Non-Thermal Plasma Couples Oxidative Stress to TRAIL Sensitization through DR5 Upregulation
Abstract
1. Introduction
2. Results
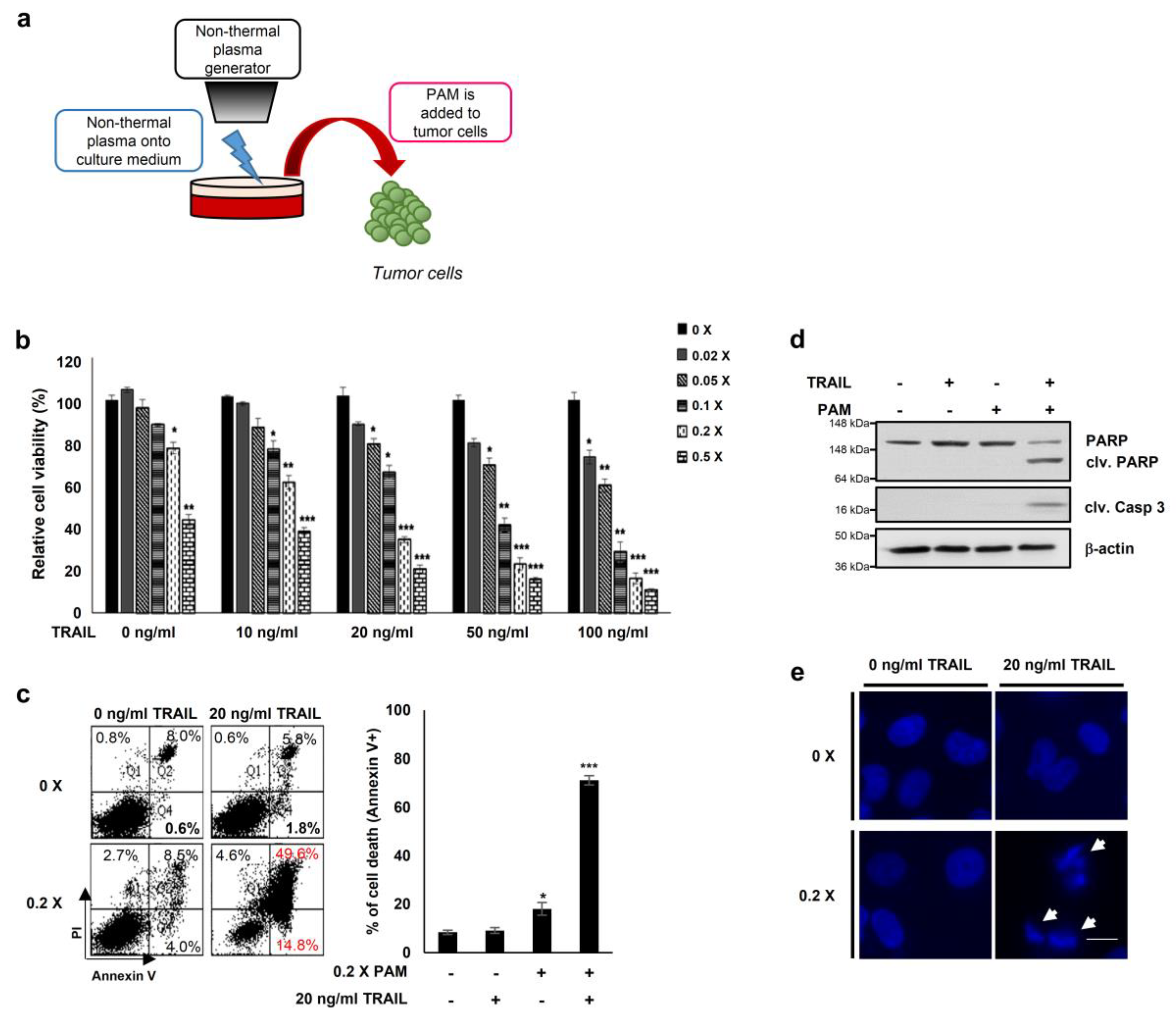
2.1. PAM Synergistically Enhances the Anticancer Efficacy of TRAIL
2.2. PAM/TRAIL Treatment Induces Apoptosis in TRAIL-Resistant Cancer Cells but Not in Normal Cells
2.3. PAM/TRAIL Treatment Induces Apoptosis via DR5 Upregulation
2.4. CHOP Mediates DR5 Upregulation Induced by PAM/TRAIL Treatment
2.5. Plasma-Activated Medium (PAM) Promotes Membrane-Bound DR5 Redistribution
2.6. ROS is Implicated in PAM/TRAIL Sensitization
2.7. PAM Sensitizes Cancer Cells to TRAIL-Induced Apoptosis via Modulation of miR-425-PTEN-Akt Axis
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Immunostaining of DR5
4.3. Cell Culture and Treatment with Plasma-Activated Medium (PAM) and TRAIL
4.4. Detection of Nuclei Condensation and Fragmentation
4.5. Quantification of Mitochondrial and Intracellular ROS
4.6. Quantification of ROS and RNS of PAM
4.7. Measurement of Intracellular Ca2+
4.8. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.9. Immunoblotting
4.10. Plasmid Construction and Transfection
4.11. Chromatin Immunoprecipitation (ChIP) Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| PAM | plasma-activated medium |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| NAC | N-acetyl-cysteine |
| GSH | glutathione |
| DR | death receptor |
| TNF | tumor necrosis factor |
| HDF | human dermal fibroblast |
| c-FLIP | cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein |
| CHOP | CCAAT/enhancer binding protein (C/EBP) homologous protein |
| SOD | superoxide dismutase |
| PTEN | phosphatase and Tensin homolog deleted on Chromosome 10 |
| mROS | mitochondrial reactive oxygen species |
References
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [PubMed]
- Voltan, R.; Secchiero, P.; Casciano, F.; Milani, D.; Zauli, G.; Tisato, V. Redox signaling and oxidative stress: Cross talk with TNF-related apoptosis inducing ligand activity. J. Biochem. Cell Biol. 2016, 81, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, L.Y.; Anderson, C.K.; Camidge, R.; Behbakhr, K.; Thorburn, A.; Ford, H.L. On the TRAIL to successful cancer therapy? Predicting and couteracting resistance against TRAIL-based tehrapeutic. Oncogene 2013, 32, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual inactivation of Akt and Erk by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, H.N.; Ashkenazi, A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003, 10, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sessler, T.; Healy, S.; Samali, A.; Szegezdi, E. Structural determinants of DISC function: New insights into death receptor-mediated apoptosis signalling. Pharmacol. Ther. 2013, 140, 186–199. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, J.Y.; Wei, W.Z.; Wu, G.S. Activation of the Akt survival pathway contributes to TRAIL resistance in cancer cells. PLoS ONE 2010, 5, e10226. [Google Scholar] [CrossRef]
