The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy
Abstract
:1. Introduction
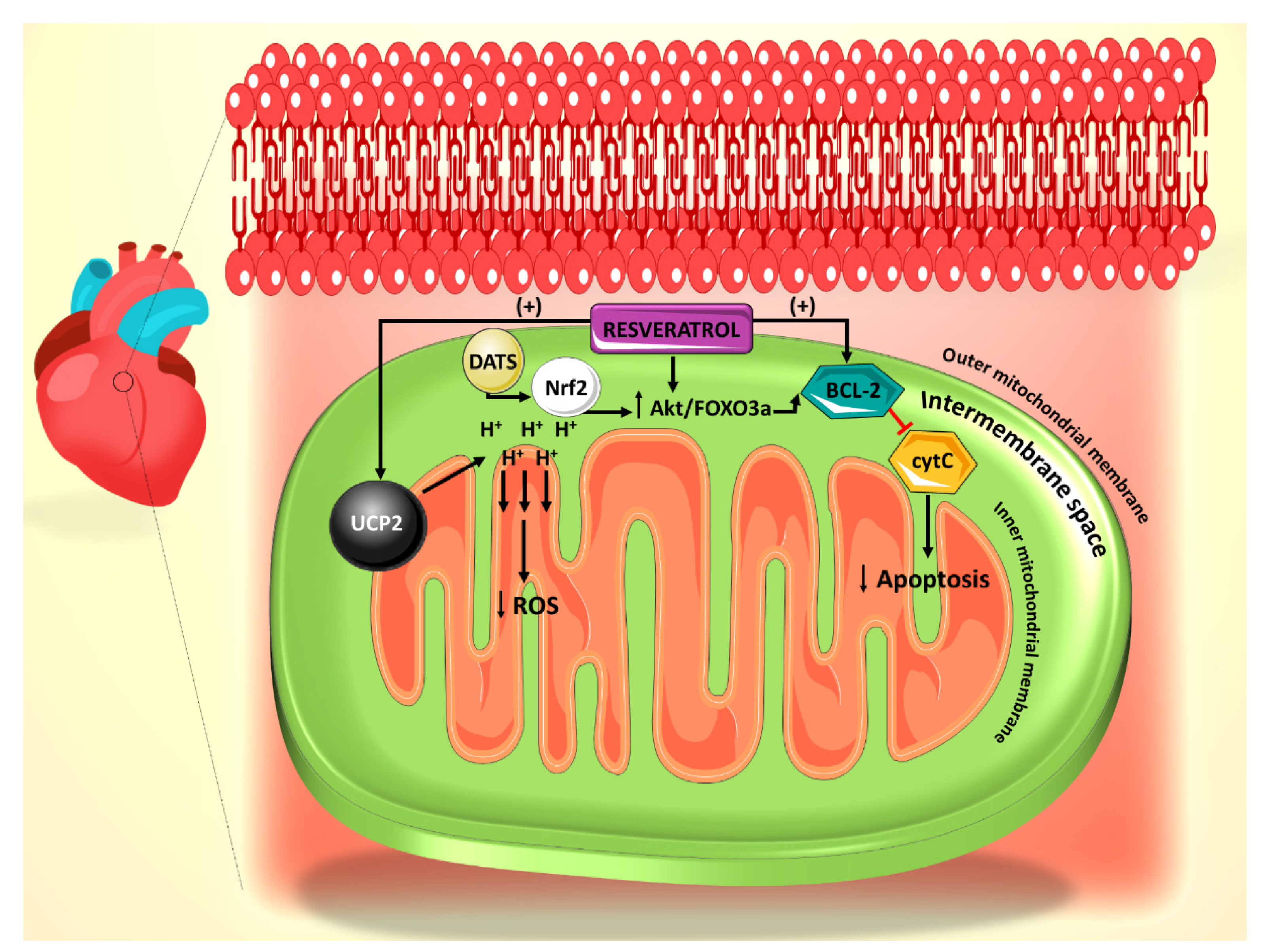
2. Effect of Resveratrol on the Mitochondrial ROS Generation and Apoptosis Pathways
3. Effect of Resveratrol on the SIRT1-Dependent Mitochondrial Biogenesis Pathway
4. Role of Resveratrol on Mitochondrial Lipid Oxidation
5. Role of Polyphenols in Autophagy–Apoptosis Interactions and Their Role in Diabetic Cardiomyopathy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aboumsallem, J.P.; Muthuramu, I.; Mishra, M.; Kempen, H.; De Geest, B. Effective Treatment of Diabetic Cardiomyopathy and Heart Failure with Reconstituted HDL (Milano) in Mice. Int. J. Mol. Sci. 2019, 20, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, I.; Nuti, R.; Picchioni, T.; Dotta, F.; Palazzuoli, A. Molecular Dysfunction and Phenotypic Derangement in Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Kang, Y.J. Cell death and diabetic cardiomyopathy. Cardiovasc. Toxicol. 2003, 3, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Liang, Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 252–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell Boil. 2011, 21, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasun, P. Treatment of Mitochondrial Diseases. In Mitochondrial Medicine; Prasun, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 15–20. [Google Scholar] [CrossRef]
- Diano, S.; Horvath, T.L. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol. Med. 2012, 18, 52–58. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochem. (Mosc.) 2005, 70, 200–214. [Google Scholar] [CrossRef]
- Eisenberg-Lerner, A.; Bialik, S.; Simon, H.-U.; Kimchi, A. Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009, 16, 966–975. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Sabu, M.; Smitha, K.; Kuttan, R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002, 83, 109–116. [Google Scholar] [PubMed]
- Brasnyó, P.; Molnar, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, Á.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols Stimulate AMP-Activated Protein Kinase, Lower Lipids, and Inhibit Accelerated Atherosclerosis in Diabetic LDL Receptor-Deficient Mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, J.-X.; Ou, J.-Y.; Dai, H.; Li, H.-Y.; Huang, W.; Hua, H.-Y.; Xie, T.; Wang, M.; Yang, Y.-G. Antioxidant and Antiapoptotic Polyphenols from Green Tea Extract Ameliorate CCl4-Induced Acute Liver Injury in Mice. Chin. J. Integr. Med. 2019, 1–9. [Google Scholar] [CrossRef]
- Fardoun, M.M.; Maaliki, D.; Halabi, N.; Iratni, R.; Bitto, A.; Baydoun, E.; Eid, A.H. Flavonoids in adipose tissue inflammation and atherosclerosis: One arrow, two targets. Clin. Sci. 2020, 134, 1403–1432. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.; Pintus, G.; El-Yazbi, A.F.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Alsamri, H.; El Hasasna, H.; Al Dhaheri, Y.; Eid, A.H.; Attoub, S.; Iratni, R. Carnosol, a Natural Polyphenol, Inhibits Migration, Metastasis, and Tumor Growth of Breast Cancer via a ROS-Dependent Proteasome Degradation of STAT3. Front. Oncol. 