocytes. Oncotarget 2017, 8, 90781–90795. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, J.; Yue, G.; Sun, M.; Li, J.; Xiu, X.; Gao, Z. Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Mol. Med. Rep. 2018, 18, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Li, T.M.; Tan, T.W.; Fong, Y.C.; Tang, C.H. Berberine reduces the metastasis of chondrosarcoma by modulating the α v β 3 integrin and the PKC δ, c-Src, and AP-1 signaling pathways. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Xia, C.; Shi, L.; Wang, S.; Zheng, X.; Hu, T.; Zhang, B. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signaling. J. Cell Mol. Med. 2014, 18, 283–292. [Google Scholar] [CrossRef]
- Liu, S.C.; Lee, H.P.; Hung, C.Y.; Tsai, C.H.; Li, T.M.; Tang, C.H. Berberine attenuates CCN2-induced IL-1β expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol. Appl. Pharmacol. 2015, 289, 20–29. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.Q.; Yu, L.; He, B.; Wu, S.H.; Zhao, Q.; Xia, S.Q.; Mei, H.J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 2015, 20, 1187–1199. [Google Scholar] [CrossRef]
- Wang, X.H.; Jiang, S.M.; Sun, Q.W. Effects of berberine on human rheumatoid arthritis fibroblast-like synoviocytes. Exp. Biol. Med. 2011, 236, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.J.; Lee, H.J.; Jo, H.S.; Nam, D.C.; Lee, Y.B.; Kang, B.H.; Moon, D.K.; Kim, D.H.; Lee, C.J.; Hwang, S.C. Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat. Korean J. Physiol. Pharmacol. 2017, 21, 19–26. [Google Scholar] [CrossRef][Green Version]
- Ko, W.C.; Lin, L.H.; Shen, H.Y.; Lai, C.Y.; Chen, C.M.; Shih, C.H. Biochanin a, a phytoestrogenic isoflavone with selective inhibition of phosphodiesterase 4, suppresses ovalbumin-induced airway hyperresponsiveness. Evid. Based Complement. Altern. Med. 2011, 2011, 635058. [Google Scholar] [CrossRef] [PubMed]
- Kole, L.; Giri, B.; Manna, S.K.; Pal, B.; Ghosh, S. Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011, 653, 8–15. [Google Scholar] [CrossRef]
- Wu, D.Q.; Zhong, H.M.; Ding, Q.H.; Ba, L. Protective effects of biochanin A on articular cartilage: In vitro and in vivo studies. BMC Complement. Altern. Med. 2014, 14, 444. [Google Scholar] [CrossRef]
- Gao, H.Y.; Wu, B.; Li, W.; Chen, D.H.; Wu, L.J. Chemical constituents of Chaenomeles sinensis (Thouin) Koehne. Chin. J. Nat. Med. 2004, 2, 351–353. [Google Scholar]
- Feng, W.Y. Metabolism of green tea catechins: An overview. Curr. Drug Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhao, Y.Q.; Ni, C.L. Chemical constituents of Acacia catechu (L.f) wild. Chin. Tradit. Herb Drugs 2005, 36, 790–792. [Google Scholar]
- Liu, X.Q.; Li, W.W.; Sheng, K.X.; Liu, J.; Chen, F.K. Studies on the chemical constituents of the n-Buoh extract of Polygonum bistorta. J. Shenyang Pharm. Univ. 2006, 23, 15–17. [Google Scholar]
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmed, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Adcocks, C.; Collin, P.; Buttle, D.J. Catechins from green tea (Camellia sinensis) inhibit bovine and human cartilage proteoglycan and type-II collagen degradation in vitro. J. Nutr. 2002, 132, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1β–induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin3-gallate inhibits interleukin-1β-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor-B (NF-B/p65) activation by inhibiting IB-α degradation. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef]
- Singh, R.; Ahmed, S.; Malemud, C.J.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate selectively inhibits interleukin-1-induced activation of mitogen activated protein kinase subgroup c-Jun-N-terminal kinase in human osteoarthritis chondrocytes. J. Orthop. Res. 2002, 21, 102–109. [Google Scholar] [CrossRef]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Goldberg, V.M.; Haqqi, T.M. Phenyl-Ntert-butylnitrone down-regulates interleukin-1-stimulated matrix metalloproteinase-13 gene expression in human chondrocytes: Suppression of c-Jun NH2- terminal kinase, p38-mitogen-activated protein kinase and activating protein-1. J. Pharmacol. Exp. Ther. 2003, 305, 981–988. [Google Scholar] [CrossRef]
- Huang, G.S.; Tseng, C.Y.; Lee, C.H.; Su, S.L.; Lee, H.S. Effects of (-)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE, and IL-8 expression induced by IL-1beta in human synovial fibroblasts. Rheumatol. Int. 2010, 30, 1197–1203. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse posttraumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef]
