Abstract
The distal lung provides an intricate structure for gas exchange in mammalian lungs. Efficient gas exchange depends on the functional integrity of lung alveoli. The cells in the alveolar tissue serve various functions to maintain alveolar structure, integrity and homeostasis. Alveolar epithelial cells secrete pulmonary surfactant, regulate the alveolar surface liquid (ASL) volume and, together with resident and infiltrating immune cells, provide a powerful host-defense system against a multitude of particles, microbes and toxicants. It is well established that all of these cells express purinergic P2 receptors and that purinergic signaling plays important roles in maintaining alveolar homeostasis. Therefore, it is not surprising that purinergic signaling also contributes to development and progression of severe pathological conditions like pulmonary inflammation, acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and pulmonary fibrosis. Within this review we focus on the role of P2 purinergic signaling in the distal lung in health and disease. We recapitulate the expression of P2 receptors within the cells in the alveoli, the possible sources of ATP (adenosine triphosphate) within alveoli and the contribution of purinergic signaling to regulation of surfactant secretion, ASL volume and composition, as well as immune homeostasis. Finally, we summarize current knowledge of the role for P2 signaling in infectious pneumonia, ALI/ARDS and idiopathic pulmonary fibrosis (IPF).
1. Introduction
Alveoli in the distal lung are the functional units for gas exchange within mammalian lungs. In the human lung, some 400 million alveoli provide an extensive surface for efficient gas exchange, whilst the very thin alveolar barrier separating blood and air entails a minimal resistance for diffusion of oxygen and carbon dioxide [1]. This intricate structure constitutes various physiological challenges for maintenance of functional integrity and tissue homeostasis, including biophysical properties at the air-liquid interphase, regulation of local alveolar fluid balance, tissue pressure and lymph flow, perfusion matching and hemostatic control, as well as clearance of inhaled particles, containment of commensal microbiota and defense against pathogenic invaders. All of this is achieved by structural and functional adaptations of the alveolar tissue and the cells within alveolar septae.
Alveolar septae are comprised of two continuous cell layers of epithelium and capillary endothelium forming the thin air-blood barrier, and an interstitial space of variable composition and thickness containing fibroblasts [2,3]. The alveolar epithelium consists of type I (ATI) and type II (ATII) alveolar epithelial cells. Both serve essential roles to maintain alveolar homeostasis [4,5,6,7]. ATI cells cover >95% of the alveolar surface [8] and are mainly renowned for their special morphology, which is perfectly designed for efficient gas exchange between the alveolus and the pulmonary capillaries [9]. Together with ATII cells, ATI cells control transepithelial ion and fluid transport to regulate the volume of the alveolar surface liquid (ASL) and prevent flooding of alveoli. The main function of ATII cells is secretion of pulmonary surfactant [10,11,12]. Surfactant reduces the surface tension at the air-liquid interphase to stabilize alveoli during exhalation and facilitate alveolar distension during lung inflation [13,14]. ATII cells are also considered stem cells within the alveolar epithelium that restore epithelial integrity after injury [15,16]. Fibroblasts secrete components of extracellular matrix to provide elastic and structural properties of the lung parenchyma. Pulmonary endothelial cells (EC) line the blood side of the alveolar barrier and are structurally adapted for maintaining blood flow, gas exchange and fluid balance, as well as the recruitment of immune cells [17].
In addition, the lung contains various immune cells for clearance of inhaled pathogens. Their number and composition vary in healthy and diseased alveoli [18]. Alveolar macrophages (AMs) are the most abundant innate immune cells in the distal lung parenchyma. AMs are loosely attached to the epithelial surface and provide a first-line of defense against pollutants and pathogenic microbes that initiate an innate immune response in the lung [3,19]. AMs are central to orchestrate the initiation and resolution of the immune response in the lung. In addition, AMs perform non-immune, tissue-specific, homeostatic functions, most notably clearance of surfactant [20]. The distal lung also harbors interstitial macrophages [20,21,22], and recent, single-cell RNA-sequencing, studies have revealed great macrophage diversity in healthy, malignant and fibrotic lung tissue [23,24]. Lungs are also a reservoir of neutrophils under a steady-state. In the event of an infection or injury, they are promptly activated and recruited to the alveolar compartment, as well as the airways [25]. Neutrophils migrate to sites of inflammation and act in various ways against pathogens including phagocytosis, neutrophil extracellular traps (NET) formation or degranulation [26]. Besides, many other cell types are recruited to the distal lung under pathological conditions.
All of the cells express purinergic P2 receptors (Table 1). Purinergic signaling plays important roles for maintaining alveolar homeostasis, but is also critically involved in development and progression of severe pathological conditions like pulmonary inflammation, acute lung injury (ALI/ARDS), fibrosis or cancer. In the subsequent sections we aim at summarizing the current state on the role of P2 receptors in the distal lung in health and disease.

Table 1.
Expression of P2 receptors within the cells of the distal lung.
2. Functional Relevance of P2 Receptor Signaling in the Distal Lung
P2 purinergic signaling is initiated through the binding of extracellular nucleotides to P2 receptors expressed on the surface of target cells. Hence, signaling via P2 receptors can be regulated by the expression of P2 receptors on target cells and/or the availability of nucleotides in the extracellular space.
P2 purinoceptors can be classified into two major families: ionotropic P2X or metabotropic P2Y receptors [55,56,57]. P2X receptors are membrane cation channels formed as homo- or heteromeric trimers from seven P2X receptor subunits (P2X(1–7)) [58,59,60]. P2X channels open within milliseconds of ATP binding. The binding of ATP between subunits causes subunits to flex together within the ectodomain and separate in the membrane spanning region so as to open a central channel [58,59,61,62]. P2X channels are essentially non-selective cation channels permeable to small monovalent and divalent cations with preferential gating for Na+, K+ and Ca2+. Receptor activation generally leads to a change in membrane potential, initiating subsequent cellular events. Besides the change of membrane potential, a major physiological mechanism by which activated P2X receptors control cellular functions is elevation in intracellular Ca2+ concentration, either directly by Ca2+ permeation and/or indirectly by activation of voltage-gated Ca2+ channels. The increase in intracellular Ca2+ activates a broad range of second messenger systems and signaling cascades, and can trigger manifold short- and long-term cellular events [63,64,65]. P2X receptor activation, conductivity and de-sensitization are also modulated by a variety of compounds including divalent cations, protons, lipids, steroids, ethanol and ivermectin [65,66]. There are a number of excellent reviews that summarize the molecular and functional properties of P2X receptors and their pharmacological targeting in great detail [59,60,65,66,67,68].
P2Y receptors are membrane bound, G-protein-coupled receptors (GPCRs) for extracellular nucleotides [56,69]. Eight P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) are currently recognized [70]. Human P2Y receptor subtypes have, in contrast to P2X receptors, different principal agonists: ATP (P2Y2 and P2Y11), ADP (P2Y1, P2Y12, and P2Y13), UTP (P2Y2 and P2Y4), UDP (P2Y6 and P2Y14), and UDPG (P2Y14). ATP may act as an antagonist or partial agonist at several P2Y receptor subtypes, including antagonism at the human P2Y4 receptors [71,72]. The eight receptors can also be divided into two subfamilies based on sequence homology and second messengers: five Gq-coupled P2Y1-like (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11) and three Gi-coupled P2Y12-like (P2Y12–P2Y14) receptors [72]. Activation of Gq-coupled receptors results in stimulation of phospholipase C followed by increases in inositol phosphates and mobilization of Ca2+ from intracellular stores. P2Y11 receptors couple in addition to Gs proteins, followed by increased adenylate cyclase activity. Receptors signaling via Gi proteins, in contrast, inhibit adenylate cyclase activity or control ion channel activity [70]. Again, excellent reviews have summarized their molecular, functional and pharmacological properties [68,70,72,73,74,75].
2.1. Expression of P2 Receptors in the Distal Lung
Within the lung, expression of a wide range of P2 receptors has been reported on either the mRNA or protein level in epithelial cells [27,28,29,30,31], fibroblasts [39,76], endothelial cells [32,33,34,35,37,38], AMs [40,41,43,44,77,78] and neutrophils [46,47,48,49,50,51,52,53,54,79,80,81] (see Table 1 for details). However, care must be taken when analyzing expression in primary isolated cells as purity of most isolations are <90%. Impurities can account for either underestimation of expression levels or the detection of non-specific expression levels.
P2 receptors are also expressed on many other cell types that are recruited to the lung, in particular under pathological conditions. These cells, mainly of the hematopoietic lineage, do not per se represent resident cell populations of the lung and do not likely adopt lung-specific differentiation properties. It is beyond the scope of this review to address them all in detail and therefore we refer to recent reviews [82,83]. Among them are monocytes, T cells, natural killer (NK) cells and regulatory T cells (Treg). Although comprehensive data on P2 receptor expression within these cells are often scarce, it seems that all of these express P2X7 receptors [82,84,85,86,87], whilst expression of other P2 receptors may vary between cell types.
Despite the widespread expression of many P2X and P2Y receptor subtypes within the cells of the distal lung, specific and significant physiological functions have only been attributed to few P2 receptor isoforms so far; in particular, P2X4, P2X7 and P2Y2 have been studies in greater detail (Table 2). P2X4 and P2Y2 receptors expressed on the alveolar epithelium are key regulators for surfactant secretion and also contribute to regulation of the alveolar surface liquid (ASL) volume. Activation of P2X7 and P2Y2 receptors expressed on immune cells is central for host defense in the alveolus (Figure 1).

Table 2.
Contribution of P2 receptor signaling to alveolar function and to the development/progression of lung diseases.
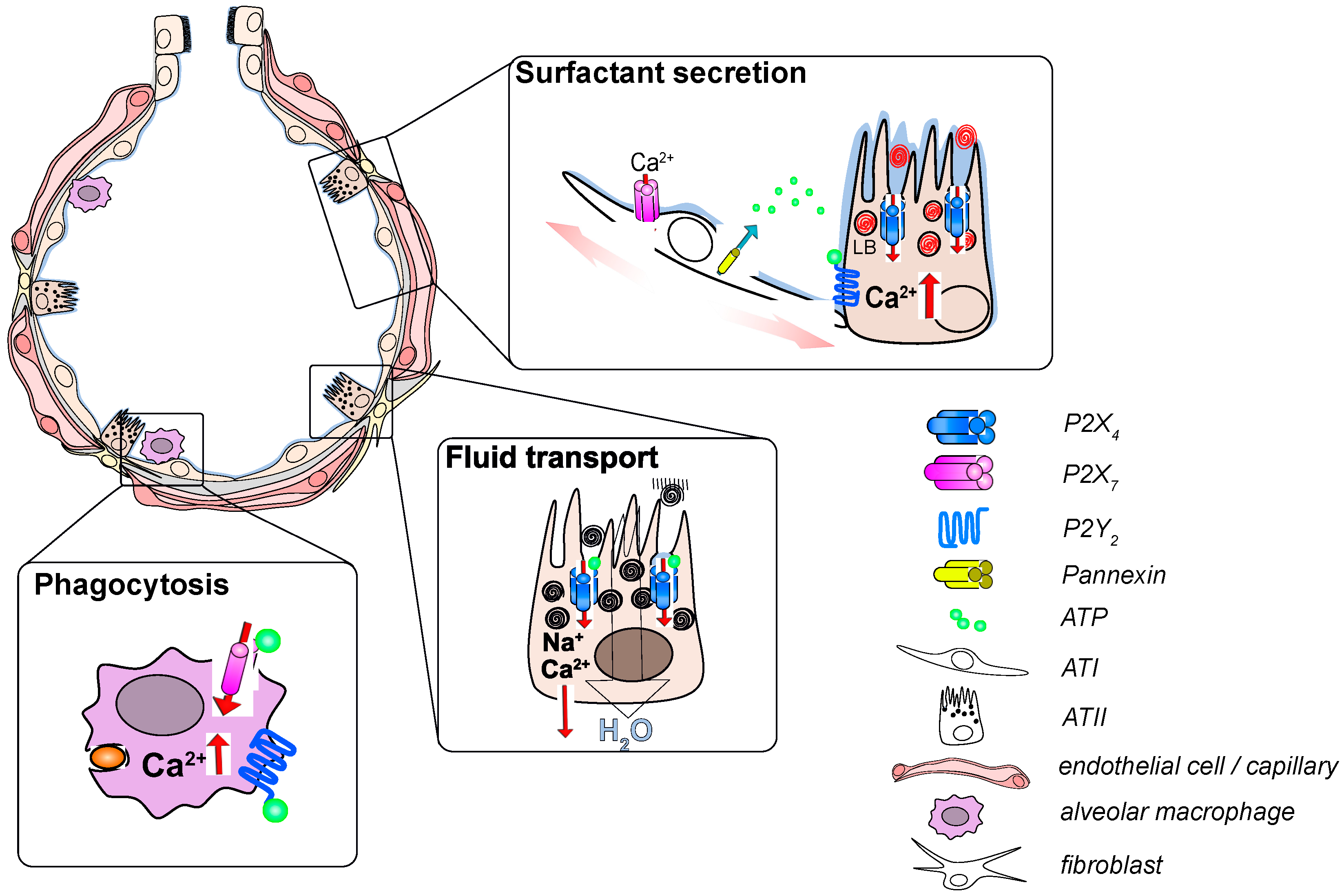
Figure 1.