2019, 9, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Dhaheri, Y.; Attoub, S.; Ramadan, G.; Arafat, K.; Bajbouj, K.; Karuvantevida, N.; AbuQamar, S.F.; Eid, A.; Iratni, R. Carnosol Induces ROS-Mediated Beclin1-Independent Autophagy and Apoptosis in Triple Negative Breast Cancer. PLoS ONE 2014, 9, e109630. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posadino, A.M.; Giordo, R.; Cossu, A.; Nasrallah, G.K.; Shaito, A.; Abou-Saleh, H.; Eid, A.H.; Pintus, G. Flavin Oxidase-Induced ROS Generation Modulates PKC Biphasic Effect of Resveratrol on Endothelial Cell Survival. Biomolecules 2019, 9, 209. [Google Scholar] [CrossRef] [Green Version]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Van Nguyen, L.H.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.M.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Yi, L.; Jin, X.; Liang, X.-Y.; Zhou, Y.; Zhang, T.; Xie, Q.; Zhou, X.; Chang, H.; Fu, Y.-J.; et al. Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 2013, 9, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; An, R. Resveratrol and sildenafil synergistically improve diabetes-associated erectile dysfunction in streptozotocin-induced diabetic rats. Life Sci. 2015, 135, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.M.; Issa, K.; Maaliki, D.; Nasser, S.A.; Baydoun, E.; Eid, A.H. Estrogen increases expression of vascular alpha 2C adrenoceptor through the cAMP/Epac/JNK/AP-1 pathway and potentiates cold-induced vasoconstriction. Vasc. Pharmacol. 2020, 106690. [Google Scholar] [CrossRef]
- Eid, A.H.; Maiti, K.; Mitra, S.; Chotani, M.A.; Flavahan, S.; Bailey, S.R.; Thompson-Torgerson, C.S.; Flavahan, N.A. Estrogen increases smooth muscle expression of α2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am. J. Physiol. Circ. Physiol. 2007, 293, H1955–H1961. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.A.; Samaha, A.A.; Baydoun, S.; Iratni, R.; Eid, A.H. Rhus coriaria L. (Sumac) Evokes Endothelium-Dependent Vasorelaxation of Rat Aorta: Involvement of the cAMP and cGMP Pathways. Front. Pharmacol. 2018, 9, 688. [Google Scholar] [CrossRef]
- Eid, A.H. cAMP Induces Adhesion of Microvascular Smooth Muscle Cells to Fibronectin via an Epac-Mediated but PKA-independent Mechanism. Cell. Physiol. Biochem. 2012, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Chotani, M.A.; Mitra, S.; Eid, A.H.; Han, S.A.; Flavahan, N.A. Distinct cAMP signaling pathways differentially regulate α2C-adrenoceptor expression: Role in serum induction in human arteriolar smooth muscle cells. Am. J. Physiol. Circ. Physiol. 2005, 288, H69–H76. [Google Scholar] [CrossRef] [Green Version]