- Ding, Q.H.; Cheng, Y.; Chen, W.P.; Zhong, H.M.; Wang, X.H. Celastrol, an inhibitor of heat shock protein 90β potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2013, 708, 1–7. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Y.; Cheng, Z.; Guo, Y.; Liu, P.; Wen, Y. Crocin exerts anti-inflammatory and anti-arthritic effects on type II collagen-induced arthritis in rats. Pharm. Biol. 2018, 56, 209–216. [Google Scholar] [CrossRef]
- Ding, Q.; Zhong, H.; Qi, Y.; Cheng, Y.; Li, W.; Yan, S.; Wang, X. Anti-arthritic effects of crocin in interleukin-1β-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm. Res. 2010, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.P.; Yang, S.H.; Chou, C.H.; Yang, K.C.; Wu, C.C.; Cheng, Y.H.; Lin, F.H. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: Inhibition of hydrogen peroxide-induced proinflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm. Res. 2010, 59, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Na, J.Y.; Song, K.B.; Choi, D.S.; Kim, J.H.; Kwon, Y.B.; Kwon, J. Protective effect of ginsenoside Rb1 on hydrogen peroxide-induced oxidative stress in rat articular chondrocytes. J. Ginseng Res. 2012, 36, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wu, D.; Zuo, Q.; Wang, Z.; Fan, W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int. Orthop. 2013, 37, 2065–2070. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Fan, W. Protection of ginsenoside Rg1 on chondrocyte from IL-1β-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol. Cell. Biochem. 2014, 392, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, H.; Shehzad, O.; Kim, Y.S.; Kim, H.P. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase- 13 expression in articular chondrocytes and prevent cartilage degradation. Eur. J. Pharmacol. 2014, 724, 145–151. [Google Scholar] [CrossRef]
- Chen, Y.J.; Tsai, K.S.; Chan, D.C.; Lan, K.C.; Chen, C.F.; Yang, R.S.; Liu, S.H. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J. Orthop. Res. 2014, 32, 573–580. [Google Scholar] [CrossRef]
- Kim, K.R.; Park, K.K.; Chun, K.S.; Chung, W.Y. Honokiol inhibits the progression of collagen-induced arthritis by reducing levels of pro-inflammatory cytokines and matrix metalloproteinases and blocking oxidative tissue damage. J. Pharmacol. Sci. 2010, 114, 69–78. [Google Scholar] [CrossRef]
- Wu, H.; Yin, Z.; Wang, L.; Li, F.; Qiu, Y. Honokiol improved chondrogenesis and suppressed inflammation in human umbilical cord derived mesenchymal stem cells via blocking nuclear factor-κB pathway. BMC Cell Biol. 2017, 18, 1–13. [Google Scholar] [CrossRef]
- Li, D.; Yuan, T.; Zhang, X.; Xiao, Y.; Wang, R.; Fan, Y.; Zhang, X. Icariin: A potential promoting compound for cartilage tissue engineering. Osteoarthr. Cartil. 2012, 20, 1647–1656. [Google Scholar] [CrossRef]
- Kang, B.J.; Ryu, J.; Lee, C.J.; Hwang, S.C. Luteolin inhibits the activity, secretion and gene expression of MMP-3 in cultured articular chondrocytes and production of MMP-3 in the rat knee. Biomol. Ther. 2014, 22, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, L.; Li, L.; Chen, S. Monotropein exerts protective effects against IL-1β-induced apoptosis and catabolic responses on osteoarthritis chondrocytes. Int. Immunopharmacol. 2014, 23, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Gao, F.; Li, M.; Wei, P. Monotropein accelerates chondrocyte progression in osteoarthritis by alleviating TNF-α induced inflammation through regulation of MAPK/NF-κB signaling pathway. Int. J. Clin. Exp. Med. 2020, 13, 417–428. [Google Scholar]
- Xie, M.X.; Long, M.; Liu, Y.; Qin, C.; Wang, Y.D. Characterization of the interaction between human serum albumin and morin. Biochim. Biophys. Acta 2006, 1760, 1184–1191. [Google Scholar] [CrossRef]
- Bartosikova, L.; Necas, I.; Suchy, V. Monitoring of antioxidative effect of morin in alloxan-induced diabetic mellitus in the laboratory rat. Acta Vet. Brno 2003, 72, 191–200. [Google Scholar] [CrossRef]
- Galvez, J.; Coelho, G.; Crespo, M.E.; Cruz, T.; Rodriguez-Cabezas, M.E.; Concha, A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment. Pharmacol. Ther. 2001, 15, 2027–2039. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, J.H.; Hwang, B.Y.; Mun, S.H.; Ko, N.Y.; Kim, K. Morin inhibits Fyn kinase in mast cells and IgE-mediated type I hypersensitivity response in vivo. Biochem. Pharmacol. 2009, 77, 1506–1512. [Google Scholar] [CrossRef]