Functional relevance of P2 receptor signaling in the alveolus. Inflation of the alveolus leads to stretch-induced release of ATP (adenosine triphosphate) from alveolar epithelial cells which activates P2Y2 receptors on ATII (type II) cells. The resulting Ca2+ release from the endoplasmatic reticulum stimulates LB exocytosis. Subsequent activation of P2X4 receptors on the limiting membrane of fused LBs results in a fusion-activated Ca2+-entry (FACE) which facilitates release of surfactant from fused LBs. FACE also results in transepithelial cation transport leading to fluid resorption from the alveolar lumen to promote activation of secreted surfactant. Activation of P2X7 and P2Y2 receptors on AMs results in an increase in intracellular Ca2+ that facilitates phagocytosis of airborne particulates.
2.2. ATP in the Alveolus
Activation of P2X and P2Y receptors in the alveolus primarily depends on extracellular ATP. ATP is present in the pulmonary hypophase [6]; however, the estimated concentration under resting conditions is in the low nM range [118], well below the EC50 values for P2 receptor activation [59,119,120]. A possible reason for this low concentration is rapid hydrolysis of extracellular ATP in the hypophase by cell surface ectonucleotidases CD39 and CD73 [121,122]. Hence, a tight temporal and spatial coordination between ATP release and ATP demand (i.e., P2 receptor activation) is essential for providing appropriate concentrations of ATP [123]. ATP release within individual alveoli can hence adapt surfactant secretion and fluid transport to local demands. ATP is released from primary ATI, ATII or immortalized alveolar cells in response to increased alveolar distension [4,5,6,124,125], or when coming in close proximity to the air-liquid interphase following a decrease in alveolar hypophase height (i.e., due to increased surface tension forces) [126]. Mechanical ventilation results in inflation-induced ATP release in the ex-vivo rat lung, likely as a result of alveolar over distension and stretch of alveolar cells [127]. Consistently, it has been demonstrated in vitro and in vivo that the stretching of alveolar cells is the most potent stimulus for surfactant secretion, likely via the release of ATP [128,129,130,131], which is released from ATI cells via pannexin hemichannels following an increase in intracellular Ca2+ levels, subsequent to activation of purinergic P2X7 receptors [5]. We recently collected evidence that mechanical distension of ATI-like cells (hAELVi) [132] results in caveolin-1 dependent activation of mechanosensitive piezo1 channels (unpublished observation) that triggers Ca2+-entry and subsequent release of ATP via pannexin hemichannels. ATP is also stored in organelles known as lamellar bodies (LBs) and released upon LB exocytosis and surfactant secretion from ATII cells [123].
The release of purine nucleotides from epithelia is also significantly increased under pathophysiological conditions resulting from chronic lung diseases or following trauma-induced damage of the alveolus [28,133,134]. In line, ATP is released following ventilator-induced lung injury (VILI) upon improperly delivered mechanical ventilation (MV) and injurious overdistension of alveoli [135]. Damaged and necrotic tissues discharge large quantities of ATP. Furthermore, immune cells release ATP via vesicular exocytosis and/or activation of connexin or pannexin hemichannels (this has recently been reviewed in detail [103]). For example, ATP is released in a positive feedback loop from phagocytes via pannexin-1 hemichannels in response to prolonged activation of P2X7 receptors. A massive ATP release due to disruption or permeabilization of cell plasma membrane (PM) saturates ATP-hydrolyzing enzymes and leads to sustained increased ATP levels. High levels of extracellular ATP act as a DAMP (danger associated molecular pattern) or “danger signal” [96,134,136]. Alternative mechanisms proposed ATP release following hypotonic swelling observed in a surrogate cell line of type II pneumocytes (A549 cells) [137], or from sympathetic/adrenergic nerves, which terminate close to ATII cells in the lung and utilize ATP as a neuro(co)transmitter [138]. However, their physiological roles in vivo need to be determined.
2.3. Surfactant Secretion
Pulmonary surfactant is synthesized in ATII cells, stored in lamellar bodies (LBs) and secreted via regulated exocytosis of LBs [139,140,141] to fulfill its biophysical, as well as immunomodulatory functions at the air-liquid interphase within alveoli [17]. The continuous presence of surfactant is crucial for lung mechanics and survival. Its deficiency causes respiratory failure, most impressively manifested as infant respiratory distress syndrome (IRDS) of newborns with immature lungs [141]. Regulated secretion of pulmonary surfactant is therefore essential for proper lung function and is correlated to elevations of the intracellular Ca2+ concentration [10]. The P2 agonist ATP has emerged as the most potent physiological agonist for surfactant secretion amongst a variety of para- or endocrine mediators [112,138,139,142,143,144,145]. ATP triggers LB exocytosis via activation of P2Y2 receptor and the subsequent increase in the intracellular Ca2+ concentration [10,12,112,142,146]. The ATP-induced Ca2+-signal in ATII cells consists of at least 2 phases, an initial, short lasting “peak” followed by a “plateau” phase. The peak is the result of inositol 1,4,5-trisphosphate (IP3)-induced Ca2+- release form intracellular stores, whereas the plateau depends on Ca2+ entry by a yet undefined mechanism [10,12]. The integrated Ca2+ signal defines the amount of LB exocytosis [12,146].
Unlike many readily soluble secretory molecules (e.g., neurotransmitters), surfactant is not immediately released following the fusion of LBs with the plasma membrane. The exocytic fusion pore constitutes a physical barrier for the release of this poorly soluble, lipoprotein-like substance. An increase in intracellular Ca2+ at the fusion site is required to expand the fusion pore [94,147,148,149]. This is mediated by activation of P2X4 receptors expressed on the membrane of LBs. ATP is stored in LBs and upon exocytosis of LBs and opening of the fusion pore, the low pH within LBs is rapidly neutralized and the luminal ATP activates the P2X4 receptors on the membrane of the fused LB [125]. This triggers a transient Ca2+-influx (FACE, fusion activated Ca2+-entry) at the site of the LB fusion and provides the Ca2+ necessary for fusion pore expansion and surfactant release [29,91,92,150]. FACE is short lasting, and P2X4 receptors need to be re-sensitized by a protonation/deprotonation cycle depending on receptor internalization and recycling [151]. It has also been suggested that P2X4 activation and FACE are part of a positive feedback mechanism, which, due to the increase of Ca2+ near the apical PM, stimulates additional LB fusions [10]. Consistently, when cells are stimulated with UTP (instead of ATP), which activates the P2Y2 but not the P2X4 receptor, the exocytic response is blunted [29].
2.4. Epithelial Fluid Transport
Efficient gas exchange and surfactant function depend on regulation of the alveolar surface liquid (ASL) volume and composition [152,153,154]. The ASL is a very thin fluid layer (an average thickness of only 0.2 µm, also termed hypophase) [155], and protects the alveolar surface from desiccation whilst providing a minimal resistance (distance) to gas exchange. Malfunctions in regulation of the ASL volume can cause severe disturbances, such as the formation of alveolar edema [156]. Vectorial ion transport across epithelia drives transepithelial fluid flux. This requires asymmetric or differential distribution of ion transporters and other membrane proteins on the apical and basolateral membrane of polarized epithelial cells. The expression and, in part, localization of several sodium, potassium and anion channels in ATI and ATII cells have been described. Yet, a definite picture of the regulation of transepithelial ion transport to maintain ASL volume under physiological, as well as pathological conditions, is still missing [152,153,154,157,158,159].
Some evidence exists for the contribution of P2 receptor signaling in the regulation of the apical fluid in airways [154,160,161,162], but much less is known about their role in alveolar fluid homeostasis. Several ion channels/transporters known to be affected by luminal P2 receptor activation and/or increases in intracellular Ca2+, such as ENaC, CFTR, Ca2+-activated Cl- channels (CaCC) or Na+/K+-ATPase [113,163,164,165,166] are expressed on alveolar epithelial cells [154]. However, direct evidence for the involvement of P2 receptors in maintaining ASL homeostasis is elusive. In general, luminal nucleotides and activation of P2Y2 receptors induce Cl-, or HCO3- secretion and inhibit ENaC-meditated Na+ absorption [113,114] resulting in fluid secretion. Within the alveoli, it is generally accepted that fluid resorption prevails to maintain the relatively thin ASL and impaired Na+ reabsorption is associated with the formation of pulmonary edema [152,153]. Hence, P2 receptor-mediated ion channel activation might not play a major role for steady-state fluid transport. It is, however, functionally linked to surfactant secretion. FACE via P2X4 drives fluid resorption in response to surfactant secretion. This P2X4 receptor-mediated, inward-rectifying cation current on the apical side results in vectorial ion transport across the epithelium, which in turn promotes apical to basolateral fluid transport [93]. The localized alveolar fluid resorption results in temporary thinning of the ASL and promotes contact between surfactant and the air-liquid interphase. This is required for adsorption of newly released surfactant into the air-liquid interface and activation of surfactant [93,167].
2.5. Host Defense
The epithelial surfaces of the lungs are in direct contact with the environment and exposed to a multitude of particles, microbes and toxicants. A multilayered physical and chemical innate host-defense system evolved to prevent their entry into lung tissue and the circulation [168]. The complex interplay between resident (e.g. epithelial cells, AMs, dendritic cells) and infiltrating immune cells is regulated by various pro- and anti-inflammatory signaling molecules [169], including extracellular nucleotides [83,170]. In particular, extracellular ATP is an endogenous danger signal to activate immune cells [171,172,173]. Whether activation culminates in inflammation depends on the actual cellular and general composition of the inflammatory microenvironment, receptor expression and state on target cells, the kinetics of nucleotide release, degradation and fate of its products. In general, the purinergic impact on inflammation is highly complex, interwoven with many other signaling cascades and the dynamic and integrative behavior of the system is not well understood. Yet, considerable knowledge has been gained with respect to single pro- and anti-inflammatory processes. While P1 adenosinergic receptors are mainly associated with anti-inflammatory outcomes, P2 receptors predominantly exert pro-inflammatory effects [82,170]. Pro-inflammatory processes affected by P2 receptor stimulation include leukocyte chemotaxis, inflammatory cell maturation or polarization and activation with increased cyto- and chemokine release, ROS (reactive oxygen species) production, hemostasis, cytotoxic and phagocytic activity. Anti-inflammatory effects mediated by P2 receptors are related to apoptotic cell death of immune cells, reduced cytokine release and inhibition of chemotaxis.
So far, P2X7 and P2Y2 receptors expressed on AMs [40,41,44] neutrophils [46,48,51,53] and infiltrating immune cells have gained the highest attention for host defense in the alveolus. Although it needs to be stated, that data on P2X7 receptor expression and function in neutrophils are ambiguous. While many studies found expression of P2X7 in neutrophils [48,49,50,51,52,81], others reported an absence of P2X7 [45,46,50]. Whether these inconsistencies resulted from differences in research protocols or account for subpopulations of neutrophils remains to be answered.
P2X7 receptor is critically involved in the sensing of cell damage when stimulated by extracellular ATP concentrations in the lower mM range, close to intracellular concentrations [101]. Efflux of K+ with subsequent depletion of intracellular K+ content is thought to provoke NLRP3 inflammasome activation in primed phagocytes [174]. The P2X7-dependent Ca2+-entry triggers an increased ROS and inflammatory lipid production [175] and activation of TNF-α converting enzyme (TACE/ADAM-17) [176]. NLRP3 inflammasome activation provokes the release of the alarmins IL-1β and IL-18 via non-classical pathways, including exosome secretion and shedding of microvesicles [177]. Other alarmins (e.g., IL-33) are also expressed in alveolar epithelial cells and alveolar macrophages [178], and are released in response to pathogen exposure and cell damage [179]. It has been shown in airways that extracellular ATP triggers release of IL-33 [180], but whether ATP and P2 signaling are involved in IL-33 release in alveoli has yet to be confirmed. Recent results, in LPS-primed macrophages, demonstrate that ATP redirects TNF-α from TACE-dependent cell membrane release to membrane packaging in shed microvesicles [181]. Such macrophage-derived microvesicles are highly pro-inflammatory in lungs [181,182]. How microvesicles are activated and release their cargo at remote target sites is not well understood, but it has been suggested that the P2X7 receptor, which is incorporated into microvesicle membranes, is involved [183].
