De Novo Assembly of the Asian Citrus Psyllid Diaphorina citri (Hemiptera: Psyllidae) Transcriptome across Developmental Stages
Abstract
:1. Introduction
2. Results
2.1. Illumina Sequencing and Assembly
2.2. Annotation of Predicted Proteins
2.3. Functional Annotation Results
2.4. Differentially Expressed Genes (DEGs)
2.5. Developmental Gene Expression of Trehalase 1 and Trehalase 2
2.6. Impact of the silencing of trehalase 1 and trehalase 2 on Gene Expression
2.7. Toxicity of in Vitro Synthesized Dstrehalase1 and Dstrehalase2
3. Discussion
4. Materials and Methods
4.1. Insect Rearing and Sample Preparation
4.2. mRNA Sequencing by ILLUMINA HiSeq
4.3. Functional Annotation Analysis of Sequencing Data
4.4. Temporal Gene Expression Analysis of Trehalase
4.5. dsRNA Synthesis
4.6. RNA Interference Assays on D. citri
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Nr | Non-redundant |
| COG | Clusters of Orthologous Groups of proteins |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| RT-qPCR | reverse transcriptase-quantitative polymerase chain reaction |
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Kuwayama, S. Die psylliden Japans. Trans. Sapporo Nat. Hist. Soc. 1908, 2, 149–189. [Google Scholar]
- Lin, K.H. Observations on yellow shoot of citrus: Etiological studies of yellow shoot of citrus. Acta Phytopathol. Sin. 1956, 2, 13–42. [Google Scholar]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Huang, M.; Beattie, G.A.; Xia, Y.; Ouyang, G.; Xiong, J. Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: A status report for China. Int. J. Pest Manag. 2006, 52, 343–352. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Hall, D.G.; Wenninger, E.J.; Hentz, M.G. Temperature studies with the Asian citrus psyllid, Diaphorina citri: Cold hardiness and temperature thresholds for oviposition. J. Insect Sci. 2011, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.H.; Tsai, J.H. Effects of temperature on biology and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Ann. Appl. Biol. 2000, 137, 201–206. [Google Scholar] [CrossRef]
- Tiwari, S.; Gondhalekar, A.D.; Mann, R.S.; Scharf, M.E.; Stelinski, L.L. Characterization of five CYP4 genes from Asian citrus psyllid and their expression levels in Candidatus Liberibacter asiaticus-infected and uninfected psyllids. Insect Mol. Biol. 2011, 20, 733–744. [Google Scholar] [CrossRef]
