Characterization and Comparison of Two Complete Plastomes of Rosaceae Species (Potentilla dickinsii var. glabrata and Spiraea insularis) Endemic to Ulleung Island, Korea
Abstract
:1. Introduction
2. Results and Discussion
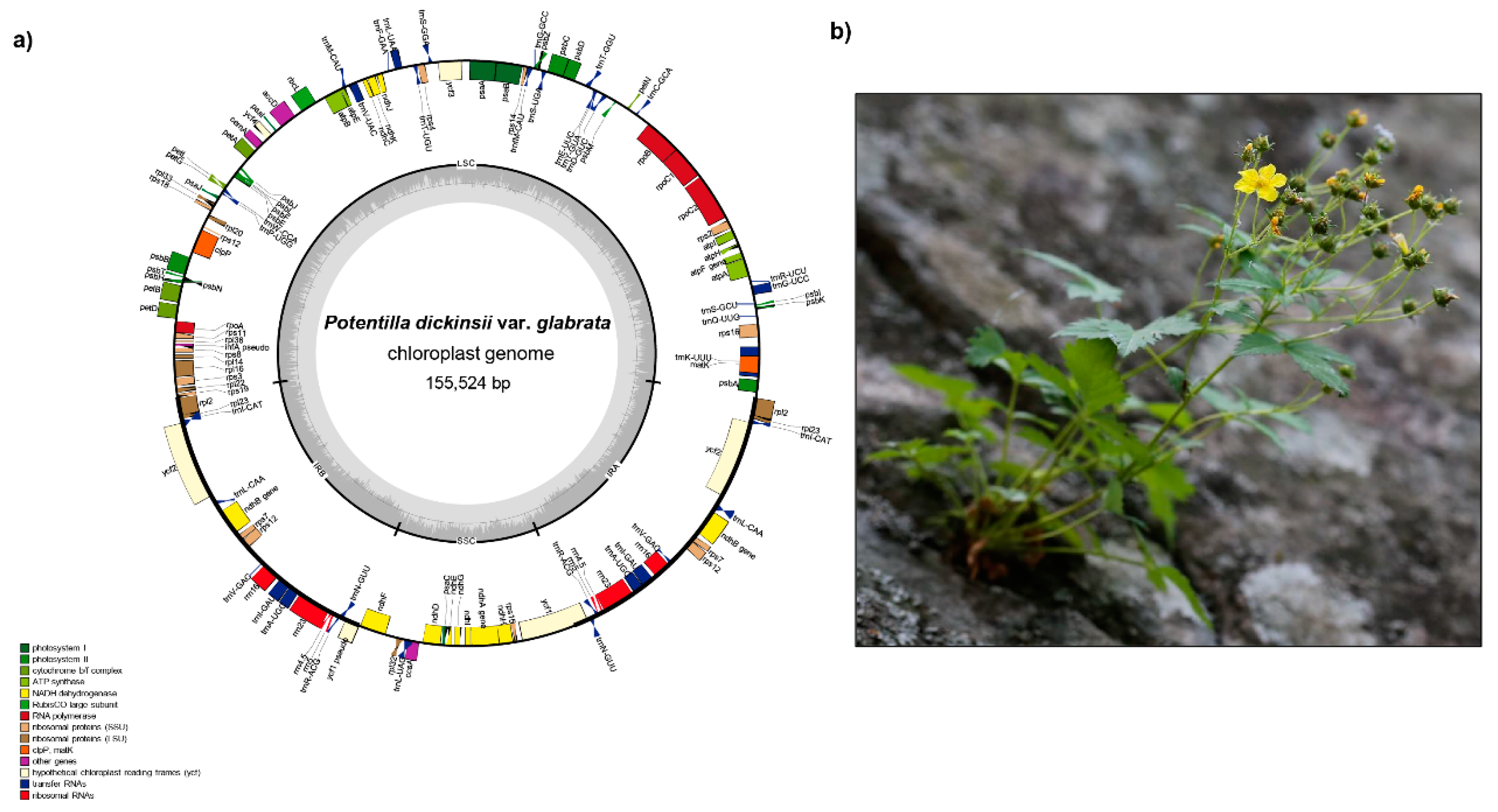
2.1. Genome Size and Features
2.2. Comparative Analysis of Genome Structure
2.3. Phylogenetic Analysis
3. Materials and Methods
3.1. Plant Sampling, DNA Isolation, and Plastome Sequencing/Annotation
3.2. Comparative Plastome Analysis
3.3. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenaut, M.; Dickinson, T.A.; et al. Phylogeney and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, economic importance, genomics. In Genetics and Genomics of Rosaceae; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–17. [Google Scholar]
- Phipps, J.B. Magnoliophyta: Picramniaceae to Rosaceae. In Flora of North America North of Mexico; Oxford University Press: New York, NY, USA; Oxford, UK, 2014; Volume 9. [Google Scholar]
- Vamosi, J.C.; Dickinson, T.A. Polyploidy and diversification: A phylogenetic investigation in Rosaceae. Int. J. Plant Sci. 2006, 167, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, T.A.; Lo, E.; Talent, N. Polyploidy, reproductive biology, and Rosaceae: Understanding evolution and making classifications. Plant Syst. Evol. 2007, 266, 59–78. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, C.-H.; Hu, Y.; Wen, J.; Li, S.; Yi, T.; Chen, H.; Xiang, J.; Ma, H. Evolution of Rosaceae fruit types based on nuclear phylogeny in the context of geological times and genome duplication. Mol. Biol. Evol. 2016, 34, 262–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforova, S.V.; Cavalieri, D.; Velasco, R.; Goremykin, V. Phylogenetic Analysis of 47 Chloroplast Genomes Clarifies the Contribution of Wild Species to the Domesticated Apple Maternal Line. Mol. Biol. Evol. 2013, 30, 1751–1760. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Khan, M.A.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, S.W.; Shaw, J.; Haberle, R.; Wen, J.; Potter, D. Diversification of almonds, peaches, plums and cherries-molecular systematics and biogeographic history of Prunus (Rosaceae). Mol. Phylogentics Evol. 2014, 76, 34–48. [Google Scholar] [CrossRef]
- Zhang, S.-D.; Jin, J.J.; Chen, S.Y.; Chase, M.W.; Soltis, D.E.; Li, H.T.; Yang, J.B.; Li, D.Z.; Yi, T.S. Diversification of Rosaceae since the late Craetaceous based on plastid phylogenomics. New Phytol. 2017, 214, 1355–1367. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K. Petrology of Ulreung volcanic island, Korea–part 1. Geology. J. Japan. AssocC Mineral Petrol Econ. Geol. 1985, 80, 128–135. [Google Scholar] [CrossRef]
- Sun, B.Y.; Stuessy, T.F. Preliminary observations on the evolution of endemic angiosperms of Ullung Island, Korea. In Evolution and Speciation of Island Plants; Stuessy, T.F., Ono, M., Eds.; Cambridge University Press: New York, NY, USA, 1998; pp. 181–202. [Google Scholar]