Prolonged stimulation of P2X7 receptors on phagocytes provokes pannexin-1 opening, subsequent ATP release [103] and causes apoptotic or pyroptotic cell death. ATP is a “find me” signal for apoptotic cell debris mediated by P2Y2 receptors expressed on monocytes [115]. Furthermore, autocrine purinergic signaling promotes chemotaxis of myeloid cells [117,184,185,186,187]. ATP release at the leading edge results in positive feedback through P2Y2 receptors [53,116]. Interestingly, Wang et al. demonstrated that LPS might act as a stop signal for neutrophil chemotaxis through autocrine P2X1 receptor activation and that P2X1 signaling was indispensable for LPS-induced neutrophil degranulation and enhanced phagocytosis [88]. In dendritic cells, functional coupling between P2X7 receptor and pannexin-1 is necessary for fast migration [86], whereas T cells rely on P2X4 receptor [94].
Inflammatory responses are augmented by platelet activation and thrombus formation [188]. Platelets release large amounts of ADP, inflammatory lipids and cytokines upon activation. ADP promotes thrombus formation by positive feedback through P2Y1 and P2Y12 receptor signaling.
Stimulation of P2Y11 receptor on immature myeloid dendritic cells leads to partial maturation with increased production of IL-10 and reduced release of IL-12p70, favoring type 2 polarization or tolerance [189]. In line with these findings, plasmacytoid dendritic cells display reduced production of type I interferons upon P2Y receptor stimulation, overall suggesting a negative regulatory role of extracellular nucleotides to contain collateral immunogenic tissue damage [190].
T-cell activation is enhanced by autocrine ATP within the immune synapse between naïve T cells and antigen-presenting cells or effector T cells and target cells [191,192,193]. This process involves P2X1, P2X4, P2X7, P2Y12 and pannexin-1 receptors for coupled ATP release [82]. Despite this activity amplification within the immune synapse of cytotoxic T lymphocytes, P2X7 receptor seems to exert an anti-inflammatory effect through its apoptosis-inducing action on cytotoxic cells in general. This has not only been shown for classical T cells, but also NK and invariant natural killer T cells (iNKT) [82]. P2X7 receptor expression in iNKT cells is vitamin A-dependent, such that vitamin A-deficiency led to iNKT overpopulation in mouse lungs and other organs [104]. The pro-apoptotic effect of nucleotides is associated with the expression level of P2X7 receptor, being highest in Tregs among T lymphocytes [87]. Tregs play an important role for the maintenance of immune homeostasis. They express high amounts of ectonucleotidases CD39 and CD73, thereby favoring an anti-inflammatory microenvironment of high adenosine and low ATP concentrations. IL-6 exposure increases ATP release by Tregs and fosters T-cell polarization into Th17 cells via P2X7 receptor [82].
3. The Role of P2 Receptors in Lung Disease
A role for P2 purinergic signaling has been reported for almost all major lung diseases [194]. A better understanding of the impact of P2 receptor signaling on the onset and progression of pulmonary pathologies will eventually help to develop targeted and efficient therapies [195,196]. Within the subsequent sections we will specifically focus on eminent diseases of the distal lung, in particular affecting alveolar function and homeostasis. These include infectious pneumonia, ALI/ARDS and pulmonary fibrosis.
3.1. Infectious Pneumonia
Tissue damage during the course of an infectious disease is due to the direct impact of the pathogen on cellular and tissue homeostasis but is also a consequence of toxic actions originating from the immune response induced to contain the invader. Therefore, the severity of disease pathologies depends on an appropriate trade-off between infectious virulence and immunogenic collateral damage.
With respect to the purinergic system, much research has focused on the role of the P2X7 receptor with its pro- and anti-inflammatory impact and this topic has recently been extensively reviewed [77,197]. For example, virus-associated ATP release increases production of type 1 interferons that limit virus replication in infected cells. This defense-mechanism was lost in P2X7 knock-out (KO) mice [102]. Tate et al. demonstrated that NLRP3 inflammasomes play a protective role early during the course of influenza A infection in mice but later turns detrimental due to immunopathogenic effects [198]. In an early study Lee et al. [99] applied replication deficient adenovirus at controlled infectious dosages to wild type (WT) or P2X7 KO mice. All animals having received low-dose adenovirus tolerated the infection well, while all wild-type animals in the high-dose group died within five days whereas 30 % in the KO groups survived. Increased survival was associated with a reduced inflammatory response due to inhibited inflammasome activation. Similar results were achieved in a model of influenza A infection [100]. Therefore, if P2X7 receptor is critically involved in the induction of detrimental hyperinflammation, inhibition of the receptor might be of therapeutic potential to limit tissue damage, whereas P2X7-signaling is protective by limiting virus replication. Interestingly, pharmacological inhibition of P2X7 receptor ameliorated influenza A pneumonia in mice after inoculation of a lethal dose of virus particles and drug application as early as day 1 post infection [199]. P2X7 receptor has also been related to pulmonary tuberculosis [77]. P2X7 receptor activity seems to be associated to the ability of infected macrophages to kill mycobacteria, although some controversies arose from the fact that P2X7 KO mice were either more or less susceptible to mycobacterial infection compared to WT mice depending on the virulence of applied mycobacterial strains [77,200,201]. In infections with highly virulent strains, P2X7 receptor activation leads to pyroptotic cell death of infected macrophages, causing inflammation and the release of bacteria, thus aggravating the disease process [201]. P2X7 receptor single-nucleotide loss-of-function polymorphisms seem to be correlated to an impaired capacity of macrophages to clear the bacterium [77]. Mawatwal et al. reported that calcimycin-mediated stimulation of P2X7 receptor improves mycobacterial elimination by enhanced autophagy in a Ca2+ and IL-12 dependent manner [96,97]. P2X7 receptor has also been described to be required for the clearance of Streptococcus pneumoniae [52]. In a recent study, Oluto et al. reported that Streptococcus pneumoniae inhibits purinergic signaling by increased internalization of P2Y2 receptors in alveolar epithelial cells [202]. This might result in reduced surfactant secretion and subsequent alveolar collapse [95].
3.2. ALI/ARDS
Acute respiratory distress syndrome (ARDS) is a clinically defined syndrome resulting from diffuse acute exudative inflammation in the lung parenchyma [203]. It arises from multiple aetiologies, but many cases present with a common histopathological picture of diffuse alveolar damage, characterized by interstitial and intra-alveolar infiltration of inflammatory cells, hyaline membranes and, depending on the disease phase, hyperplasia of ATII cells and fibrotic organization of intra-alveolar edematous material. This detrimental inflammatory response can be triggered by direct impact to the lung such as in blunt chest trauma, pneumonia or a setting of ischemia-reperfusion injury or indirectly, during the course of systemic inflammatory states like in polytrauma, sepsis or acute pancreatitis. The involvement of the purinergic system in the pathogenesis of ALI/ARDS and VILI is well established. Much about the role of P2 receptors in acute inflammation has already been described in previous sections.
There is compelling evidence from KO experiments and pharmacological interferences that P2X7 is relevant for the pathogenesis of ARDS [98,105,106,107,108,109,110,111,204,205] (Figure 2). A hallmark of ARDS is excessive invasion and activation of neutrophils (PMN) in the alveolar space. The release of toxic mediators from PMNs causes alveolar barrier breakdown, which is central for developing ARDS. Antagonism of the P2X7 receptor with AZ106006120, or KO of the receptor, reduced neutrophil infiltration and pro-inflammatory cytokine levels in a mouse model of ALI [98]. Li et al. recently reported that mesenchymal stem cell-derived exosomes ameliorated lung injury in a rat model of blunt chest trauma, and that this was mediated through downregulation of P2X7 receptor by microRNA-124-3p, a constituent of these exosomes [110].
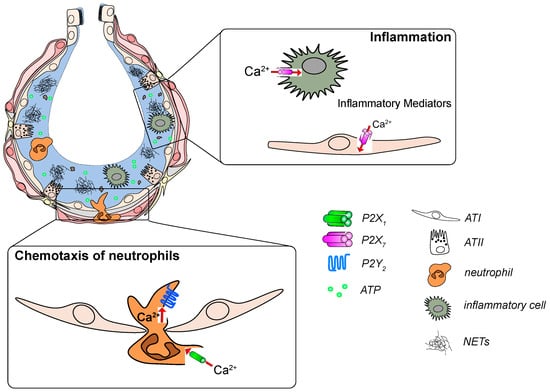
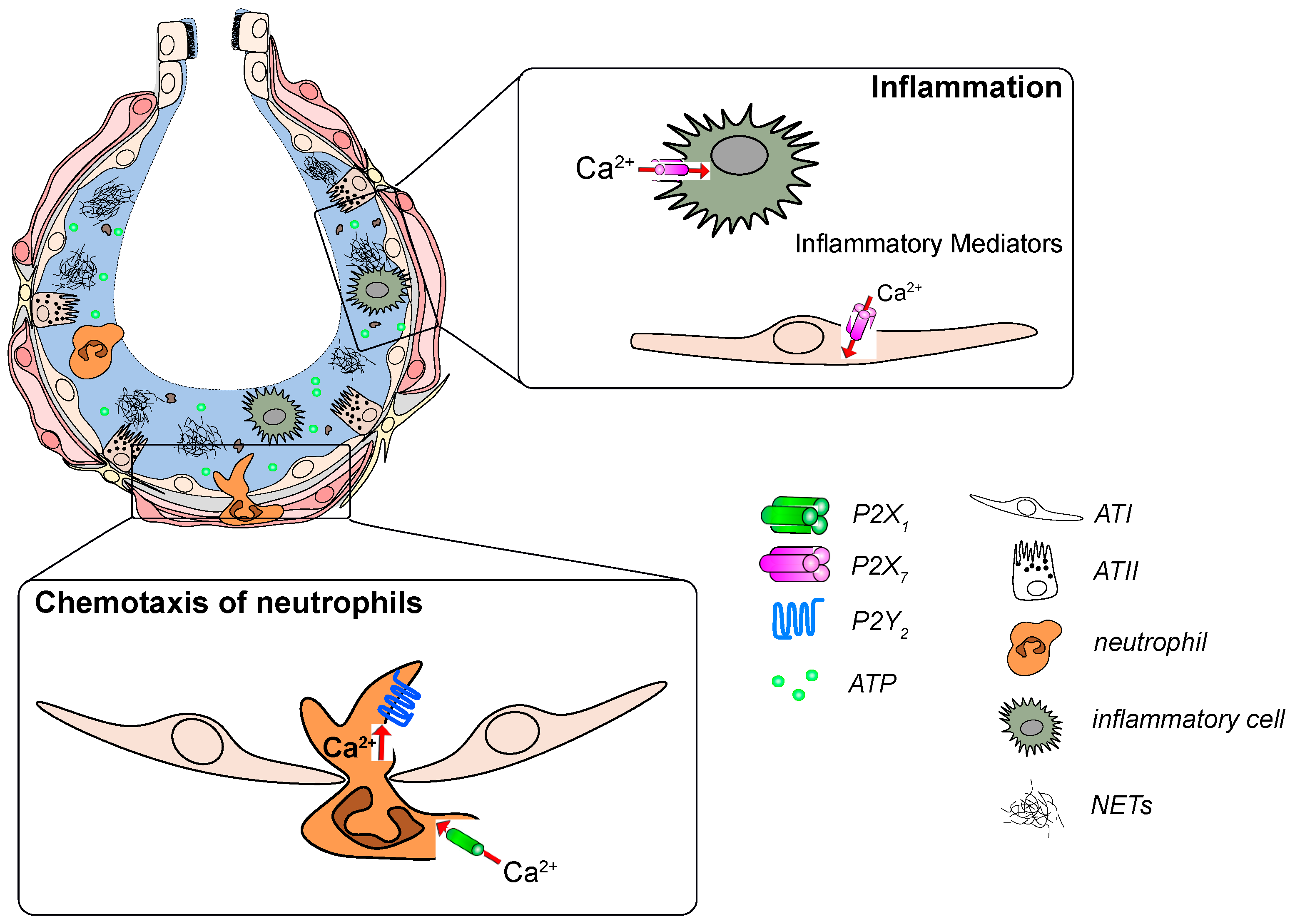
Figure 2.