- Trivedi, R.; Maurya, R.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef]
- Kholoussi, N.M.; El-Nabi, S.E.; Esmaiel, N.N.; Abd El-Bary, N.M.; El-Kased, A.F. Evaluation of Bax and Bak gene mutations and expression in breast cancer. Biomed. Res. Int. 2014, 2014, 249372. [Google Scholar] [CrossRef]
- Amelio, I.; Gostev, M.; Knight, R.A.; Willis, A.E.; Melino, G.; Antonov, A.V. DRUG-SURV: A resource for repositioning of approved and experimental drugs in oncology based on patient survival information. Cell Death Dis. 2014, 5, e1051. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Mishra, D.P. Trailing TRAIL resistance: Novel targets for TRAIL sensitization in cancer cells. Front. Oncol. 2015, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, X.; Zheng, S.; Wong, Y.S.; Chen, T. Selenocysteins derivative overcomes TRAIL resistance in melanoma cells: Evidence for ROS-depedent synergism and sinaling crosstalk. Oncotarget 2014, 5, 7431–7445. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Woo, S.M.; Choi, K.S.; Kwon, T. Acrolein sensitizes human renal cancer Caki cells to TRAIL-induced apoptosis via ROS-mediated up-regulation of death receptor-5 (DR5) and down-regulation of Bcl-2. Exp. Cell Res. 2011, 317, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Kang, Y.J.; Kim, E.H.; Lee, J.A.; Lim, J.H.; Kwon, T.K.; Choi, K.S. Monensin, a polyeher ionophore antibiotic, overcomes TRAIL resistance in glioma cells via endoplasmic reticulum stress, DR5 upregulation and c-FLIP downregulation. Carcinogenesis 2013, 34, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; Guilian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancerbinding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, J.H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef]
- Kim, K.I.; Ahn, H.J.; Lee, J.H.; Yang, S.S.; Lee, J.S. Cellular membrane collapse by atmospheric-pressure plasma jet. Appl. Phys. Lett. 2014, 104, 13701. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Park, H.J.; Yang, S.S.; Choi, K.S.; Lee, J.S. Anti-cancer efficacy of nonthermal plasma dissolved in a liquid, lquid plasma in heterogeneous cancer cells. Sci. Rep. 2016, 6, 29020. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Park, H.J.; Hwang, S.Y.; Lee, J.S.; Yang, S.S. Anticancer efficacy of long-term stored plasma-activated medium. Appl. Sci. 2019, 9, 801. [Google Scholar] [CrossRef]
- Kim, E.H.; Yoon, M.J.; Kim, S.U.; Kwon, T.K.; Shon, S.; Choi, K.S. Arsenic trioxide sensitizes human glioma cells, but not normal astrocytes, to TRAIL-induced apoptosis via CCAAT/enhancer-binding protein homologous protein-dependent DR5 up-regulation. Cancer Res. 2008, 68, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Su, R.Y.; Chi, K.H.; Huang, D.Y.; Tai, M.H.; Lin, W.W. 15-deoxy-Delta12,14-prostaglandin J2 up-regulates death receptor 5 gene expression in HCT116 cells: Involvement of reactive oxygen species and C/EBP homologous transcription factor gene transcription. Mol. Cancer Ther. 2008, 7, 3429–3440. [Google Scholar] [CrossRef]