- Serikawa, R.H.; Backus, E.A.; Rogers, M.E. Effects of soil-applied imidacloprid on Asian citrus psyllid (Hemiptera: Psyllidae) feeding behavior. J. Econ. Entomol. 2012, 105, 1492–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gowda, S.; Killiny, N. Double-stranded RNA delivery through soaking mediates silencing of the muscle protein 20 and increases mortality to the Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2017, 73, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Liu, Z.Q.; Guo, W.; Guo, M.J.; Chen, S.M.; Yang, C.X.; Zhang, Y.J.; Pan, H.P. Oral delivery of dsHvlwr is a feasible method for management of the pest Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Insect Sci. 2020. [Google Scholar] [CrossRef]
- Lü, J.; Guo, W.; Chen, S.M.; Guo, M.J.; Qiu, B.L.; Yang, C.X.; Zhang, Y.J.; Pan, H.P. Double-stranded RNAs targeting HvRPS18 and HvRPL13 reveal potential targets for pest management of the 28-spotted ladybeetle, Henosepilachna vigintioctopunctata. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef]
- Lü, J.; Guo, M.J.; Chen, S.M.; Noland, J.E.; Guo, W.; Sang, W.; Qi, Y.X.; Qiu, B.L.; Zhang, Y.J.; Yang, C.X.; et al. Double-stranded RNA targeting vATPase B reveals a potential target for pest management of Henosepilachna vigintioctopunctata. Pestic. Biochem. Phys. 2020. [Google Scholar] [CrossRef]
- Lü, J.; Liu, Z.Q.; Guo, W.; Guo, M.J.; Chen, S.M.; Li, H.L.; Yang, C.X.; Zhang, Y.J.; Pan, H.P. Feeding delivery of dsHvSnf7 is a promising method for management of the pest Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Insects 2020, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Reese, J.; Christenson, M.K.; Leng, N.; Saha, S.; Cantarel, B.; Lindeberg, M.; Tamborindeguy, C.; MacCarthy, J.; Weaver, D.; Trease, A.J.; et al. Characterization of the Asian citrus psyllid transcriptome. J. Genomics 2014, 2, 54–58. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Zhao, L.N.; Yang, M.M.; Shen, Q.D.; Liu, X.J.; Shi, Z.K.; Wang, S.G.; Tang, B. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Rep. 2016, 6, 27841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Wei, P.; Zhao, L.N.; Shi, Z.K.; Shen, Q.D.; Yang, M.M.; Xie, G.Q.; Wang, S.G. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Hamada, M.; Floreancig, P.; Nakabachi, A. Diaphorin, a polyketide synthesized by an intracellular symbiont of the Asian citrus psyllid, is potentially harmful for biological control agents. PLoS ONE 2019, 14, e0216319. [Google Scholar] [CrossRef] [Green Version]
- Nakabachi, A.; Malenovský, I.; Gjonov, I.; Hirose, Y. 16S rRNA sequencing detected Profftella, Liberibacter, Wolbachia, and Diplorickettsia from relatives of the Asian citrus psyllid. Microb. Ecol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.I.; Tuda, M.; Takasu, K.; Furuya, N.; Imamura, Y.; Kim, S.; Tashiro, K.; Iiyama, K.; Tavares, M.; Amaral, A.C. Unique clade of alphaproteobacterial endosymbionts induces complete