P2 receptor signaling in development and progression of acute respiratory distress syndrome (ARDS). Increased ATP levels act as a “danger signal” in the damaged alveolus. ATP activates P2X7 receptors on immune and epithelial cells to promote the release of inflammatory mediators. ATP is also sensed by P2Y2 and P2X1 receptors on neutrophils which leads to their infiltration into the alveolus and subsequent activation.
In addition, the P2Y12 receptor is involved in the pathogenesis of ARDS most likely through its effects on platelet activation and aggregation [206,207]. P2Y1 and P2Y14 have been described to be relevant for platelet induced-leukocyte migration into LPS-treated lungs [208] but P2Y1 was not critically involved in lung injury in a model of LPS-induced peritonitis [209]. Specific blocking of P2X1 channels improves transfusion-related acute lung injury in the mouse [89]. P2X4 receptor has failed to demonstrate a significant contribution to the final outcome in a mouse model of traumatic lung injury [210]. Dixit et al. reported recently that the non-specific P2-antagonist suramin alleviated systemic and lung inflammation as well as lung injury in mouse models of acute pancreatitis [211]. Further, they observed increased plasma levels of ATP, suggesting that ATP was involved in remote activation of the pulmonary immune system, and indeed i.v. apyrase treatment significantly reduced inflammation and lung injury. Conflicting to this hypothesis is that systemic administration of the P2 agonist ATPγS has been shown to protect against LPS-induced lung injury [212], which might be related to chemotaxis inhibiting effects of systemic ATP on neutrophils [213].
Since patients suffering from ARDS often require ventilatory support, VILI is a complication that eventually aggravates ARDS [136,214]. An early study reported the involvement of P2Y receptors in the pathogenesis of VILI [204]. Hasan et al. suggested that desensitization of P2Y2 and P2X4 receptors might contribute to the development of VILI [95]. Zheng et al. presented results that in a two-hit mouse model of Pseudomonas aeruginosa pneumonia with high-pressure ventilation a specific P2Y6 receptor antagonist partially attenuated inflammation and lung injury without interfering with the ability of the immune system to clear the bacteria [215].
3.3. Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible and fatal disease. The conceptual model for the pathogenesis of IPF postulates that recurrent micro-injuries to ageing alveolar epithelium result in aberrant epithelial–fibroblast communication, the induction of matrix-producing myofibroblasts, and considerable extracellular matrix accumulation and remodeling of lung interstitium [216,217,218]. Ultimately, this leads to destruction of the overall alveolar architecture, strong impairment of lung function and eventually death of the patient [216,219].
Nucleotides like ATP and UTP are released from injured epithelial cells [28,133,134,136] and ATP levels are significantly increased in bronchoalveolar lavage (BAL) fluid from IPF patients [117,134]. In line, ATP was increased in a murine bleomycin-induced pulmonary fibrosis model [117,134].
It was also found that P2Y2 receptor expression was up-regulated on BAL fluid macrophages and blood neutrophils derived from IPF patients [117], whereas P2Y6 receptor expression was upregulated on lung structural cells in the alveolar space of IPF patients but not on BAL cells [220]. Both, P2Y2 and P2Y6 receptor expression were upregulated in the lung tissue of animals with bleomycin-induced pulmonary fibrosis and activation of P2Y2 or P2Y6 receptor was associated with enhanced proliferation of human and murine lung fibroblasts [117,220] (Figure 3). P2Y2 receptor activation also stimulated the migration of primary lung fibroblasts [117]. Moreover, ATP increased the numbers of neutrophils and macrophages in the lungs of WT animals whereas no changes were seen in P2Y2-deficient mice [117]. It has recently been shown that monocyte-derived macrophages are recruited to the lung and that selective inhibition thereof ameliorated lung fibrosis [24]. P2Y2- as well as P2Y6-deficient animals were found to be partially protected from bleomycin-induced pulmonary inflammation and fibrosis [117].
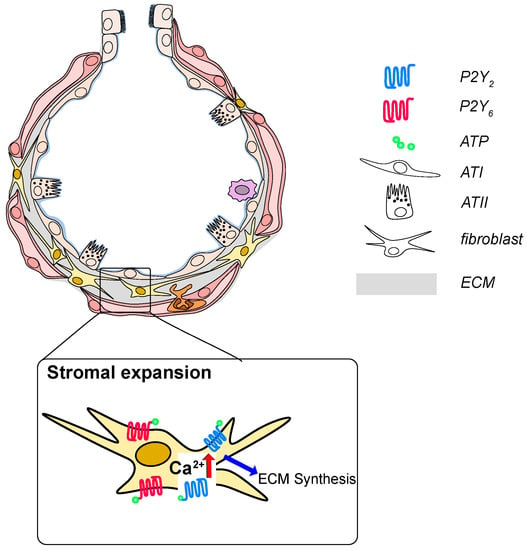
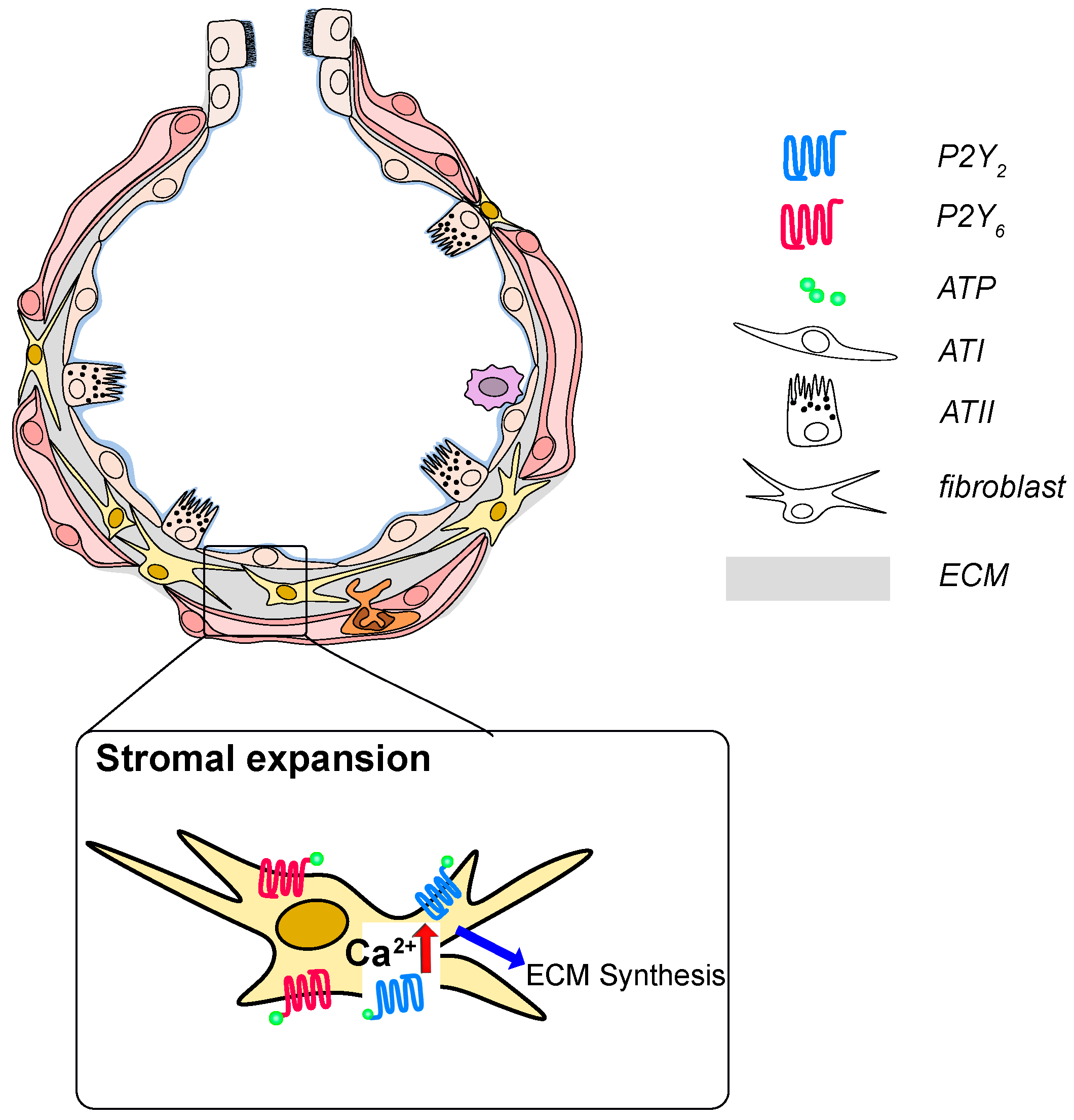
Figure 3.
P2 receptor signaling in development and progression of idiopathic pulmonary fibrosis (IPF) ATP and UTP are released from injured epithelial cells and are increase in the BAL fluid from IPF patients. Activation of P2Y2 or P2Y6 on fibroblasts results in fibroblast proliferation, migration and likely excessive deposition of extracellular matrix constituents.
However, data from animal models have to be taken with caution. Moreover, animal models of lung fibrosis do not recapitulate IPF [217]. The widely used bleomycin model triggers an early inflammatory response followed by fibrotic remodeling at a later stage [221], yet IPF does appear to follow a pathogenic sequence of secondary and modulatory immune activation [222]. Hence, it is difficult to dissect the impact of the P2 receptor expression/activation on inflammation or fibrotic changes; moreover, P2Y2 as well as P2Y6 receptor activation, have been associated with pulmonary inflammation [117,220].
3.4. Pulmonary Arterial Hypertension (PAH)
Patients with PAH suffer increased vascular resistance and high pulmonary arterial pressure. In PAH smooth muscle cells (SMC) proliferate in small peripheral pulmonary arteries and ECs form plexiform lesions within the lung tissue [223].
In lungs of patients with idiopathic PAH (IPAH), a decreased expression of CD39 by ECs in plexiform lesions and primary isolated ECs was detected [38,91]. Decreased expression of CD39 leads to an increased ATP level, which could also be measured in the plasma of patients with IPAH [90]. Downregulation of CD39 expression was observed in lung tissue, as well as in primary isolated EC from IPAH patients [38,90]. On the other hand, circulating EC micro particles from IPAH patients have an increased expression of CD39 [224].
Despite CD39 downregulation, in-vitro studies of isolated EC from IPAH patients also showed an upregulated P2Y11 receptor expression on protein, but not mRNA level [38]. These changes in the purinergic system lead to an apoptosis resistant phenotype of EC, which could be reversed by downregulation of the P2Y11 receptor using siRNA. Furthermore, it was shown that the attenuated CD39 expression and increased ATP level increases SMC migration and proliferation, promoting vascular remodeling [38].
On the basis of the downregulation of CD39, a mouse model for IPAH was established where CD39-/- mice were housed under hypoxic conditions [90]. In this model, as well as in tissue samples from IPAH patients, an increased P2X1 receptor expression was found [90]. Contribution of the P2X1 receptor to IPAH could be demonstrated by rescue experiments with NF279, a P2X1 receptor antagonist [90]. NF279 was able to prevent elevation of pulmonary arterial pressure [90].
Further, for PAH due to BMPR2 (Bone morphogenetic protein receptor type II, serine/threonine receptor kinase) loss-of-function mutations or downregulation in pulmonary ECs, RNA sequencing and Ca2+ imaging data showed a link to the P2 receptor Ca2+-signalosome [32].
4. Conclusion and Outlook
It is well established that P2 receptors are widely expressed on the cells in the lung. Extracellular ATP and P2 receptor-dependent signaling is central to fundamental mechanisms maintaining alveolar homeostasis, in particular surfactant secretion and alveolar host defense. It is not surprising therefore that, in recent years, the contribution of P2 receptors and P2-mediated signaling has gained widespread attention for the pathophysiology of alveolar diseases. A better understanding thereof can ultimately culminate in the development of targeted therapeutics. For example, it has been shown that P2X7 receptor antagonists reduced neutrophil infiltration and proinflammatory cytokine levels [98]. Various P2X7 receptor antagonists are currently in development for the clinic [173]. Also, other P2 receptor agonists and antagonists have already been approved as therapeutics for various diseases, some of which may well be relevant for the treatment of lung diseases in the future. Diquafosol, a long-acting P2Y2 receptor agonist has been approved for the treatment of dry eye disease [225]. P2Y12 receptor antagonists (clopidogrel, prasugrel, cangrelor and ticagrelor) have become an important class of antithrombotic drugs blocking P2Y12 receptor-mediated platelet aggregation [196,226,227]. Successful clinical trials have been completed for application of the P2X3 receptor antagonist gefapixant in chronic cough and other inflammatory conditions [196,228].