- Yamaghci, H.; Wang, H.G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004, 279, 45495–45502. [Google Scholar] [CrossRef]
- Grassme, H.; Schwarz, H.; Gulbins, E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem. Biophys. Res. Commun. 2001, 284, 1016–1030. [Google Scholar] [CrossRef]
- Pang, L.; Fu, T.M.; Zhao, W.; Zhao, L.; Chen, W.; Qiu, C.; Liu, W.; Liu, Z.; Piai, A.; Fu, Q.; et al. Higher-order clustering of the transmembrane anchor of DR5 drives signaling. Cell 2019, 176, 1477–1489. [Google Scholar] [CrossRef]
- Lu, M.; Lawrence, D.A.; Marsters, S.; Acosta-Alvear, D.; Kimmig, P.; Mendez, A.S.; Paton, A.W.; Paton, J.C.; Walter, P.; Ashkenazi, A. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 2014, 345, 98–101. [Google Scholar] [CrossRef]
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Wang, Z.; Gu, X.; Fan, Y.; Zhang, W.; Xu, L.; Zhang, J.; Cai, D. NF-κB-depedent microRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1β induction. Mol. Cancer 2014, 13, 40. [Google Scholar] [CrossRef]
- Li, C.; Song, L.; Zhang, Z.; Bai, X.X.; Cui, M.F.; Ma, L.J. MicroRNA-21 promotes TGF-β1 induced epithelial-mesenchymal transition in gastric cancer through upregulation PTEN expression. Oncotarget 2016, 7, 66989–67003. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.L.; Gaudet, S.; Albeck, J.G.; Burke, J.M.; Sorger, P.K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 2009, 459, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Leva, G.D.; Romano, G.; Nuovo, G.; Shu, S.S.; Ngankeu, A.; Taccioli, C.; Pichiorri, F.; Alder, H.; Secchiero, P.; et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009, 16, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Karasaki, M.; Ochiai, T.; Suzuki-Karasaki, Y. Crosstalk between mitochondrial ROS and depolarization in the potentiation of TRAIL-induced apoptosis in human tumor cells. Int. J. Oncol. 2014, 44, 616–628. [Google Scholar] [CrossRef]
- Bortner, C.D.; Gomez-Angelats, M.; Cidlowski, J.A. Plasma membrane depolarization without repolarization is an early molecular event in anti-Fas-induced apoptosis. J. Biol. Chem. 2001, 276, 4304–4314. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, X.; Feng, S.; Cheng, W.; Tang, B.; Shi, Y.L.; Hua, Z.C. Plasma membrane depolarization and Na,K-ATPase impairment induced by mitochondrial toxins augment leukemia cell apoptosis via a novel mitochondrial amplification mechanism. Biochem. Pharmacol. 2009, 78, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Cheng, W.; Shen, W.; Shu, L.; Zhao, J.; Zhang, J.; Hua, Z.C. Impairment of Na+,K+-ATPase in CD95(APO-1)-induced human T-cell leukemia cell apoptosis mediated by glutathione depletion and generation of hydrogen peroxide. Leukemia 2007, 21, 1669–1678. [Google Scholar] [CrossRef]