- Sun, B.-Y.; Shin, H.; Hyun, J.-O.; Kim, Y.-D.; Oh, S.-H. Vascular Plants of Dokdo and Ulleungdo Islands in Korea; The National Institute of Biological Resources, GeoBook Publishing Co.: Seoul, Korea, 2014. [Google Scholar]
- Stuessy, T.F.; Jakubowsky, G.; Gómez, R.S.; Pfosser, M.; Schlüter, P.M.; Fer, T.; Sun, B.-Y.; Kato, H. Anagenetic evolution in island plants. J. Biogeogr. 2006, 33, 1259–1265. [Google Scholar] [CrossRef]
- Givnish, T.J.; Sytsma, K.J.; Smith, J.F.; Hahn, W.J. Thorn-like prickles and heterophylly in Cyanea: Adaptations to extinct avian browsers on Hawaii? Proc. Natl. Acad. Sci. USA 1994, 91, 2810–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfosser, M.; Jakubowsky, G.; Schluter, P.M.; Fer, T.; Kato, H.; Stuessy, T.F.; Sun, B.Y. Evolution of Dystaenia takesimana (Apiaceae), endemic to Ullung Island, Korea. Plant Syst. Evol. 2006, 256, 159–170. [Google Scholar] [CrossRef]
- Pfosser, M.; Guzy-Wrobelska, J.; Sun, B.Y.; Stuessy, T.F.; Sugawara, T.; Fujii, N. The origin of species of Acer (Sapindaceae) endemic to Ullung Island, Korea. Syst. Bot. 2002, 27, 351–367. [Google Scholar]
- Takayama, K.; Sun, B.Y.; Stuessy, T.F. Genetic consequences of anagenetic speciation in Acer okamotoanum (Sapindaceae) on Ullung Island, Korea. Ann. Bot. 2012, 109, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, K.; Sun, B.Y.; Stuessy, T.F. Anagenetic speciation in Ullung Island, Korea: Genetic diversity and structure in the island endemic species, Acer takesimense (Sapindaceae). J. Plant Res. 2013, 126, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.Y.; Pak, J.-H.; Maki, M.; Kim, S.-C. Multiple origins and the population genetic structure of Rubus takesimensis (Rosaceae) on Ulleung Island: Implications for the genetic consequences of anagenetic speciation. PLoS ONE 2019, 14, e0222707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, T. Notulae ad plantas Japoniae et Koreae XVII. Bot. Mag. Tokyo 1918, 32, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Nakai, T. Report on the Vegetation of the Island Ooryongto or Dagelet Island; The Government of Chosen: Seoul, Korea, 1919.
- Oh, S.Y. Floral studies on the vascular plants of the Dagelet Island. Res. Rev. Kyungpook Natl. Univ. 1978, 25, 131–201. [Google Scholar]
- Lee, W.T.; Yang, I.S. The flora of Ulreung Is. and Dogdo Island. Rep. KACN 1981, 19, 61–95. [Google Scholar]
- National Institute of Biological Resources. Spiraea insularis. In Korean Red List of Threatened Species, 2nd ed.; Suh, M.-H., Lee, B.-Y., Kim, S.T., Park, C.-H., Oh, H.-K., Kim, H.-Y., Lee, J.-H., Lee, S.Y., Eds.; Ministry of environment: Incheon, Korea, 2014; p. 141. [Google Scholar]
- Kim, C.H.; Kim, T.J.; Sun, B. Taxonomic identities of some endemic Korean vascular plants. Korean J. Plant Taxon. 2000, 30, 355–361. [Google Scholar] [CrossRef]