Apart from the development of specific drugs, a detailed understanding of the pathophysiology is required to bring P2 receptor therapies to the distal lung. Much of the current knowledge has been derived through use of animal models, which offer great possibilities to investigate the role of specific P2 receptors using adequate knock-out models. However, caution is required when translating findings from animal studies to the human lung. Animal models for pulmonary disease do not recapitulate the full spectrum of human pathophysiology. This is a result of differences in anatomy, as well as physiology [221,229,230]. It will be important to assess the implication of P2 receptor signaling in human lungs or lung tissue from healthy and diseased donors, but availability and access to such tissue often pose a major limitation. In-vitro models offer alternatives to increase our understanding of how the human lung maintains homeostasis and how dysregulation of specific cellular processes leads to disease, especially in hard-to-study lung regions like the human alveolus. Such models can provide powerful, scalable screening platforms to test pharmaceuticals, and can act as an important preclinical step that bridges the gap between drug testing in rodent models and human clinical trials. Recently developed alveolar “lung-on-a-chip” systems recapitulate structural, functional and mechanical elements of the unique biophysical and cellular architecture of the human alveolus in vitro [231,232,233,234,235]. Leveraging these in-vitro models will certainly help in deciphering the molecular and cellular mechanisms driving pathophysiological alterations and thereby accelerate the discovery of novel therapeutic targets [230].
Author Contributions
Writing—original draft preparation, E.W., M.F. (Michael Fauler), G.F. and M.F. (Manfred Frick); writing—review and editing, E.W., M.F. (Michael Fauler), G.F. and M.F. (Manfred Frick); visualization, E.W., M.F. (Michael Fauler), G.F. and M.F. (Manfred Frick); funding acquisition, M.F. (Manfred Frick). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-IDs: 278012962 (GRK “Pulmosens”), 251293561 (Collaborative Research Center (CRC) 1149) and 175083951 (to M.F.).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALI/ARDS | Acute Lung Injury/Acute Respiratory Distress Syndrome |
| AM | Alveolar macrophage |
| ASL | Alveolar surface liquid (thin fluid layer, also termed hypophase) |
| ATI | Alveolar type I epithelial cell |
| ATII | Alveolar type II epithelial cell |
| BAL | Bronchoalveolar lavage |
| CaCC | Ca2+-activated Cl- channels |
| CD39 | Ectonucleotidase, converts ATP into AMP |
| CD73 | Ectonucleotidase, dephosphorylates AMP into adenosine |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| ENaC | Epithelial Na+ channel |
| FACE | Fusion activated Ca2+-entry |
| Hypophase | Thin fluid layer covering the luminal alveolar surface |
| Inflammasome | Cytosolic multiprotein oligomers, involved in inflammatory responses |
| LB | Lamellar Body, surfactant storing organelle in alveolar type II epithelial cells |
| NK | Natural killer cell |
| NLRP3 | Nucleotide-binding domain leucine-rich repeat (NLR) and pyrin domain containing receptor 3, Pattern recognition receptor |
| Treg | Regulatory T cell |
References
- Ochs, M.; Nyengaard, J.R.; Jung, A.; Knudsen, L.; Voigt, M.; Wahlers, T.; Richter, J.; Gundersen, H.J.G. The Number of Alveoli in the Human Lung. Am. J. Respir. Crit. Care Med. 2004. [Google Scholar] [CrossRef]
- Weibel, E.R. Lung morphometry: The link between structure and function. Cell Tissue Res. 2017, 367, 413–426. [Google Scholar] [CrossRef]
- Hsia, C.C.W.; Hyde, D.M.; Weibel, E.R. Lung structure and the intrinsic challenges of gas exchange. Compr. Physiol. 2016. [Google Scholar] [CrossRef]
- Isakson, B.E.; Seedorf, G.J.; Lubman, R.L.; Evans, W.H.; Boitano, S. Cell-Cell Communication in Heterocellular Cultures of Alveolar Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2003. [Google Scholar] [CrossRef]
- Mishra, A.; Chintagari, N.R.; Guo, Y.; Weng, T.; Su, L.; Liu, L. Purinergic P2X7 receptor regulates lung surfactant secretion in a paracrine manner. J. Cell. Sci. 2011. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Reigada, D.; Mitchell, C.H.; Bates, S.R.; Margulies, S.S.; Koval, M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L489–L496. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C. Alveolar Type I Cells: Molecular Phenotype and Development. Annu. Rev. Physiol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Crapo, J.D.; Barry, B.E.; Gehr, P.; Bachofen, M.; Weibel, E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982, 126, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R. On the tricks alveolar epithelial cells play to make a good lung. Am. J. Respir. Crit. Care Med. 2015. [Google Scholar] [CrossRef]
- Dietl, P.; Haller, T.; Frick, M. Spatio-temporal aspects, pathways and actions of Ca2+ in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium 2012, 52, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dietl, P.; Haller, T.; Mair, N.; Frick, M. Mechanisms of surfactant exocytosis in alveolar type II cells in vitro and in vivo. News Physiol. Sci. 2001. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.; Eschertzhuber, S.; Haller, T.; Mair, N.; Dietl, P. Secretion in alveolar type II cells at the interface of constitutive and regulated exocytosis. Am. J. Respir. Cell Mol. Biol. 2001. [Google Scholar] [CrossRef]
- Parra, E.; Pérez-Gil, J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem. Phys. Lipids 2015. [Google Scholar] [CrossRef] [PubMed]
- Goerke, J. Pulmonary surfactant: Functions and molecular composition. Biochim. Biophys. Acta Mol. Basis Dis. 1998. [Google Scholar] [CrossRef]
- Rao Tata, P.; Rajagopal, J. Plasticity in the lung: Making and breaking cell identity. Development 2017, 144, 755–766. [Google Scholar] [CrossRef]
- Olajuyin, A.M.; Zhang, X.; Ji, H.L. Alveolar type 2 progenitor cells for lung injury repair. Cell Death Discov. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.; Ochs, M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Marsland, B.J. Lung Homeostasis: Influence of Age, Microbes, and the Immune System. Immunity 2017, 46, 549–561. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell. Immunol. 2018. [Google Scholar] [CrossRef]
- Garbi, N.; Lambrecht, B.N. Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflugers Arch. Eur. J. Physiol. 2017, 469, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Evren, E.; Ringqvist, E.; Willinger, T. Origin and ontogeny of lung macrophages: From mice to humans. Immunology 2019. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.M.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.I.; Anekalla, K.R.; Joshi, N.; Williams, K.J.N.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, G.K. Neutrophils in the lung: “The first responders”. Cell Tissue Res. 2018, 371, 577–588. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, N.; Narasaraju, T.; Chen, J.; McFarland, L.R.; Scott, M.; Liu, L. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem. Biophys. Res. Commun. 2004, 319, 774–780. [Google Scholar] [CrossRef]
- Belete, H.A.; Hubmayr, R.D.; Wang, S.; Singh, R.D. The role of purinergic signaling on deformation induced injury and repair responses of alveolar epithelial cells. PLoS ONE 2011. [Google Scholar] [CrossRef]
- Miklavc, P.; Mair, N.; Wittekindt, O.H.; Haller, T.; Dietl, P.; Felder, E.; Timmler, M.; Frick, M. Fusion-activated Ca 2+ entry via vesicular P2X 4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 14503–14508. [Google Scholar] [CrossRef]
- Olotu, C.; Kiefmann, M.; Ronneburg, C.; Lehmensiek, F.; Cuvenhaus, A.; Meidl, V.; Goetz, A.E.; Kiefmann, R. Analysis of purine receptor expression and functionality in alveolar epithelial cells. Purinergic Signal. 2020. [Google Scholar] [CrossRef]
- Rice, W.R.; Burton, F.M.; Fiedeldey, D.T. Cloning and expression of the alveolar type II cell P2u-purinergic receptor. Am. J. Respir. Cell Mol. Biol. 1995. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Lüneburg, N.; Stage, A.; Schmitz, M.; Körbelin, J.; Harbaum, L.; Matuszcak, C.; Mienert, J.; Bokemeyer, C.; Böger, R.H.; et al. The P2-receptor-mediated Ca2+ signalosome of the human pulmonary endothelium—Implications for pulmonary arterial hypertension. Purinergic Signal. 2019, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Qi, Z.; Sokabe, M.; Ando, J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Schwiebert, L.M.; Rice, W.C.; Kudlow, B.A.; Taylor, A.L.; Schwiebert, E.M. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2002, 282, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sokabe, T.; Ohura, N.; Nakatsuka, H.; Kamiya, A.; Ando, J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 793–803. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Zemskov, E.A.; Gonzales, J.; Gorshkov, B.A.; Sridhar, S.; Chakraborty, T.; Lucas, R.; Verin, A.D. Extracellular β-nicotinamide adenine dinucleotide (β-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. J. Cell. Physiol. 2010, 223, 215–223. [Google Scholar] [CrossRef]
- Zemskov, E.; Lucas, R.; Verin, A.D.; Umapathy, N.S. P2Y receptors as regulators of lung endothelial barrier integrity. J. Cardiovasc. Dis. Res. 2011, 2, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Helenius, M.H.; Vattulainen, S.; Orcholski, M.; Aho, J.; Komulainen, A.; Taimen, P.; Wang, L.; De Jesus Perez, V.A.; Koskenvuo, J.W.; Alastalo, T.P. Suppression of endothelial CD39/ENTPD1 is associated with pulmonary vascular remodeling in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1046–L1057. [Google Scholar] [CrossRef]
- Janssen, L.J.; Farkas, L.; Rahman, T.; Kolb, M.R.J. ATP stimulates Ca2+-waves and gene expression in cultured human pulmonary fibroblasts. Int. J. Biochem. Cell Biol. 2009, 41, 2477–2484. [Google Scholar] [CrossRef]
- Myrtek, D.; Müller, T.; Geyer, V.; Derr, N.; Ferrari, D.; Zissel, G.; Dürk, T.; Sorichter, S.; Luttmann, W.; Kuepper, M.; et al. Activation of Human Alveolar Macrophages via P2 Receptors: Coupling to Intracellular Ca 2+ Increases and Cytokine Secretion. J. Immunol. 2008, 181, 2181–2188. [Google Scholar] [CrossRef]
- Kessler, S.; Clauss, W.G.; Günther, A.; Kummer, W.; Fronius, M. Expression and functional characterization of P2X receptors in mouse alveolar macrophages. Pflugers Arch. Eur. J. Physiol. 2011, 462, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Bowler, J.W.; Bailey, R.J.; North, R.A.; Surprenant, A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br. J. Pharmacol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Stokes, L.; Surprenant, A. Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur. J. Immunol. 2009, 39, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Stokes, L.; Surprenant, A. Purinergic P2Y 2 Receptors Induce Increased MCP-1/CCL2 Synthesis and Release from Rat Alveolar and Peritoneal Macrophages. J. Immunol. 2007, 179, 6016–6023. [Google Scholar] [CrossRef]
- Vaughan, K.R.; Stokes, L.; Prince, L.R.; Marriott, H.M.; Meis, S.; Kassack, M.U.; Bingle, C.D.; Sabroe, I.; Surprenant, A.; Whyte, M.K.B. Inhibition of Neutrophil Apoptosis by ATP Is Mediated by the P2Y 11 Receptor. J. Immunol. 2007, 179, 8544–8553. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Raible, D.G.; McDermott, L.J.; Pelleg, A.; Schulman, E.S. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: Activation of purinergic P2Y receptors. J. Allergy Clin. Immunol. 2001, 107, 849–855. [Google Scholar] [CrossRef]
- Lecut, C.; Frederix, K.; Johnson, D.M.; Deroanne, C.; Thiry, M.; Faccinetto, C.; Marée, R.; Evans, R.J.; Volders, P.G.A.; Bours, V.; et al. P2X 1 Ion Channels Promote Neutrophil Chemotaxis through Rho Kinase Activation. J. Immunol. 2009, 183, 2801–2809. [Google Scholar] [CrossRef]