- La Rovere, R.M.; Roest, G.; Bultynchk, G.; Parys, J.B. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium. 2016, 60, 74–87. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Kroemer, G.; Dallaporta, B.; Resche-Rigon, M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 1998, 60, 619–642. [Google Scholar] [CrossRef]
- Suzuki, Y.; Inoue, T.; Murai, M.; Suzuki-Karasaki, M.; Ochiai, T.; Ra, C. Depolarization potentiates TRAIL-induced apoptosis in human melanoma cells: Role for ATP-sensitive K+ channels and endoplasmic reticulum stress. Int. J. Oncol. 2012, 41, 465–475. [Google Scholar] [CrossRef]
- Testa, U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia 2004, 18, 1176–1199. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, F.C.; Lawrence, D.A.; Chuntharapai, A.; Schow, P.; Kim, K.J.; Ashkenazi, A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 2000, 12, 611–620. [Google Scholar] [CrossRef]
- Sprick, M.R.; Weigand, M.A.; Rieser, E.; Rauch, C.T.; Juo, P.; Blenis, J.; Krammer, P.H.; Walczak, H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Z.; Xie, L.; DeWitt, J.P.; Chen, Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016, 23, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hawke, N.; Baldwin, A.S. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006, 13, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 2006, 25, 6717–6730. [Google Scholar] [CrossRef]
- Ryo, A.; Suizu, F.; Yoshida, Y.; Perrem, K.; Liou, Y.C.; Wulf, G.; Rottapel, R.; Yamaoka, S.; Lu, K.P. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 2003, 12, 1413–1426. [Google Scholar] [CrossRef]
- Joo, J.H.; Jetten, A.M. NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway. J. Biol. Chem. 2008, 283, 16391–16399. [Google Scholar] [CrossRef]
- Nowak, D.E.; Tian, B.; Jamaluddin, M.; Boldogh, I.; Vergara, L.A.; Choudhary, S.; Brasier, A.R. RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol. Cell Biol. 2008, 28, 3623–3638. [Google Scholar] [CrossRef]
- Hochrainer, K.; Racchumi, G.; Anrather, J. Site-specific phosphorylation of the p65 protein subunit mediates selective gene expression by differential NF-κB and RNA polymerase II promoter recruitment. J. Biol. Chem. 2013, 288, 285–293. [Google Scholar] [CrossRef]
- Huang, C.Y.; Fong, Y.C.; Lee, C.Y.; Chen, M.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem. Pharmacol. 2009, 77, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Msaki, A.; Sanchez, A.M.; Koh, L.F.; Barre, B.; Rocha, S.; Perkins, N.D.; Johnson, R.F. The role of RelA (p65) threonine 505 phosphorylation in the regulation of cell growth, survival and migration. Mol. Biol. Cell 2011, 22, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Ahir, M.; Bhattacharya, S.; Kamakar, S.; Mukhopadhyay, A.; Mukherjee, S.; Ghosh, S.; Chattopadhyay, S.; Patra, P.; Adhikary, A. Tailoerd-CuO-nanowire decorated with folic acid mediated coupling of the mitochondrial-ROS generation and