- Chung, T.H. Korean Flora; Shinjisa: Seoul, Korea, 1957. [Google Scholar]
- Lee, S. Physocarpus. In Genera of Vascular Plants of Korea; Park, C.W., Ed.; Academy: Seoul, Korea, 2007; pp. 538–539. [Google Scholar]
- Lee, B.C. Rare Plants Data Book in Korea; Korea National Arboretum: Pocheon, Korea, 2008. [Google Scholar]
- Oh, S.H.; Potter, D. Molecular phylogenetic systematics and biogeography of tribe Neillieae (Rosaceae) using DNA sequences of cpDNA, rDNA, and LEAFY. Am. J. Bot. 2005, 92, 179–192. [Google Scholar] [CrossRef]
- Oh, S.-H.; Chen, L.; Kim, S.-H.; Kim, Y.-D.; Shin, H.C. Phylogenetic Relationship of Physocarpus insularis (Rosaceae) Endemic on Ulleung Island: Implications for Conservation Biology. J. Plant Biol. 2010, 53, 94–105. [Google Scholar] [CrossRef]
- Shin, H.C.; Kim, Y.-D.; Oh, S.-H. A New Combination in Spiraea (Rosaceae) from Ulleung Island, Korea. Novon 2011, 21, 373–374. [Google Scholar] [CrossRef]
- Naruhashi, N. Rosoideae. In Flora of Japan, Vol. IIb; Iwatsuki, K., Boufford, D.E., Ohba, H., Eds.; Kodansha: Tokyo, Japan, 2003; pp. 145–212. [Google Scholar]
- Heo, K.-I.; Lee, S.R.; Kim, Y.S.; Park, J.S.; Lee, S.T. Taxonomic studies of the tribe Potentilleae (Rosaceae) in Korea. Korean J. Plant Taxon. 2019, 49, 28–69. [Google Scholar] [CrossRef] [Green Version]
- Asker, S. Studies in Apomictic and Sexual Potentilla L.; Berlingska Boktrycheriet: Lund, Denmark, 1971; pp. 2–7. [Google Scholar]
- Soják, J. Notes on Potentilla I. Hybridogenous species derived from intersectional hybrids of sect. Niveae× sect. Multifidae. Bot. Jahrb. Syst. 1986, 106, 145–210. [Google Scholar]
- Eriksen, B. Mating systems in two species of Potentilla from Alaska. Folia Geobot. Phytotax. 1996, 31, 333–344. [Google Scholar] [CrossRef]
- Bock, R. Structure, function, and inheritance of plastid genomes. In Cell and Molecular Biology of Plastids; Bock, R., Ed.; Topics in Current Genetics; Springer: Berlin, Germany, 2007; Volume 19, pp. 29–63. [Google Scholar]
- Jansen, R.K.; Cai, Z.; Raubeson, L.A.; Daniell, H.; dePamphilis, C.W.; Leebens-Mack, J.; Müller, K.F.; Guisinger-Bellian, M.; Haberle, R.C.; Hansen, A.K.; et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA 2007, 104, 19369–19374. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Q.; Zuo, Y.J.; Zhu, X.X.; Liao, S.; Ma, J.S. Complete chloroplast genomes and comparative analysis of sequences evolution among seven Aristolochia (Aristolochiaceae) medicinal species. Int. J. Mol. Sci. 2019, 20, 1045. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.-S.; Yang, J.Y.; Yang, T.-J.; Kim, S.-C. Evolutionary comparison of the chloroplast genome in the woody Sonchus alliance (Asteraceae) on the Canary Islands. Genes 2019, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L.; Jaiswal, P.; Mockaitis, K.; Liston, A.; Mane, S.P.; et al. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 2010, 43, 109–116. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K.; Saski, C.; Lee, S.B.; Hansen, A.K.; Daniell, H. Complete plastid genome sequences of three rosids (Castanea, Prunus, Theobroma): Evidence for at least two independent transfers of rpl22 to the nucleus. Mol. Biol. Evol. 2011, 28, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Terakami, S.; Matsumura, Y.; Kurita, K.; Kanamori, H.; Katayose, Y.; Yamamoto, T.; Katayama, H. Complete sequence of the chloroplast genome from pear (Pyrus pyrifoliae): Genome structure and comparative analysis. Tree Genet. Genomes 2012, 8, 841–854. [Google Scholar] [CrossRef]
- Yang, J.Y.; Pak, J.-H.; Kim, S.-C. The complete chloroplast genome sequence of Korean raspberry Rubus crataegifolius (Rosaceae). Mitochondrial DNA B 2017, 2, 793–794. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.Y.; Pak, J.-H.; Kim, S.-C. The complete plastome sequence of Rubus takesimensis endemic to Ulleung Island, Korea: Insights into molecular evolution of anagenetically derived species in Rubus (Rosaceae). Gene 2018, 668, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Pak, J.-H.; Kim, S.-C. Chloroplast genome of critically endangered Cotoneaster wilsonii (Rosaceae) endemic to Ulleung Island, Korea. Mitochondrial DNA B 2019, 4, 3892–3893. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.H.; Kim, S.-C. Comparative analysis of the complete chloroplast genome sequences of three closely related East-Asian wild Roses (Rosa sect. Synstylae; Rosaceae). Genes 2019, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Cheon, K.S.; Kim, K.A.; Jang, S.K.; Yoo, K.O. Complete chloroplast genome sequence of Campanula takesimana (Campanulaceae), an endemic to Korea. Mitochondrial DNA B 2014, 1, 184–185. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, W.; Pak, J.-H. The complete plastid genome sequence of Chrysanthemum lucidum (Asteraceae): An endemic species of Ulleung Island of Korea. Mitochondrial DNA B 2018, 3, 476–477. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-S.; Jin, D.-P.; Park, J.-W.; Choi, B.-H. Complete chloroplast genome of Fagus multinervis, a beech species endemic to Ulleung Island in South Korea. Mitochondrial DNA B 2019, 4, 1698–1699. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Hwang, Y.-J.; Lee, S.-C.; Yang, T.-J.; Lim, K.-B. The complete chloroplast genome sequence of Lilium hansonii Leichtlin ex D. D. T. Moore. Mitochondrial DNA A 2016, 27, 3678–3679. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, W.; Pak, J.-H.; Kim, S.-C. Complete chloroplast genome of Ulleung Island endemic basswood, Tilia insularis (Malvaceae) in Korea. Mitochondrial DNA B 2018, 3, 605–606. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.Y.; Chung, J.-M.; Kim, S.-C. Complete chloroplast genome of Ulleung Island endemic, Epilobium ulleungensis (Onagraceae), in Korea. Mitochondrial DNA B 2018, 3, 703–704. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.Y.; Lee, W.; Pak, J.-H.; Kim, S.-C. Complete chloroplast genome of Ulleung Island endemic pokeweed, Phytolacca insularis (Phytolaccaceae), in Korea. Mitochondrial DNA B 2019, 4, 8–9. [Google Scholar] [CrossRef]