- Alkayed, F.; Kashimata, M.; Koyama, N.; Hayashi, T.; Tamura, Y.; Azuma, Y. P2Y11 purinoceptor mediates the ATP-enhanced chemotactic response of rat neutrophils. J. Pharmacol. Sci. 2012, 120, 288–295. [Google Scholar] [CrossRef]
- Suh, B.-C.; Kim, J.-S.; Namgung, U.; Ha, H.; Kim, K.-T. P2X 7 Nucleotide Receptor Mediation of Membrane Pore Formation and Superoxide Generation in Human Promyelocytes and Neutrophils. J. Immunol. 2001, 166, 6754–6763. [Google Scholar] [CrossRef]
- Martel-Gallegos, G.; Rosales-Saavedra, M.T.; Reyes, J.P.; Casas-Pruneda, G.; Toro-Castillo, C.; Pérez-Cornejo, P.; Arreola, J. Human neutrophils do not express purinergic P2X7 receptors. Purinergic Signal. 2010, 6, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shukla, A.; Namiki, S.; Insel, P.A.; Junger, W.G. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J. Leukoc. Biol. 2004, 76, 245–253. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef]
- Scrivens, M.; Dickenson, J.M. Functional expression of the P2Y14 receptor in human neutrophils. Eur. J. Pharmacol. 2006, 543, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G. Purinoceptors: Are there families of P2X and P2Y purinoceptors? Pharmacol. Ther. 1994, 64, 445–475. [Google Scholar] [CrossRef]
- Burnstock, G.; Kennedy, C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen. Pharmacol. 1985, 16, 433–440. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M., VI. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar] [PubMed]
- North, R.A. P2X receptors. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2016. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002. [Google Scholar] [CrossRef] [PubMed]
- Surprenant, A.; North, R.A. Signaling at Purinergic P2X Receptors. Annu. Rev. Physiol. 2009. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Evans, R.J. ATP-Gated P2X Receptor Channels: Molecular Insights into Functional Roles. Annu. Rev. Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Petersen, O.H.; Michalak, M.; Verkhratsky, A. Calcium signalling: Past, present and future. Cell Calcium 2005. [Google Scholar] [CrossRef]
- Kaczmarek-Hájek, K.; Lörinczi, É.; Hausmann, R.; Nicke, A. Molecular and functional properties of P2X receptors-recent progress and persisting challenges. Purinergic Signal. 2012. [Google Scholar] [CrossRef] [PubMed]
- Coddou, C.; Yan, Z.; Obsil, T.; Huidobro-Toro, J.P.; Stojilkovic, S.S. Activation and Regulation of Purinergic P2X Receptor Channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef]
- Habermacher, C.; Dunning, K.; Chataigneau, T.; Grutter, T. Molecular structure and function of P2X receptors. Neuropharmacology 2016. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Müller, C.E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006. [Google Scholar] [CrossRef]
- Von Kügelgen, I.; Hoffmann, K. Pharmacology and structure of P2Y receptors. Neuropharmacology 2016. [Google Scholar] [CrossRef]
- Kennedy, C.; Qi, A.D.; Herold, C.L.; Harden, T.K.; Nicholas, R.A. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4. Mol. Pharmacol. 2000, 57, 926–931. [Google Scholar] [PubMed]
- Jacobson, K.A.; Paoletta, S.; Katritch, V.; Wu, B.; Gao, Z.G.; Zhao, Q.; Stevens, R.C.; Kiselev, E. Nucleotides acting at P2Y receptors: Connecting structure and function. Mol. Pharmacol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Von Kugelgen, I.; Harden, T.K. Molecular Pharmacology, Physiology, and Structure of the P2Y Receptors. No Adv. Pharmacol. 2011. [Google Scholar] [CrossRef]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Von Kügelgen, I. Pharmacology of P2Y receptors. Brain Res. Bull. 2019. [Google Scholar] [CrossRef] [PubMed]
- Homolya, L.; Watt, W.C.; Lazarowski, E.R.; Koller, B.H.; Boucher, R.C. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y2 receptor (-/-) mice. J. Biol. Chem. 1999. [Google Scholar] [CrossRef]
- Savio, L.E.B.; Mello, P. de A.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 receptor in inflammatory diseases: Angel or demon? Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Dagvadorj, J.; Shimada, K.; Chen, S.; Jones, H.D.; Tumurkhuu, G.; Zhang, W.; Wawrowsky, K.A.; Crother, T.R.; Arditi, M. Lipopolysaccharide Induces Alveolar Macrophage Necrosis via CD14 and the P2X7 Receptor Leading to Interleukin-1α Release. Immunity 2015, 42, 640–653. [Google Scholar] [CrossRef]
- Moore, D.J.; Murdock, P.R.; Watson, J.M.; Faull, R.L.M.; Waldvogel, H.J.; Szekeres, P.G.; Wilson, S.; Freeman, K.B.; Emson, P.C. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: Possible role in neuroimmune function. Mol. Brain Res. 2003, 118, 10–23. [Google Scholar] [CrossRef]
- Darbousset, R.; Delierneux, C.; Mezouar, S.; Hego, A.; Lecut, C.; Guillaumat, I.; Riederer, M.A.; Evans, R.J.; Dignat-George, F.; Panicot-Dubois, L.; et al. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood 2014, 124, 2575–2585. [Google Scholar] [CrossRef]
- Lucattelli, M.; Cicko, S.; Müller, T.; Lommatzsch, M.; De Cunto, G.; Cardini, S.; Sundas, W.; Grimm, M.; Zeiser, R.; Dürk, T.; et al. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 2011, 44, 423–429. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Le Duc, D.; Schulz, A.; Lede, V.; Schulze, A.; Thor, D.; Brüser, A.; Schöneberg, T. P2Y Receptors in Immune Response and Inflammation. Adv. Immunol. 2017, 136, 85–121. [Google Scholar]
- Gorini, S.; Callegari, G.; Romagnoli, G.; Mammi, C.; Mavilio, D.; Rosano, G.; Fini, M.; Di Virgilio, F.; Gulinelli, S.; Falzoni, S.; et al. ATP secreted by endothelial cells blocks CX 3CL1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y 11 receptor activation. Blood 2010, 116, 4492–4500. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A. New insights of P2X7 receptor signaling pathway in alveolar functions. J. Biomed. Sci. 2013, 20, 26. [Google Scholar] [CrossRef]
- Saéz, P.J.; Vargas, P.; Shoji, K.F.; Harcha, P.A.; Lennon-Duméni, A.M.; Saéz, J.C. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptors. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Aswad, F.; Dennert, G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell. Immunol. 2006, 243, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, W.; Xu, X.; Xiong, Y.; Zhang, Y.; Zhang, H.; Sun, B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc. Natl. Acad. Sci. USA 2017, 114, 4483–4488. [Google Scholar] [CrossRef]
- El Mdawar, M.B.; Maître, B.; Magnenat, S.; Gachet, C.; Hechler, B.; de la Salle, H. The ATP-gated P2X 1 ion channel contributes to the severity of antibody-mediated Transfusion-Related Acute Lung Injury in mice. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Visovatti, S.H.; Hyman, M.C.; Goonewardena, S.N.; Anyanwu, A.C.; Kanthi, Y.; Robichaud, P.; Wang, J.; Petrovic-Djergovic, D.; Rattan, R.; Burant, C.F.; et al. Purinergic dysregulation in pulmonary hypertension. Am. J. Physiol. Hear. Circ. Physiol. 2016, 311, H286–H298. [Google Scholar] [CrossRef] [PubMed]
- Murrell-Lagnado, R.D.; Frick, M. P2X4 and lysosome fusion. Curr. Opin. Pharmacol. 2019, 47, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Neuland, K.; Sharma, N.; Frick, M. Synaptotagmin-7 links fusion-activated Ca2+ entry and fusion pore dilation. J. Cell Sci. 2014, 127, 5218–5227. [Google Scholar] [CrossRef]
- Thompson, K.E.; Korbmacher, J.P.; Hecht, E.; Hobi, N.; Wittekindt, O.H.; Dietl, P.; Kranz, C.; Frick, M. Fusion-activated cation entry (FACE) via P2X4 couples surfactant secretion and alveolar fluid transport. FASEB J. 2013, 27, 1772–1783. [Google Scholar] [CrossRef]
- Ledderose, C.; Liu, K.; Kondo, Y.; Slubowski, C.J.; Dertnig, T.; Denicoló, S.; Arbab, M.; Hubner, J.; Konrad, K.; Fakhari, M.; et al. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J. Clin. Invest. 2018, 128, 3583–3594. [Google Scholar] [CrossRef]
- Hasan, D.; Satalin, J.; van der Zee, P.; Kollisch-Singule, M.; Blankman, P.; Shono, A.; Somhorst, P.; den Uil, C.; Meeder, H.; Kotani, T.; et al. Excessive extracellular ATP desensitizes P2Y2 and P2X4 ATP receptors provoking surfactant impairment ending in ventilation-induced lung injury. Int. J. Mol. Sci. 2018, 19, 1185. [Google Scholar] [CrossRef] [PubMed]
- Mawatwal, S.; Behura, A.; Ghosh, A.; Kidwai, S.; Mishra, A.; Deep, A.; Agarwal, S.; Saha, S.; Singh, R.; Dhiman, R. Calcimycin mediates mycobacterial killing by inducing intracellular calcium-regulated autophagy in a P2RX7 dependent manner. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Mawatwal, S.; Behura, A.; Mishra, A.; Singh, R.; Dhiman, R. Calcimycin induced IL-12 production inhibits intracellular mycobacterial growth by enhancing autophagy. Cytokine 2018, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Guo, Y.; Zhang, L.; More, S.; Weng, T.; Chintagari, N.R.; Huang, C.; Liang, Y.; Pushparaj, S.; Gou, D.; et al. A Critical Role for P2X7 Receptor–Induced VCAM-1 Shedding and Neutrophil Infiltration during Acute Lung Injury. J. Immunol. 2016, 197, 2828–2837. [Google Scholar] [CrossRef]
- Lee, B.H.; Hwang, D.M.; Palaniyar, N.; Grinstein, S.; Philpott, D.J.; Hu, J. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Leyva-Grado, V.H.; Ermler, M.E.; Schotsaert, M.; Gonzalez, M.G.; Gillespie, V.; Lim, J.K.; García-Sastrea, A. Contribution of the purinergic receptor P2X7 to development of lung immunopathology during influenza virus infection. MBio 2017. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Coutinho-Silva, R. Purinergic signalling, DAMPs and inflammation. Am. J. Physiol. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H.; Wang, L.; Zhang, N.; Huang, H.; Xiong, Q.; Yan, Y.; Wu, N.; Ren, H.; Han, H.; et al. Virus-Triggered ATP Release Limits Viral Replication through Facilitating IFN-β Production in a P2X7-Dependent Manner. J. Immunol. 2017, 199, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, C.H. Control of Tissue-Resident Invariant NKT Cells by Vitamin A Metabolites and P2X7-Mediated Cell Death. J. Immunol. 2019, 203, 1189–1197. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, J.; Bian, H.; Cai, J.; Zhu, G. Suppression of P2X7/NF-κB pathways by Schisandrin B contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Arch. Pharm. Res. 2016, 39, 499–507. [Google Scholar] [CrossRef]
- Cicko, S.; Köhler, T.C.; Korcan Ayata, C.; Müller, T.; Ehrat, N.; Meyer, A.; Hossfeld, M.; Zech, A.; Di Virgilio, F.; Idzko, M. Extracellular ATP is a danger signal activating P2X7 receptor in a LPS mediated inflammation (ARDS/ALI). Oncotarget 2018, 9, 30635–30648. [Google Scholar] [CrossRef] [PubMed]
- Cicko, S.; Meyer, A.; Ehrat, N.; Ayata, C.K.; Zech, A.; Hossfeld, M.; Idzko, M. Extracellular ATP is a danger signal activating P2X7 Receptor in a LPS mediated inflammation (ARDS). ERS 2015, 46. [Google Scholar] [CrossRef]
- Galam, L.; Rajan, A.; Failla, A.; Soundararajan, R.; Lockey, R.F.; Kolliputi, N. Deletion of P2X7 attenuates hyperoxia-induced acute lung injury via inflammasome suppression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, 572–581. [Google Scholar] [CrossRef]
- Ibrahim, M.; Wang, X.; Puyo, C.A.; Montecalvo, A.; Huang, H.J.; Hachem, R.R.; Andreetti, C.; Menna, C.; Chen, R.; Krupnick, A.S.; et al. Human recombinant apyrase therapy protects against canine pulmonary ischemia-reperfusion injury. J. Hear. Lung Transplant. 2015, 34, 247–253. [Google Scholar] [CrossRef]
- Li, Q.C.; Liang, Y.; Su, Z.B. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L1107–L1117. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Chen, X.; Shi, Q.; Su, W.; Zhao, H. SOCS-1 Suppresses Inflammation Through Inhibition of NALP3 Inflammasome Formation in Smoke Inhalation-Induced Acute Lung Injury. Inflammation 2018, 41, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.A. Regulation of surfactant secretion. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2001. [Google Scholar] [CrossRef]