miR425-PTEN axis in furnishing potent anti-cancer activity in human tripe negative breast carcinoma cells. Biomaterials 2016, 76, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.R.; Ravindran, J.; Aggarwal, B.B. ROS and CHOP are critical for dibenzylideneacetone to sensitize tumor cells to TRAIL through induction of death receptors and downregulation of cell survival proteins. Cancer Res. 2011, 71, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Prasad, S.; Ravindran, J.; Yadav, V.R.; Aggarwal, B.B. Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic. Biol. Med. 2012, 53, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of nitric oxide production in biological systems by using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]





| Cell Line | PAM/TRAIL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | TRAIL | zVAD | DR5/Fc | NAC | PAM | Vehicle | zVAD | DR5/Fc | NAC | |
| HeLa | 100 ± 2.3 | 99.1 ± 1.6 | 96.4 ± 4.3 | 92.7 ± 0.5 | 93.6 ± 3.3 | 69.0 ± 1.6 ** | 25.6 ± 0.5 *** | 53.3 ± 1.8 *** | 55.3 ± 3.9 * | 40.2 ± 3.3 *** |
| A549 | 100 ± 1.3 | 99.0 ± 0.6 | 103.8 ± 6.1 | 101.0 ± 4.8 | 106.1 ± 2.7 | 78.2 ± 0.8 * | 39.9 ± 0.2 *** | 58.8 ± 1.0 ** | 61.7 ± 3.2 *** | 56.6 ± 2.2 *** |
| HepG2 | 100 ± 2.7 | 96.7 ± 2.8 | 108.7 ± 5.0 | 95.8 ± 2.9 | 108.7 ± 7.6 | 76.5 ± 3.3 * | 32.8 ± 0.6 *** | 44.1 ± 0.2 ** | 45.9 ± 2.3 *** | 48.9 ± 2.3 *** |
| Cell Line | PAM/TRAIL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | TRAIL | zVAD | DR5/Fc | NAC | PAM | Vehicle | zVAD | DR5/Fc | NAC | |
| HeLa | 7.0 ± 0.5 | 7.7 ± 0.3 | 8.2 ± 1.3 | 8.3 ± 1.3 | 8.2 ± 0.9 | 21.1 ± 1.4 * | 64.3 ± 1.6 *** | 27.4 ± 1.2 * | 19.2 ± 1.1 | 27.2 ± 2.0 * |
| A549 | 9.6 ± 0.2 | 14.9 ± 0.5 | 11.6 ± 0.6 | 12.0 ± 0.4 | 11.6 ± 1.1 | 24.6 ± 0.4 * | 61.3 ± 2.1 *** | 31.7 ± 1.4 * | 15.6 ± 0.6 | 29.4 ± 1.4 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.Y.; Nguyen, N.H.; Kim, T.J.; Lee, Y.; Kang, M.A.; Lee, J.-S. Non-Thermal Plasma Couples Oxidative Stress to TRAIL Sensitization through DR5 Upregulation. Int. J. Mol. Sci. 2020, 21, 5302. https://doi.org/10.3390/ijms21155302
Hwang SY, Nguyen NH, Kim TJ, Lee Y, Kang MA, Lee J-S. Non-Thermal Plasma Couples Oxidative Stress to TRAIL Sensitization through DR5 Upregulation. International Journal of Molecular Sciences. 2020; 21(15):5302. https://doi.org/10.3390/ijms21155302
Chicago/Turabian StyleHwang, Soon Young, Ngoc Hoan Nguyen, Tae Jung Kim, Youngsoo Lee, Mi Ae Kang, and Jong-Soo Lee. 2020. "Non-Thermal Plasma Couples Oxidative Stress to TRAIL Sensitization through DR5 Upregulation" International Journal of Molecular Sciences 21, no. 15: 5302. https://doi.org/10.3390/ijms21155302
APA StyleHwang, S. Y., Nguyen, N. H., Kim, T. J., Lee, Y., Kang, M. A., & Lee, J.-S. (2020). Non-Thermal Plasma Couples Oxidative Stress to TRAIL Sensitization through DR5 Upregulation. International Journal of Molecular Sciences, 21(15), 5302. https://doi.org/10.3390/ijms21155302