- Kim, H.T.; Pak, J.-H.; Kim, J.S. The complete chloroplast genome sequence of Acer takesimense (Sapindaceae), an endemic to Ullenung Island of Korea. Mitochondrial DNA B 2019, 4, 1531–1532. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.-S.; Yang, J.Y.; Kim, S.-C. Complete chloroplast genome of Ulleung Island endemic flowering cherry, Prunus takesimensis (Rosaceae), in Korea. Mitochondrial DNA B 2018, 3, 274–275. [Google Scholar] [CrossRef]
- Gil, H.-Y.; Kim, S.-C. The plastome sequence of Ulleung Rowan, Sorbus ulleungensis (Rosaceae), a new endemic species on Ulleung Island, Korea. Mitochondrial DNA B 2018, 3, 284–285. [Google Scholar] [CrossRef]
- Yang, J.Y.; Takayama, K.; Pak, J.-H.; Kim, S.-C. Comparison of the Whole-Plastome Sequence between the Bonin Islands Endemic Rubus boninensis and Its Close Relative, Rubus trifidus (Rosaceae), in the Southern Korean Peninsula. Genes 2019, 10, 774. [Google Scholar] [CrossRef] [Green Version]
- Ravi, V.; Khurana, J.P.; Tyagi, A.K.; Khurana, P. An update on chloroplast genomes. Plant Syst. Evol. 2008, 271, 101–122. [Google Scholar] [CrossRef]
- Morton, B.R. Selection on the codon bias of chloroplast and cyanelle genes in different plant and algal lineages. J. Mol. Evol. 1998, 46, 449–459. [Google Scholar] [CrossRef]
- Rabah, S.O.; Lee, C.; Hajrah, N.H.; Makki, R.M.; Alharby, H.F.; Alhebshi, A.M.; Sabir, J.; Jansen, R.K.; Ruhlman, T.A. Plastome sequencing of ten nonmodel crop species uncovers a large insertion of mitochondrial DNA in cashew. Plant Genome 2017, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinard, D.; Myburg, A.A.; Mizrachi, E. The plastid and mitochondrial genomes of Eucalyptus grandis. BMC Genom. 2019, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Yang, J.Y.; Park, J.S.; Yamada, T.; Maki, M.; Kim, S.-C. Comparison of Whole Plastome Sequences between Thermogenic Skunk Cabbage Symplocarpus renifolius and Nonthermogenic S. nipponicus (Orontioideae; Araceae) in East Asia. Int. J. Mol. Sci. 2019, 20, 4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evolut. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z. PAML: A program package for phylogenetic analysis by maximum likelihood. Bioinformatics 1997, 13, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Makałowski, W.; Boguski, M.S.; Hughes, A.L.; Yeager, M. Synonymous and nonsynonymous substitution distances are correlated in mouse and rat genes. J. Mol. Evol. 1998, 47, 119–121. [Google Scholar] [CrossRef]
- Sloan, D.B.; Triant, D.A.; Forrester, N.J.; Bergner, L.M.; Wu, M.; Taylor, D.R. A recurring syndrome of accelerated plastid genome evolution in the angiosperm tribe Sileneae (Caryophyllaceae). Mol. Phylogenet. Evol. 2014, 72, 82–89. [Google Scholar] [CrossRef]