- Motawea, H.K.B.; Jeyaraj, S.C.; Eid, A.H.; Mitra, S.; Unger, N.T.; Ahmed, A.A.E.; Flavahan, N.A.; Chotani, M.A. Cyclic AMP-Rap1A signaling mediates cell surface translocation of microvascular smooth muscle α2C-adrenoceptors through the actin-binding protein filamin-2. Am. J. Physiol. Physiol. 2013, 305, C829–C845. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraj, S.C.; Unger, N.T.; Eid, A.H.; Mitra, S.; El-Dahdah, N.P.; Quilliam, L.A.; Flavahan, N.A.; Chotani, M.A. Cyclic AMP-Rap1A signaling activates RhoA to induce α(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am. J. Physiol. Physiol. 2012, 303, C499–C511. [Google Scholar] [CrossRef] [Green Version]
- Eid, A.H.; Chotani, M.A.; Mitra, S.; Miller, T.J.; Flavahan, N.A. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle alpha2C-adrenoceptors. Am. J. Physiol. Circ. Physiol. 2008, 295, H266–H272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Wu, R.-X.; Zhao, L.; Li, J.; Guo, H.-T.; Fan, R.; Cui, Y.; Wang, Y.-M.; Yue, S.-Q.; Pei, J.-M. Resveratrol Attenuates Ischemia/Reperfusion Injury in Neonatal Cardiomyocytes and Its Underlying Mechanism. PLoS ONE 2012, 7, e51223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, J.; Wang, H.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3β and mitochondrial permeability transition pore. Eur. J. Pharmacol. 2009, 604, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badran, A.; Baydoun, E.; Samaha, A.; Pintus, G.; Mesmar, J.; Iratni, R.; Issa, K.; Eid, A.H. Marjoram Relaxes Rat Thoracic Aorta via a PI3-K/eNOS/cGMP Pathway. Biomolecules 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.; Samaha, A.A.; Ballan, S.; Saleh, A.I.; Iratni, R.; Eid, A.H. Salvia fruticosa Induces Vasorelaxation in Rat Isolated Thoracic Aorta: Role of the PI3K/Akt/eNOS/NO/cGMP Signaling Pathway. Sci. Rep. 2017, 7, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fardoun, M.M.; Nassif, J.; Issa, K.; Baydoun, E.; Eid, A.H. Raynaud’s Phenomenon: A Brief Review of the Underlying Mechanisms. Front. Pharmacol. 2016, 7, 438. [Google Scholar] [CrossRef] [Green Version]
- Jardim, F.R.; De Rossi, F.T.; Nascimento, M.X.; Barros, R.G.D.S.; Borges, P.A.; Prescilio, I.C.; De Oliveira, M.R. Resveratrol and Brain Mitochondria: A Review. Mol. Neurobiol. 2017, 55, 2085–2101. [Google Scholar] [CrossRef]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free. Radic. Boil. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Gambardella, J.; Sorriento, D.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; Napolitano, L.; Trimarco, B.; Iaccarino, G.; Santulli, G. Functional role of mitochondria in arrhythmogenesis. In Mitochondrial Dynamics in Cardiovascular Medicine; Springer: Cham, Switzerland, 2017; pp. 191–202. [Google Scholar]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.-X.; Marks, A.R. Mitochondrial oxidative stress promotes atrial fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Yang, R.; Yang, J.; Zhou, L. Mitochondrial Dysfunction-Associated Arrhythmogenic Substrates in Diabetes Mellitus. Front. Physiol. 2018, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Diao, J.; Wei, J.; Yan, R.; Fan, G.; Lin, L.; Chen, M. Effects of resveratrol on regulation on UCP2 and cardiac function in diabetic rats. J. Physiol. Biochem. 2018, 75, 39–51. [Google Scholar] [CrossRef]