cytoplasmic incompatibility in the coconut beetle. Proc. Natl. Acad. Sci. USA 2017, 114, 6110–6115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.W.; Luan, J.B.; Li, J.M.; Bao, Y.Y.; Zhang, C.X.; Liu, S.S. De novo characterization of a whitefly transcriptome and analysis of its gene expression during development. BMC Genomics 2010, 11, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, G.M.; Dou, W.; Niu, J.Z.; Jiang, H.B.; Yang, W.J.; Jia, F.X.; Hu, F.; Cong, L.; Wang, J.J. Transcriptome analysis of the oriental fruit fly (Bactrocera dorsalis). PLoS ONE 2011, 6, e29127. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef]
- Liu, F.Z.; Yang, B.; Zhang, A.H.; Ding, D.R.; Wang, G.R. Plant-mediated RNAi for controlling Apolygus lucorum. Front. Plant Sci. 2019, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.J.; Kang, S.; Zhu, X.; Xia, J.X.; Wu, Q.J.; Wang, S.L.; Xie, W.; Zhang, Y.J. The novel ABC transporter ABCH1 is a potential target for RNAi-based insect pest control and resistance management. Sci. Rep. 2015, 5, 13728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vélez, A.M.; Fishilevich, E.; Rangasamy, M.; Khajuria, C.; Mccaskill, D.G.; Pereira, A.E.; Gandra, P.; Frey, M.; Worden, S.E.; Whitlock, S.L.; et al. Control of western corn rootworm via RNAi traits in maize: Lethal and sublethal effects of Sec23 dsRNA. Pest Manag. Sci. 2020, 76, 1500–1512. [Google Scholar] [CrossRef] [Green Version]
- Asano, N. Glycosidase inhibitors: Update and perspectives on practical use. Glycobiology 2003, 13, 93R–104R. [Google Scholar] [CrossRef]
- Chen, J.; Tang, B.; Chen, H.X.; Yao, Q.; Huang, X.F.; Chen, J.; Zhang, D.W.; Zhang, W.Q. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA interference. PLoS ONE 2010, 5, e10133. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2014, 25, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Qin, Z.; Shi, Z.K.; Wang, S.; Guo, X.J.; Wang, S.G.; Zhang, F. Trehalase in Harmonia axyridis (Coleoptera: Coccinellidae): Effects on beetle locomotory activity and the correlation with trehalose metabolism under starvation conditions. Appl. Entomol. Zool. 2014, 49, 255–264. [Google Scholar] [CrossRef]
- Whyard, S.; Singh, A.D.; Wong, S. Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol. 2009, 39, 824–832. [Google Scholar] [CrossRef]