- Cheng, H.; Li, J.; Zhang, H.; Cai, B.; Gao, Z.; Qiao, Y.; Mi, L. The complete chloroplast genome sequence of strawberry (Fragaria×ananassa Duch.) and comparison with related species of Rosaceae. PeerJ 2017, 5, e3919. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Shi, F.X.; Li, M.R.; Liu, B.; Wen, J.; Xiao, H.X.; Li, L.F. Positive selection driving cytoplasmic genome evolution of the medicinally important ginseng plant genus Panax. Front. Plant Sci. 2018, 9, 359. [Google Scholar] [CrossRef]
- Li, P.; Lou, G.; Cai, X.; Zhang, B.; Cheng, Y.; Wang, H. Comparison of the complete plastomes and the phylogenetic analysis of Paulownia species. Sci. Rep. 2020, 10, 2225. [Google Scholar] [CrossRef] [Green Version]
- Piot, A.; Hackel, J.; Christin, P.A.; Besnard, G. One-third of the plastid genes evolved under positive selection in PACMAD grasses. Planta 2018, 247, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Jiang, M.; Chen, H.; Huang, L.; Liu, C. Complete plastome sequence of Iodes cirrhosa Turcz., the first in the Icacinaceae, comparative genomic analyses and possible split of Idoes species in response to climate changes. PeerJ 2019, 7, e6663. [Google Scholar] [CrossRef] [PubMed]
- Burri, R.; Salamin, N.; Studer, R.A.; Roulin, A.; Fumagalli, L. Adaptive divergence of ancient gene duplicates in the avian MHC class II beta. Mol. Biol. Evol. 2010, 27, 2360–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbino, D.R.; Birney, E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008, 18, 821–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Bock, R. Organellar genome DRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2009, 25, 1451–1452. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software v7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP v6: DNA sequence polymorphismanalysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis v7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Sharp, P.M.; Li, W.H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J. Mol. Evol. 1986, 24, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol. Rev. 1983, 47, 1–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mower, J.P. The PREP suite: Predictive RNA editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res. 2009, 37, W253–W259. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]





| Taxa | Potentilla dickinsii var. glabrata | Spiraea insularis | Cotoneaster wilsonii | Prunus takesimensis | Rubus takesimensis | Sorbus ulleungensis |
|---|---|---|---|---|---|---|
| Total cpDNA size (bp) | 155,524 | 158,637 | 159,999 | 157,948 | 155,760 | 159,632 |
| GC content (%) | 37.0% | 36.9% | 36.6% | 36.7% | 37.1% | 36.5% |
| LSC size (bp)/GC content (%) | 85,213/34.8% | 86,997/34.8% | 87,868/34.2% | 85,959/34.6% | 85,402/35.1% | 88,003/34.2% |