- Fang, W.-J.; Wang, C.-J.; He, Y.; Zhou, Y.-L.; Peng, X.-D.; Liu, S.-K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol. Sin. 2017, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide activates mitochondrial uncoupling proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2016, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinnally, K.W.; Peixoto, P.M.; Ryu, S.-Y.; Dejean, L.M. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, E.; Griparic, L.; Shurland, D.-L.; Van Der Bliek, A.M. Dynamin-related Protein Drp1 Is Required for Mitochondrial Division in Mammalian Cells. Mol. Boil. Cell 2001, 12, 2245–2256. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, M.; Torres, G.; Wu, S.; Ouyang, C.; Xie, Z.; Zou, M. Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes 2016, 66, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef]
- Ikeda, Y.; Shirakabe, A.; Maejima, Y.; Zhai, P.; Sciarretta, S.; Toli, J.; Nomura, M.; Mihara, K.; Egashira, K.; Ohishi, M.; et al. Endogenous Drp1 Mediates Mitochondrial Autophagy and Protects the Heart against Energy Stress. Circ. Res. 2014, 116, 264–278. [Google Scholar] [CrossRef]
- Sharp, W.W.; Fang, Y.H.; Han, M.; Zhang, H.J.; Hong, Z.; Banathy, A.; Morrow, E.; Ryan, J.J.; Archer, S.L. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: Therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2013, 28, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Huang, A.; Yan, J.; Liu, B.; Liu, Q.; Zhang, J.; Zhang, X.; Ou, C.; Luo, C.-F. Resveratrol Ameliorates Cardiac Dysfunction by Inhibiting Apoptosis via the PI3K/Akt/FoxO3a Pathway in a Rat Model of Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. 2017, 70, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zha, W.; Ke, Z.; Min, Q.; Li, C.; Sun, H.; Liu, C. Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway. J. Diabetes Res. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wang, C.-C.; Lai, T.-Y.; Tsu, H.-N.; Wang, C.-H.; Liang, H.-Y.; Kuo, W.-W. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Cai, L. Diabetic Cardiomyopathy and Its Prevention by Nrf2: Current Status. Diabetes Metab. J. 2014, 38, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, Y.; Kalra, N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007, 247, 167–181. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Qian, J.; Sun, C.; Zhang, H.; Ye, S.; Chen, T.; Xu, Z.; Wang, J.; Huang, W.-J.; Liang, G. An Aza resveratrol–chalcone derivative 6b protects mice against diabetic cardiomyopathy by alleviating inflammation and oxidative stress. J. Cell. Mol. Med. 2018, 22, 1931–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-G.; Zhu, W.; Tao, J.-P.; Xin, P.; Liu, M.-Y.; Li, J.-B.; Wei, M. Resveratrol protects cardiomyocytes from oxidative stress through SIRT1 and mitochondrial biogenesis signaling pathways. Biochem. Biophys. Res. Commun. 2013, 438, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Poornima, I.G.; Parikh, P.; Shannon, R.P. Diabetic cardiomyopathy: The search for a unifying hypothesis. Circ. Res. 2006, 98, 596–605. [Google Scholar] [CrossRef]