- Leipziger, J. Control of epithelial transport via luminal P2 receptors. Am. J. Physiol. Ren. Physiol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Novak, I. Purinergic signalling in epithelial ion transport: Regulation of secretion and absorption. Acta Physiol. 2011. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G. Purinergic regulation of neutrophil chemotaxis. Cell. Mol. Life Sci. 2008, 65, 2528–2540. [Google Scholar] [CrossRef]
- Müller, T.; Fay, S.; Vieira, R.P.; Harry, K.Q.; Cicko, S.; Ayata, K.; Zissel, G.; Goldmann, T.; Lungarella, G.; Ferrari, D.; et al. The purinergic receptor subtype P2Y2 mediates chemotaxis of neutrophils and fibroblasts in fibrotic lung disease. Oncotarget 2017, 8, 35962–35972. [Google Scholar] [CrossRef]
- Bove, P.F.; Grubb, B.R.; Okada, S.F.; Ribeiro, C.M.P.; Rogers, T.D.; Randell, S.H.; O’Neal, W.K.; Boucher, R.C. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J. Biol. Chem. 2010. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Watt, W.C.; Stutts, M.J.; Boucher, R.C.; Harden, T.K. Pharmacological selectivity of the cloned human P2U-purinoceptor: Potent activation by diadenosine tetraphosphate. Br. J. Pharmacol. 1995. [Google Scholar] [CrossRef]
- Brunschweiger, A.; Muller, C. P2 Receptors Activated by Uracil Nucleotides—An Update. Curr. Med. Chem. 2006. [Google Scholar] [CrossRef]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Sesma, J.I.; Seminario-Vidal, L.; Kreda, S.M. Molecular Mechanisms of Purine and Pyrimidine Nucleotide Release. Adv. Pharmacol. 2011. [Google Scholar] [CrossRef]
- Fois, G.; Winkelmann, V.E.; Bareis, L.; Staudenmaier, L.; Hecht, E.; Ziller, C.; Ehinger, K.; Schymeinsky, J.; Kranz, C.; Frick, M. ATP is stored in lamellar bodies to activate vesicular P2X4 in an autocrine fashion upon exocytosis. J. Gen. Physiol. 2018, 150, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.; Boudreault, F.; Adam, D.; Brochiero, E.; Grygorczyk, R. Type 2 secretory cells are primary source of ATP release in mechanically stretched lung alveolar cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Grygorczyk, R.; Boudreault, F.; Tan, J.J.; Ponomarchuk, O.; Sokabe, M.; Furuya, K. Mechanosensitive ATP release in the lungs: New insights from real-time luminescence imaging studies. Curr. Top. Membr. 2019, 83, 45–76. [Google Scholar] [CrossRef]
- Ramsingh, R.; Grygorczyk, A.; Solecki, A.; Cherkaoui, L.S.; Berthiaume, Y.; Grygorczyk, R. Cell deformation at the air-liquid interface induces Ca2+-dependent ATP release from lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011. [Google Scholar] [CrossRef]
- Furuya, K.; Tan, J.J.; Boudreault, F.; Sokabe, M.; Berthiaume, Y.; Grygorczyk, R. Real-time imaging of inflation-induced ATP release in the ex vivo rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L956–L969. [Google Scholar] [CrossRef] [PubMed]
- Dietl, P.; Frick, M.; Mair, N.; Bertocchi, C.; Haller, T. Pulmonary consequences of a deep breath revisited. Biol. Neonate 2004. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.; Bertocchi, C.; Jennings, P.; Haller, T.; Mair, N.; Singer, W.; Pfaller, W.; Ritsch-Marte, M.; Dietl, P. Ca2+ entry is essential for cell strain-induced lamellar body fusion in isolated rat type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, H.R.; Dobbs, L.G. The effects of mechanical forces on lung functions. Respir. Physiol. 2000. [Google Scholar] [CrossRef]
- Edwards, Y.S. Stretch stimulation: Its effects on alveolar type II cell function in the lung. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2001. [Google Scholar] [CrossRef]
- Kuehn, A.; Kletting, S.; De Souza Carvalho-Wodarz, C.; Repnik, U.; Griffiths, G.; Fischer, U.; Meese, E.; Huwer, H.; Wirth, D.; May, T.; et al. Human alveolar epithelial cells expressing tight junctions to model the air-blood barrier. ALTEX 2016, 33, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M. Purines in tissue inflammation and fibrosis. Purinergic Signal. 2014. [Google Scholar] [CrossRef]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.J.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.B.; Douillet, C.D.; Mahler, S.A.; Husain, S.A.; Boucher, R.C. Adenosine triphosphate is released during injurious mechanical ventilation and contributes to lung edema. J. Trauma 2003. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Blankman, P.; Nieman, G.F. Purinergic signalling links mechanical breath profile and alveolar mechanics with the pro-inflammatory innate immune response causing ventilation-induced lung injury. Purinergic Signal. 2017, 13, 363–386. [Google Scholar] [CrossRef]
- Tatur, S.; Groulx, N.; Orlov, S.N.; Grygorczyk, R. Ca2+-dependent ATP release from A549 cells involves synergistic autocrine stimulation by coreleased uridine nucleotides. J. Physiol. 2007. [Google Scholar] [CrossRef]
- Dietl, P.; Liss, B.; Felder, E.; Miklavc, P.; Wirtz, H. Lamellar body exocytosis by cell stretch or purinergic stimulation: Possible physiological roles, messengers and mechanisms. Cell. Physiol. Biochem. 2010, 25, 1–12. [Google Scholar] [CrossRef]
- Andreeva, A.V.; Kutuzov, M.A.; Voyno-Yasenetskaya, T.A. Regulation of surfactant secretion in alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293. [Google Scholar] [CrossRef]
- Olmeda, B.; Martínez-Calle, M.; Pérez-Gil, J. Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann. Anat. 2017, 209, 78–92. [Google Scholar] [CrossRef]
- Perez-Gil, J.; Weaver, T.E. Pulmonary surfactant pathophysiology: Current models and open questions. Physiology 2010, 25, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chander, A.; Fisher, A.B. Regulation of lung surfactant secretion. Am. J. Physiol. Lung Cell. Mol. Physiol. 1990. [Google Scholar] [CrossRef]
- Rice, W.R. Effects of Extracellular ATP on Surfactant Secretion. Ann. N. Y. Acad. Sci. 1990. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Rooney, S.A. Purinoceptor agonists stimulate phosphatidylcholine secretion in primary cultures of adult rat type II pneumocytes. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1987. [Google Scholar] [CrossRef]
- Griese, M.; Gobran, L.I.; Rooney, S.A. ATP-stimulated inositol phospholipid metabolism and surfactant secretion in rat type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 1991. [Google Scholar] [CrossRef]
- Haller, T.; Auktor, K.; Frick, M.; Mair, N.; Dietl, A.P. Threshold calcium levels for lamellar body exocytosis in type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999, 277, L893–L900. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Frick, M.; Haller, T.; Bernet, S.; Ritsch-Marte, M.; Dietl, P. Mechanical forces impeding exocytotic surfactant release revealed by optical tweezers. Biophys. J. 2003, 84, 1344–1351. [Google Scholar] [CrossRef]
- Haller, T.; Dietl, P.; Pfaller, K.; Frick, M.; Mair, N.; Paulmichl, M.; Hess, M.W.; Fürst, J.; Maly, K. Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 2001, 155, 279–289. [Google Scholar] [CrossRef]
- Miklavc, P.; Ehinger, K.; Sultan, A.; Felder, T.; Paul, P.; Gottschalk, K.-E.E.; Frick, M. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J. Cell Sci. 2015, 128, 1193–1203. [Google Scholar] [CrossRef]
- Miklavc, P.; Frick, M.; Wittekindt, O.H.; Haller, T.; Dietl, P. Fusion-Activated Ca2+ entry: An “active zone” of elevated Ca2+ during the postfusion stage of lamellar body exocytosis in rat Type II Pneumocytes. PLoS ONE 2010. [Google Scholar] [CrossRef]
- Fois, G.; Föhr, K.J.; Kling, C.; Fauler, M.; Wittekindt, O.H.; Dietl, P.; Frick, M. P2X4 receptor re-sensitization depends on a protonation/deprotonation cycle mediated by receptor internalization and recycling. J. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewski, R.; Matalon, S.; Collawn, J.F. Ion channels of the lung and their role in disease pathogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L859–L872. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, O.H.; Dietl, P. Aquaporins in the lung. Pflugers Arch. Eur. J. Physiol. 2019, 471, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, M.I.; Richter, K.; Fronius, M. Ion transport by pulmonary epithelia. J. Biomed. Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Bastacky, J.; Lee, C.Y.C.; Goerke, J.; Koushafar, H.; Yager, D.; Kenaga, L.; Speed, T.P.; Chen, Y.; Clements, J.A. Alveolar lining layer is thin and continuous: Low-temperature scanning electron microscopy of rat lung. J. Appl. Physiol. 1995, 79, 1615–1628. [Google Scholar] [CrossRef]
- Berthiaume, Y.; Matthay, M.A. Alveolar edema fluid clearance and acute lung injury. Respir. Physiol. Neurobiol. 2007. [Google Scholar] [CrossRef]
- Kemp, P.J.; Kim, K.J. Spectrum of ion channels in alveolar epithelial cells: Implications for alveolar fluid balance. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, L.G.; Johnson, M.D. Alveolar epithelial transport in the adult lung. Respir. Physiol. Neurobiol. 2007. [Google Scholar] [CrossRef]
- Peteranderl, C.; Sznajder, J.I.; Herold, S.; Lecuona, E. Inflammatory responses regulating alveolar ion transport during pulmonary infections. Front. Immunol. 2017. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Makhlina, M.; Martsen, E.; Thomas, E.J.; Lethem, M.I.; Boucher, R.C. Permeabilization via the P2X7 purinoreceptor reveals the presence of a Ca2+ -activated Cl- conductance in the apical membrane of murine tracheal epithelial cells. J. Biol. Chem. 2000. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Schreiber, R.; Cook, D. Mechanisms for the inhibition of amiloride-sensitive Na+ absorption by extracellular nucleotides in mouse trachea. Pflugers Arch. Eur. J. Physiol. 2002. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Kunzelmann, K. Purinergic P2Y 6 receptors induce Ca 2+ and CFTR dependent Cl—Secretion in mouse trachea. Cell. Physiol. Biochem. 2005. [Google Scholar] [CrossRef] [PubMed]
- Blaug, S.; Rymer, J.; Jalickee, S.; Miller, S.S. P2 purinoceptors regulate calcium-activated chloride and fluid transport in 31EG4 mammary epithelia. Am. J. Physiol. Cell Physiol. 2003. [Google Scholar] [CrossRef]
- Pochynyuk, O.; Bugaj, V.; Vandewalle, A.; Stockand, J.D. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am. J. Physiol. Ren. Physiol. 2008. [Google Scholar] [CrossRef]
- Faria, D.; Schreiber, R.; Kunzelmann, K. CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflugers Arch. Eur. J. Physiol. 2009. [Google Scholar] [CrossRef]
- Jin, W.; Hopfer, U. Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: Elevated cytosolic Ca2+ is not required. Am. J. Physiol. Cell Physiol. 1997. [Google Scholar] [CrossRef]
- Miklavc, P.; Thompson, K.E.; Frick, M. A new role for P2X4 receptors as modulators of lung surfactant secretion. Front. Cell. Neurosci. 2013, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Hartl, D.; Tirouvanziam, R.; Laval, J.; Greene, C.M.; Habiel, D.; Sharma, L.; Yildirim, A.Ö.; Dela Cruz, C.S.; Hogaboam, C.M. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J. Innate Immun. 2018. [Google Scholar] [CrossRef]
- Le, T.T.T.; Berg, N.K.; Harting, M.T.; Li, X.; Eltzschig, H.K.; Yuan, X. Purinergic signaling in pulmonary inflammation. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Vuerich, M. Purinergic signaling in the immune system. Auton. Neurosci. Basic Clin. 2015. [Google Scholar] [CrossRef]