| IR size (bp)/GC content (%) | 25,827/42.8% | 26,365/42.5% | 26,399/42.6% | 26,436/42.5% | 25,781/42.8% | 26,402/42.6% |
| SSC size (bp)/GC content (%) | 18,657/30.7% | 18,910/30.5% | 19,335/30.3% | 19,117/30.2% | 18,750/31.1% | 18,824/30.5% |
| Number of genes | 131 | 132 | 131 | 131 | 131 | 131 |
| Number of protein-coding genes | 84 | 84 | 84 | 84 | 84 | 84 |
| Number of tRNA genes | 37 | 37 | 37 | 37 | 37 | 37 |
| Number of rRNA genes | 8 | 8 | 8 | 8 | 8 | 8 |
| Number of duplicated genes | 17 | 17 | 17 | 17 | 17 | 17 |
| Number of single intron genes | 15 (intron loss in atpF) | 16 | 16 | 16 | 15 (intron loss in atpF) | 16 |
| Number of two intron genes | 2 | 2 | 2 | 2 | 2 | 2 |
| Accession Number | MT412406 | MT412405 | NC046834 | NC039379 | NC037991 | NC03702 |
| Gene Name | Models | np | ln L | Likelihood Ratio Test p-Value | Positively Selected Sites |
|---|---|---|---|---|---|
| accD | M8 | 14 | −3164.733948 | 0.053131782 | 134 M 0.971 * |
| M7 | 12 | −3167.668928 | |||
| ndhB | M8 | 14 | −2187.954632 | 0.000001793 | 371 S 0.974 * |
| M7 | 12 | −2201.186056 | |||
| ndhD | M8 | 14 | −2896.605326 | 0.031007257 | 42 I 0.959 * |
| M7 | 12 | −2900.078860 | |||
| ndhF | M8 | 14 | −4982.146816 | 0.000002084 | 643 S 0.952 *; 649 Q 0.990 *; 671 G 0.994 ** |
| M7 | 12 | −4995.227947 | |||
| rbcL | M8 | 14 | −2701.384653 | 0.000000000 | 86 H 0.980 *; 142 T 0.990 **; 247 C 0.999 **; 279 S 0.990 **; 475 L 0.998 ** |
| M7 | 12 | −2729.389370 | |||
| rps3 | M8 | 14 | −1248.202111 | 0.000949733 | 97 T 0.952 *; 118 A 0.953 * |
| M7 | 12 | −1255.161441 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Kang, G.-H.; Pak, J.-H.; Kim, S.-C. Characterization and Comparison of Two Complete Plastomes of Rosaceae Species (Potentilla dickinsii var. glabrata and Spiraea insularis) Endemic to Ulleung Island, Korea. Int. J. Mol. Sci. 2020, 21, 4933. https://doi.org/10.3390/ijms21144933
Yang J, Kang G-H, Pak J-H, Kim S-C. Characterization and Comparison of Two Complete Plastomes of Rosaceae Species (Potentilla dickinsii var. glabrata and Spiraea insularis) Endemic to Ulleung Island, Korea. International Journal of Molecular Sciences. 2020; 21(14):4933. https://doi.org/10.3390/ijms21144933
Chicago/Turabian StyleYang, JiYoung, Gi-Ho Kang, Jae-Hong Pak, and Seung-Chul Kim. 2020. "Characterization and Comparison of Two Complete Plastomes of Rosaceae Species (Potentilla dickinsii var. glabrata and Spiraea insularis) Endemic to Ulleung Island, Korea" International Journal of Molecular Sciences 21, no. 14: 4933. https://doi.org/10.3390/ijms21144933
APA StyleYang, J., Kang, G.-H., Pak, J.-H., & Kim, S.-C. (2020). Characterization and Comparison of Two Complete Plastomes of Rosaceae Species (Potentilla dickinsii var. glabrata and Spiraea insularis) Endemic to Ulleung Island, Korea. International Journal of Molecular Sciences, 21(14), 4933. https://doi.org/10.3390/ijms21144933