- Huang, G.; Lu, H.; Hao, A.; Ng, D.C.H.; Ponniah, S.; Guo, K.; Lufei, C.; Zeng, Q.; Cao, X. GRIM-19, a Cell Death Regulatory Protein, Is Essential for Assembly and Function of Mitochondrial Complex I. Mol. Cell. Boil. 2004, 24, 8447–8456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nat. 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef]
- Alvarez-Guardia, D.; Palomer, X.; Coll, T.; Davidson, M.M.; Chan, T.O.; Feldman, A.M.; Laguna, J.C.; Vazquez-Carrera, M. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc. Res. 2010, 87, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.W.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor delta. Pharmacol. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef]
- Ding, G.; Fu, M.; Qin, Q.; Lewis, W.; Kim, H.W.; Fukai, T.; Bacanamwo, M.; Chen, Y.E.; Schneider, M.D.; Mangelsdorf, D.J.; et al. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc. Res. 2007, 76, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liu, J.; Li, Y.; Wu, S.; Luo, J.; Yang, H.; Subbiah, R.; Chatham, J.; Zhelyabovska, O.; Yang, Q. Peroxisome proliferator-activated receptor {delta} is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ. Res. 2010, 106, 911–919. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2006, 291, H1489–H1506. [Google Scholar] [CrossRef]
- Rijzewijk, L.J.; Van Der Meer, R.W.; Lamb, H.J.; De Jong, H.W.; Lubberink, M.; Romijn, J.A.; Bax, J.J.; De Roos, A.; Twisk, J.W.; Heine, R.J.; et al. Altered Myocardial Substrate Metabolism and Decreased Diastolic Function in Nonischemic Human Diabetic Cardiomyopathy: Studies with cardiac positron emission tomography and magnetic resonance imaging. J. Am. Coll. Cardiol. 2009, 54, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Beaudoin, M.-S.; Perry, C.G.R.; Arkell, A.M.; Chabowski, A.; Simpson, J.A.; Wright, D.C.; Holloway, G.P. Impairments in mitochondrial palmitoyl-CoA respiratory kinetics that precede development of diabetic cardiomyopathy are prevented by resveratrol in ZDF rats. J. Physiol. 2014, 592, 2519–2533. [Google Scholar] [CrossRef] [Green Version]
- Novgorodov, S.A.; Riley, C.L.; Yu, J.; Keffler, J.A.; Clarke, C.J.; Van Laer, A.O.; Baicu, C.F.; Zile, M.R.; Gudz, T.I. Lactosylceramide contributes to mitochondrial dysfunction in diabetes. J. Lipid Res. 2016, 57, 546–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, P.; Schaffer, J.E. Metabolic stress in the myocardium: Adaptations of gene expression. J. Mol. Cell. Cardiol. 2012, 55, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Zhao, Y.; He, M.; Li, H.; Fan, J.; Nie, X.; Yan, M.; Chen, C.; Wang, D.-W. MiR-30c/PGC-1β protects against diabetic cardiomyopathy via PPARα. Cardiovasc. Diabetol. 2019, 18, 7. [Google Scholar] [CrossRef]