- Morandini, A.; Savio, L.; Coutinho-Silva, R. The role of p2x7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed. J. 2014. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Falzoni, S.; Di Virgilio, F. The P2X7 receptor-interleukin-1 liaison. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Krautloher, A.; Ramírez-Fernández, A.; Nicke, A. P2X7 Interactions and Signaling—Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- Barberà-Cremades, M.; Gómez, A.I.; Baroja-Mazo, A.; Martínez-Alarcón, L.; Martínez, C.M.; de Torre-Minguela, C.; Pelegrín, P. P2X7 receptor induces tumor necrosis factor-α converting enzyme activation and release to boost TNF-α production. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Dubyak, G.R. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell. Microbiol. 2012, 14, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 2015. [Google Scholar] [CrossRef]
- Scott, I.C.; Majithiya, J.B.; Sanden, C.; Thornton, P.; Sanders, P.N.; Moore, T.; Guscott, M.; Corkill, D.J.; Erjefält, J.S.; Cohen, E.S. Interleukin-33 is activated by allergen- and necrosis-associated proteolytic activities to regulate its alarmin activity during epithelial damage. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J. Immunol. 2011. [Google Scholar] [CrossRef]
- Soni, S.; O’Dea, K.P.; Tan, Y.Y.; Cho, K.; Abe, E.; Romano, R.; Cui, J.; Ma, D.; Sarathchandra, P.; Wilson, M.R.; et al. ATP redirects cytokine trafficking and promotes novel membrane TNF signaling via microvesicles. FASEB J. 2019, 33, 6442–6455. [Google Scholar] [CrossRef]
- Soni, S.; Wilson, M.R.; O’Dea, K.P.; Yoshida, M.; Katbeh, U.; Woods, S.J.; Takata, M. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax 2016, 71, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Pizzirani, C.; Ferrari, D.; Chiozzi, P.; Adinolfi, E.; Sandonà, D.; Savaglio, E.; Di Virgilio, F. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood 2007, 109, 3856–3864. [Google Scholar] [CrossRef] [PubMed]
- Kronlage, M.; Song, J.; Sorokin, L.; Isfort, K.; Schwerdtle, T.; Leipziger, J.; Robaye, B.; Conley, P.B.; Kim, H.C.; Sargin, S.; et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal. 2010, 3. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, Y.; Ledderose, C.; Li, L.; Junger, W.G. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J. Biol. Chem. 2013, 288, 22650–22657. [Google Scholar] [CrossRef]
- Bao, Y.; Ledderose, C.; Graf, A.F.; Brix, B.; Birsak, T.; Lee, A.; Zhang, J.; Junger, W.G. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J. Cell Biol. 2015, 210, 1153–1164. [Google Scholar] [CrossRef]
- Li, P.; Cao, J.; Chen, Y.; Wang, W.; Yang, J. Apyrase protects against allergic airway inflammation by decreasing the chemotactic migration of dendritic cells in mice. Int. J. Mol. Med. 2014, 34, 269–275. [Google Scholar] [CrossRef]
- Thomas, M.R.; Storey, R.F. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- Burnstock, G.; Boeynaems, J.M. Purinergic signalling and immune cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar]
- Shin, A.; Toy, T.; Rothenfusser, S.; Robson, N.; Vorac, J.; Dauer, M.; Stuplich, M.; Endres, S.; Cebon, J.; Maraskovsky, E.; et al. P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood 2008, 111, 3062–3069. [Google Scholar] [CrossRef]
- Ledderose, C.; Bao, Y.; Lidicky, M.; Zipperle, J.; Li, L.; Strasser, K.; Shapiro, N.I.; Junger, W.G. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J. Biol. Chem. 2014, 289, 25936–25945. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, C.; Bao, Y.; Zhang, J.; Junger, W.G. Novel method for real-time monitoring of ATP release reveals multiple phases of autocrine purinergic signalling during immune cell activation. Acta Physiol. 2015, 213, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Ploia, C.; Anselmi, F.; Sarukhan, A.; Viola, A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 2014, 33, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Brouns, I.; Adriaensen, D.; Timmermans, J.-P. Purinergic signaling in the airways. Pharmacol. Rev. 2012. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The purinergic system as a pharmacological target for the treatment of immune-mediated inflammatory diseases. Pharmacol. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling: Therapeutic developments. Front. Pharmacol. 2017, 8, 1–55. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Tate, M.D.; Ong, J.D.H.; Dowling, J.K.; McAuley, J.L.; Robertson, A.B.; Latz, E.; Drummond, G.R.; Cooper, M.A.; Hertzog, P.J.; Mansell, A. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Rosli, S.; Kirby, F.J.; Lawlor, K.E.; Rainczuk, K.; Drummond, G.R.; Mansell, A.; Tate, M.D. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br. J. Pharmacol. 2019, 176, 3834–3844. [Google Scholar] [CrossRef]
- Santos, A.A.; Rodrigues-Junior, V.; Zanin, R.F.; Borges, T.J.; Bonorino, C.; Coutinho-Silva, R.; Takyia, C.M.; Santos, D.S.; Campos, M.M.; Morrone, F.B. Implication of purinergic P2X7 receptor in M. tuberculosis infection and host interaction mechanisms: A mouse model study. Immunobiology 2013, 218, 1104–1112. [Google Scholar] [CrossRef]
- Amaral, E.P.; Ribeiro, S.C.M.; Lanes, V.R.; Almeida, F.M.; de Andrade, M.R.M.; Bomfim, C.C.B.; Salles, É.M.; Bortoluci, K.R.; Coutinho-Silva, R.; Hirata, M.H.; et al. Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis. PLoS Pathog. 2014. [Google Scholar] [CrossRef] [PubMed]
- Olotu, C.; Lehmensiek, F.; Koch, B.; Kiefmann, M.; Riegel, A.K.; Hammerschmidt, S.; Kiefmann, R. Streptococcus pneumoniae inhibits purinergic signaling and promotes purinergic receptor P2Y2 internalization in alveolar epithelial cells. J. Biol. Chem. 2019, 294, 12795–12806. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Amaya, F.; Hashimoto, S.; Ueno, H.; Beppu, S.; Mizuta, M.; Shime, N.; Ishizaka, A.; Hashimoto, S. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: An experimental study. Respir. Res. 2008, 9, 1–13. [Google Scholar] [CrossRef]
- Reutershan, J.; Vollmer, I.; Stark, S.; Wagner, R.; Ngamsri, K.; Eltzschig, H.K. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009, 23, 473–482. [Google Scholar] [CrossRef]
- Mansour, A.; Bachelot-Loza, C.; Nesseler, N.; Gaussem, P.; Gouin-Thibault, I. P2Y12 inhibition beyond thrombosis: Effects on inflammation. Int. J. Mol. Sci. 2020, 21, 1391. [Google Scholar] [CrossRef]
- Liverani, E.; Rico, M.C.; Yaratha, L.; Tsygankov, A.Y.; Kilpatrick, L.E.; Kunapuli, S.P. LPS-induced systemic inflammation is more severe in P2Y 12 null mice. J. Leukoc. Biol. 2014, 95, 313–323. [Google Scholar] [CrossRef]
- Amison, R.T.; Arnold, S.; O’Shaughnessy, B.G.; Cleary, S.J.; Ofoedu, J.; Idzko, M.; Page, C.P.; Pitchford, S.C. Lipopolysaccharide (LPS) induced pulmonary neutrophil recruitment and platelet activation is mediated via the P2Y1 and P2Y14 receptors in mice. Pulm. Pharmacol. Ther. 2017, 45, 62–68. [Google Scholar] [CrossRef]
- Liverani, E. Lung injury during LPS-induced inflammation occurs independently of the receptor P2Y1. Purinergic Signal. 2017, 13, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.; Wagner, K.; Weber, S.; Gröger, M.; Wepler, M.; McCook, O.; Scheuerle, A.; Stahl, B.; Huber-Lang, M.; Jung, B.; et al. Role of the purinergic receptor P2XR4 after blunt chest trauma in cigarette smoke-exposed mice. Shock 2017, 47, 193–199. [Google Scholar] [CrossRef]
- Dixit, A.; Cheema, H.; George, J.; Iyer, S.; Dudeja, V.; Dawra, R.; Saluja, A.K. Extracellular release of ATP promotes systemic inflammation during acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G463–G475. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, I.A.; Mirzapoiazova, T.; Moreno-Vinasco, L.; Sammani, S.; Garcia, J.G.N.; Verin, A.D. Protective effect of purinergic agonist ATPγS against acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294. [Google Scholar] [CrossRef]
- Li, X.; Kondo, Y.; Bao, Y.; Staudenmaier, L.; Lee, A.; Zhang, J.; Ledderose, C.; Junger, W.G. Systemic Adenosine Triphosphate Impairs Neutrophil Chemotaxis and Host Defense in Sepsis. Crit. Care Med. 2017, 45, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Hoegl, S.; Burns, N.; Angulo, M.; Francis, D.; Osborne, C.M.; Mills, T.W.; Blackburn, M.R.; Eltzschig, H.K.; Vohwinkel, C.U. Capturing the multifactorial nature of ARDS—“Two-hit” approach to model murine acute lung injury. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, Y.; Islam, D.; Wen, X.Y.; Wu, S.; Streutker, C.; Luo, A.; Li, M.; Khang, J.; Han, B.; et al. Dual effects of human neutrophil peptides in a mouse model of pneumonia and ventilator-induced lung injury. Respir. Res. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Bagnato, G.; Harari, S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur. Respir. Rev. 2015, 24, 102–114. [Google Scholar] [CrossRef]
- Wuyts, W.A.; Agostini, C.; Antoniou, K.M.; Bouros, D.; Chambers, R.C.; Cottin, V.; Egan, J.J.; Lambrecht, B.N.; Lories, R.; Parfrey, H.; et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur. Respir. J. 2013. [Google Scholar] [CrossRef]
- Müller, T.; Fay, S.; Vieira, R.P.; Karmouty-Quintana, H.; Cicko, S.; Ayata, C.K.; Zissel, G.; Goldmann, T.; Lungarella, G.; Ferrari, D.; et al. P2Y6 receptor activation promotes inflammation and tissue remodeling in pulmonary fibrosis. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Moore, B.B.; Lawson, W.E.; Oury, T.D.; Sisson, T.H.; Raghavendran, K.; Hogaboam, C.M. Animal models of fibrotic lung disease. Am. J. Respir. Cell Mol. Biol. 2013, 49, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Günther, S.; Dorfmüller, P.; Perros, F.; Girerd, B.; Garcia, G.; Jaïs, X.; Savale, L.; Artaud-Macari, E.; Price, L.C.; et al. Pulmonary arterial hypertension. Orphanet J. Rare Dis. 2013, 8, 97. [Google Scholar] [CrossRef]
- Visovatti, S.H.; Hyman, M.C.; Bouis, D.; Neubig, R.; McLaughlin, V.V.; Pinsky, D.J. Increased CD39 nucleotidase activity on microparticles from patients with idiopathic pulmonary arterial hypertension. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ohashi, Y.; Watanabe, H.; Tsubota, K. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: A Japanese phase 2 clinical trial. Ophthalmology 2012. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.E.; Baqi, Y.; Namasivayam, V. Agonists and antagonists for purinergic receptors. In Methods in Molecular Biology; Humana: New York, NY, USA, 2020. [Google Scholar]
- Baqi, Y.; Müller, C.E. Antithrombotic P2Y 12 receptor antagonists: Recent developments in drug discovery. Drug Discov. Today 2019. [Google Scholar] [CrossRef] [PubMed]
- Abdulqawi, R.; Dockry, R.; Holt, K.; Layton, G.; Mccarthy, B.G.; Ford, A.P.; Smith, J.A. P2X3 receptor antagonist (AF-219) in refractory chronic cough: A randomised, double-blind, placebo-controlled phase 2 study. Lancet 2015. [Google Scholar] [CrossRef]
- Martin, T.R.; Matute-Bello, G. Experimental Models and Emerging Hypotheses for Acute Lung Injury. Crit. Care Clin. 2011. [Google Scholar] [CrossRef]
- Miller, A.J.; Spence, J.R. In vitro models to study human lung development, disease and homeostasis. Physiology 2017. [Google Scholar] [CrossRef]
- Potkay, J.A.; Magnetta, M.; Vinson, A.; Cmolik, B. Bio-inspired, efficient, artificial lung employing air as the ventilating gas. Lab. Chip 2011. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Yuan Hsin, H.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010. [Google Scholar] [CrossRef] [PubMed]
- Huh, D. A human breathing lung-on-a-chip. Ann. Am. Thorac. Soc. 2015. [Google Scholar] [CrossRef] [PubMed]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab. Chip 2015. [Google Scholar] [CrossRef] [PubMed]
- Konar, D.; Devarasetty, M.; Yildiz, D.V.; Atala, A.; Murphy, S.V. Lung-On-A-Chip Technologies for Disease Modeling and Drug Development. Biomed. Eng. Comput. Biol. 2016. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).