- Chen, W.P.; Wang, Y.L.; Tang, J.L.; Hu, P.F.; Bao, J.P.; Wu, L.D. Morin inhibits interleukin 1β induced nitric oxide and prostaglandin E2 production in human chondrocytes. Int. Immunopharmacol. 2012, 12, 447–452. [Google Scholar] [CrossRef]
- Sultana, F.; Rasool, M. A Novel therapeutic approach targeting rheumatoid arthritis by combined administration of morin, a dietary flavanol and non-Steroidal anti-inflammatory drug indomethacin with reference to pro-inflammatory cytokines, inflammatory enzymes, RANKL and transcription factors. Chem. Biol. Interact. 2015, 230, 58–70. [Google Scholar]
- Chen, W.P.; Hu, P.F.; Bao, J.P.; Wu, L.D. Morin exerts antiosteoarthritic properties: An in vitro and in vivo study. Exp. Biol. Med. 2012, 237, 380–386. [Google Scholar] [CrossRef]
- Long, C.; Yang, J.; Yang, H.; Li, X.; Wang, G. Attenuation of renal ischemia/reperfusion injury by oleanolic acid preconditioning via its antioxidant, anti-inflammatory, and anti-apoptotic activities. Mol. Med. Rep. 2016, 13, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Kim, S.W.; Kim, D.S.; Lee, J.Y.; Lee, S.; Oh, H.M.; Ha, Y.S.; Yoo, J.; Park, P.H.; Shin, T.Y.; et al. Oleanolic acid acetate inhibits rheumatoid arthritis by modulating T cell immune responses and matrix-degrading enzymes. Toxicol. Appl. Pharmacol. 2016, 290, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Lee, H.J.; Kim, K.T.; Hwang, S.C.; Lee, C.J.; Park, J.S. Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo. Korean J. Physiol. Pharmacol. 2017, 21, 197–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Onodera, S.; Kaneda, K.; Mizue, Y.; Koyama, Y.; Fujinaga, M.; Nishihira, J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J. Biol. Chem. 2000, 275, 444–450. [Google Scholar] [CrossRef]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [Google Scholar] [CrossRef]
- Lin, S.K.; Kok, S.H.; Yeh, F.T.; Kuo, M.Y.; Lin, C.C.; Wang, C.C. MEK/ERK and signal transducer and activator of transcription signaling pathways modulate oncostatin M-stimulated CCL2 expression in human osteoblasts through a common transcription factor. Arthritis Rheum. 2004, 50, 785–793. [Google Scholar] [CrossRef]
- Therkleson, T. Ginger compress therapy for adults with osteoarthritis. J. Adv. Nurs. 2010, 66, 2225–2233. [Google Scholar] [CrossRef]
- Srivastava, K.C.; Mustafa, T. Ginger (Zingiber officinale ) in rheumatism and musculoskeletal disorders. Med. Hypotheses 1992, 39, 342–348. [Google Scholar] [CrossRef]
- Kundu, J.K.; Na, H.K.; Surh, Y.J. Ginger-derived phenolic substances with cancer preventive and therapeutic potential. Forum Nutr. 2009, 61, 182–192. [Google Scholar]
- Ahn, S.I.; Lee, J.K.; Youn, H.S. Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol. Cells 2009, 27, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.S.; Simon, O.; Shelly, J.; Gardener, M. 6-Shogaol reduced chronic inflammatory response in the knees of rats treated with complete Freund’s adjuvant. BMC Pharmacol. 2006, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Villalvilla, A.; da Silva, J.A.; Largo, R.; Gualillo, O.; Vieira, P.C.; Herrero-Beaumont, G.; Gómez, R. 6-Shogaol inhibits chondrocytes’ innate immune responses and cathepsin-K activity. Mol. Nutr. Food Res. 2014, 58, 256–266. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayakumar, T.; Saravana Bhavan, P.; Sheu, J.-R. Molecular Targets of Natural Products for Chondroprotection in Destructive Joint Diseases. Int. J. Mol. Sci. 2020, 21, 4931. https://doi.org/10.3390/ijms21144931
Jayakumar T, Saravana Bhavan P, Sheu J-R. Molecular Targets of Natural Products for Chondroprotection in Destructive Joint Diseases. International Journal of Molecular Sciences. 2020; 21(14):4931. https://doi.org/10.3390/ijms21144931
Chicago/Turabian StyleJayakumar, Thanasekaran, Periyakali Saravana Bhavan, and Joen-Rong Sheu. 2020. "Molecular Targets of Natural Products for Chondroprotection in Destructive Joint Diseases" International Journal of Molecular Sciences 21, no. 14: 4931. https://doi.org/10.3390/ijms21144931
APA StyleJayakumar, T., Saravana Bhavan, P., & Sheu, J.-R. (2020). Molecular Targets of Natural Products for Chondroprotection in Destructive Joint Diseases. International Journal of Molecular Sciences, 21(14), 4931. https://doi.org/10.3390/ijms21144931