- Lai, L.; Wang, M.; Martin, O.J.; Leone, T.C.; Vega, R.B.; Han, X.; Kelly, D.P. A role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J. Biol. Chem. 2014, 289, 2250–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. Sirt1 acts in association with PPAR to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2010, 90, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavandero, S.; Chiong, M.; Rothermel, B.A.; Hill, J.A. Autophagy in cardiovascular biology. J. Clin. Investig. 2015, 125, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Woollard, J.R.; Ebrahimi, B.; Crane, J.A.; Jordan, K.L.; Lerman, A.; Wang, S.-M.; Lerman, L.O. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arter. Thromb. Vasc. Boil. 2012, 32, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.L.-H.; Chiang, S.; Kalinowski, D.S.; Bae, D.-H.; Sahni, S.; Richardson, D.R. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxidative Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef] [Green Version]
- Nishida, K.; Yamaguchi, O.; Otsu, K. Crosstalk Between Autophagy and Apoptosis in Heart Disease. Circ. Res. 2008, 103, 343–351. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.-F.; Ke, Z.-Q.; Yao, Q.; Guo, S.; Liu, C. Resveratrol Modulates Apoptosis and Autophagy Induced by High Glucose and Palmitate in Cardiac Cells. Cell. Physiol. Biochem. 2018, 46, 2031–2040. [Google Scholar] [CrossRef]
- Oberstein, A.; Jeffrey, P.D.; Shi, Y. Crystal Structure of the Bcl-XL-Beclin 1 Peptide Complex: Beclin 1 is a novel BH3-only protein. J. Boil. Chem. 2007, 282, 13123–13132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strappazzon, F.; Rudan, M.V.; Campello, S.; Nazio, F.; Florenzano, F.; Fimia, G.M.; Piacentini, M.; Levine, B.; Cecconi, F. Mitochondrial BCL-2 inhibits AMBRA1-induced autophagy. EMBO J. 2011, 30, 1195–1208. [Google Scholar] [CrossRef]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-Mediated Phosphorylation of Bcl-2 Regulates Starvation-Induced Autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Chen, Y.; Huang, S.-W.; Hu, P.-F.; Tang, L.-J. Regulation of autophagy by tea polyphenols in diabetic cardiomyopathy. J. Zhejiang Univ. Sci. B 2018, 19, 333–341. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhagani, H.; Nasser, S.A.; Dakroub, A.; El-Yazbi, A.F.; Eid, A.A.; Kobeissy, F.; Pintus, G.; Eid, A.H. The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 4962. https://doi.org/10.3390/ijms21144962
Bhagani H, Nasser SA, Dakroub A, El-Yazbi AF, Eid AA, Kobeissy F, Pintus G, Eid AH. The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy. International Journal of Molecular Sciences. 2020; 21(14):4962. https://doi.org/10.3390/ijms21144962
Chicago/Turabian StyleBhagani, Humna, Suzanne A. Nasser, Ali Dakroub, Ahmed F. El-Yazbi, Assaad A. Eid, Firas Kobeissy, Gianfranco Pintus, and Ali H. Eid. 2020. "The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy" International Journal of Molecular Sciences 21, no. 14: 4962. https://doi.org/10.3390/ijms21144962
APA StyleBhagani, H., Nasser, S. A., Dakroub, A., El-Yazbi, A. F., Eid, A. A., Kobeissy, F., Pintus, G., & Eid, A. H. (2020). The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy. International Journal of Molecular Sciences, 21(14), 4962. https://doi.org/10.3390/ijms21144962