- Bolger, A.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with hisat, stringtie and ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level-the DESeq Package; European Molecular Biology Laboratory (EMBL): Heidelberg, Germany, 2012. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.P.; Yang, X.W.; Bidne, K.; Hellmich, R.L.; Siegfried, B.D.; Zhou, X.G. Dietary risk assessment of v-ATPase A dsRNAs on monarch butterfly larvae. Front. Plant Sci. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bin, S.; Pu, X.; Shu, B.; Kang, C.; Luo, S.; Tang, Y.; Wu, Z.; Lin, J. Selection of reference genes for optimal normalization of quantitative real-time polymerase chain reaction results for Diaphorina citri adults. J. Econ. Entomol. 2019, 112, 355–363. [Google Scholar] [CrossRef] [Green Version]


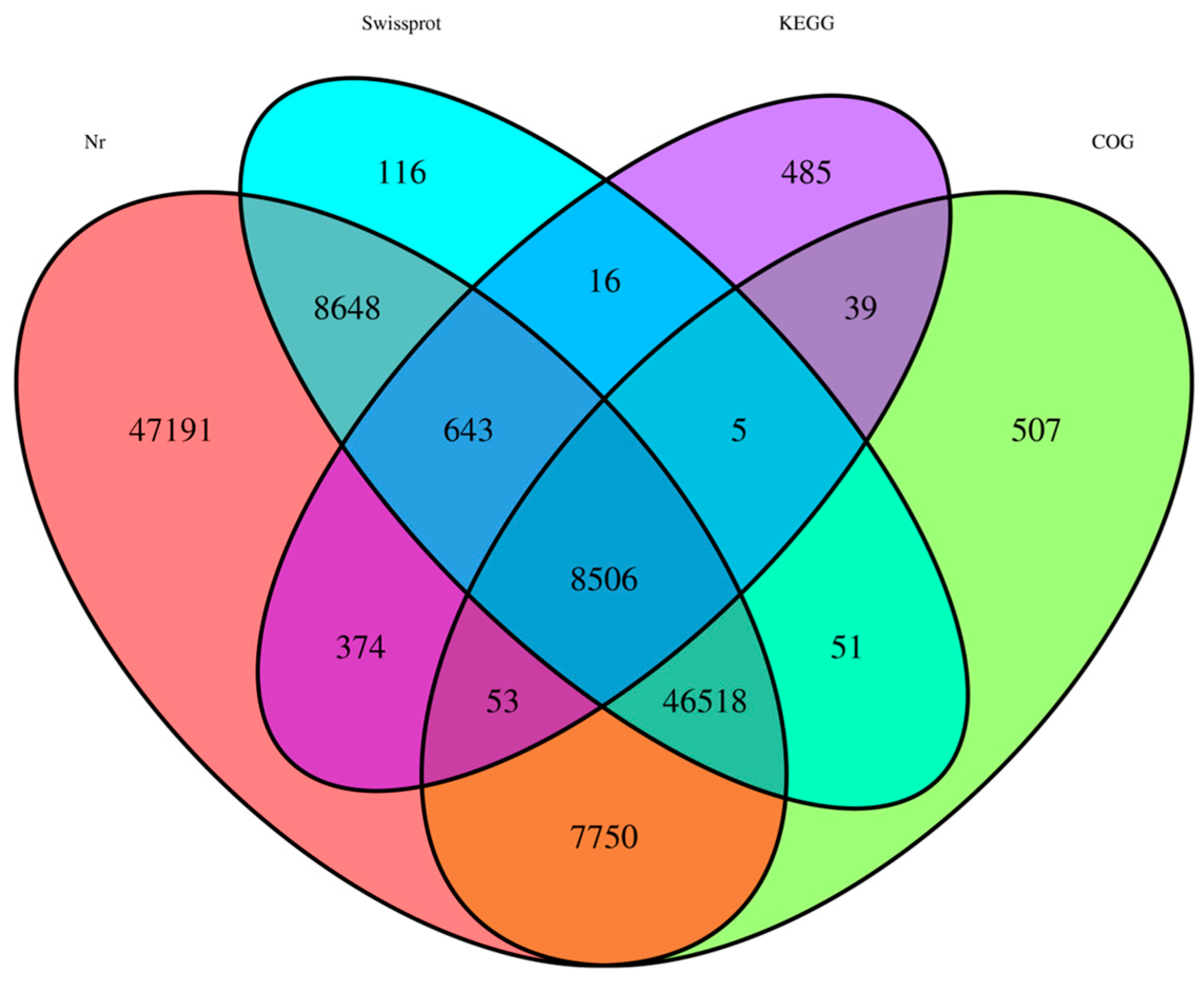







| Database | Nr | SwissProt | COG | KEGG | Total |
|---|---|---|---|---|---|
| Unigenes | 119,683 | 64,503 | 63,429 | 10,121 | 120,902 |
| Type | Total Sequences | GC Percentage | N50 | Max Length | Min Length | Average Length | Total Assembled Bases |
|---|---|---|---|---|---|---|---|
| Contig | 39,787,301 | 42.54% | 49 | 19,067 | 25 | 58.24 | 2,317,181,757 |
| Unigene | 354,726 | 39.43% | 1733 | 19,592 | 201 | 925.65 | 328,351,381 |
| Name of Primers | Primer Sequences (5′-3′) |
|---|---|
| dstrehalase1-F | TAATACGACTCACTATAGGGGGGCGTGATCGAGAACATAA |
| dstrehalase1-R | TAATACGACTCACTATAGGGGATGAACCACCGACTGGAAA |
| dstrehalase2-F | TAATACGACTCACTATAGGGCTTTGAAGCAGGCAATGAGC |
| dstrehalase2 -R | TAATACGACTCACTATAGGGGCACAATCTGTTGCCGATTT |
| RT-qPCR-trehalase1-F | TTTCCAGTCGGTGGTTCATC |
| RT-qPCR-trehalase1-R | CCACCATTCGCTCAGATAGTT |
| RT-qPCR-trehalase2-F | CGAGCCTTGCTCACCAATAA |
| RT-qPCR-trehalase2-R | CTCGGGTTGGAGGTGAAATC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Ou, D.; Guo, W.; Lü, J.; Guo, C.; Qiu, B.; Pan, H. De Novo Assembly of the Asian Citrus Psyllid Diaphorina citri (Hemiptera: Psyllidae) Transcriptome across Developmental Stages. Int. J. Mol. Sci. 2020, 21, 4974. https://doi.org/10.3390/ijms21144974
Yang C, Ou D, Guo W, Lü J, Guo C, Qiu B, Pan H. De Novo Assembly of the Asian Citrus Psyllid Diaphorina citri (Hemiptera: Psyllidae) Transcriptome across Developmental Stages. International Journal of Molecular Sciences. 2020; 21(14):4974. https://doi.org/10.3390/ijms21144974
Chicago/Turabian StyleYang, Chunxiao, Da Ou, Wei Guo, Jing Lü, Changfei Guo, Baoli Qiu, and Huipeng Pan. 2020. "De Novo Assembly of the Asian Citrus Psyllid Diaphorina citri (Hemiptera: Psyllidae) Transcriptome across Developmental Stages" International Journal of Molecular Sciences 21, no. 14: 4974. https://doi.org/10.3390/ijms21144974
APA StyleYang, C., Ou, D., Guo, W., Lü, J., Guo, C., Qiu, B., & Pan, H. (2020). De Novo Assembly of the Asian Citrus Psyllid Diaphorina citri (Hemiptera: Psyllidae) Transcriptome across Developmental Stages. International Journal of Molecular Sciences, 21(14), 4974. https://doi.org/10.3390/ijms21144974





