Gangliosides and Neuroblastomas
Abstract
1. Introduction to Neuroblastoma and the Biological Importance of Gangliosides
2. Ganglioside Structure and Biosynthesis
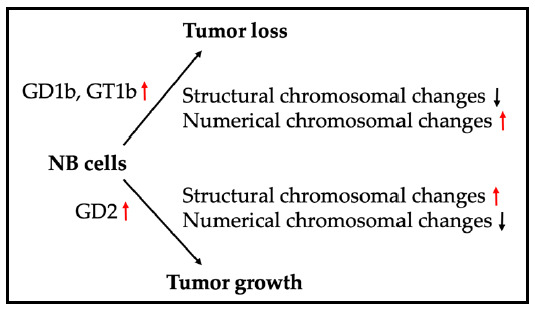
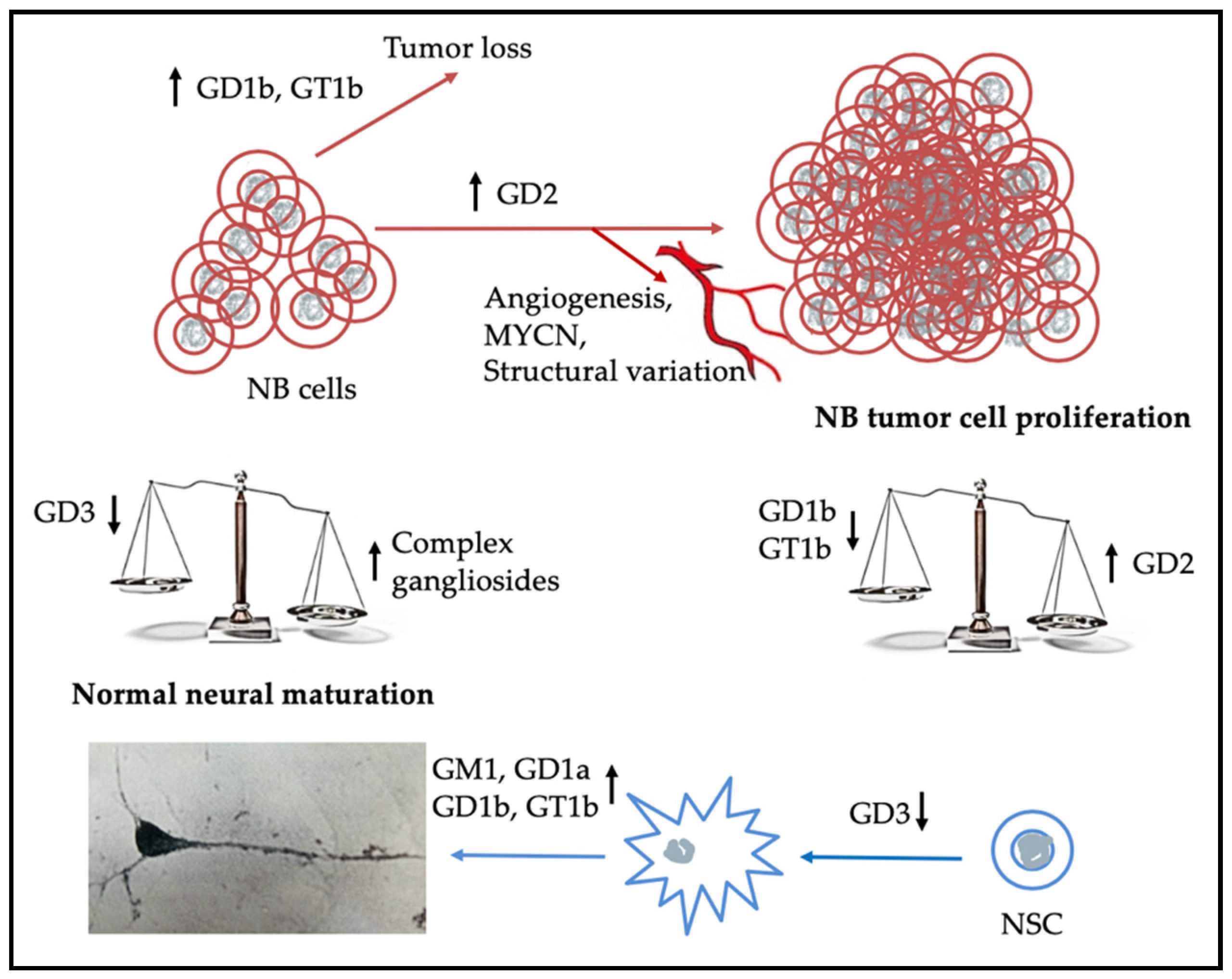
3. Gangliosides in Neuroblastoma
4. Gangliosides in Glycolipid Enriched Microdomains (Lipid Rafts)
5. Gangliosides as Therapeutic Targets in Neuroblastoma
6. Examples of Additional Prognostic Markers and How They May Act Synergistically with Gangliosides in NB
7. Summary
Funding
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ALK | anaplastic lymphoma kinase |
| CNS | central nervous system |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular-signal-regulated kinase |
| FAC | focal adhesion kinase |
| FAPP2 | 4-phosphate adaptor protein 2 |
| Fc | fragment crystallizable region of an antibody |
| Fv | fragment variable region of an antibody |
| GSL | glycosphingolipid |
| GM | monosialoganglioside |
| GD | disialoganglioside |
| GT | trisialoganglioside |
| HDAC | histone deacetylase |
| mTOR | mammalian target of rapamycin |
| MYCN | myelocytomatosis viral oncogene neuroblastoma derived homolog |
| NB | neuroblastoma |
| NEU | sialidase (neuraminidase) |
| NMDA | N-methyl-d-aspartic acid |
| NSC | neural stem cell |
| TLR2 | toll like receptor 2 |
| TrkA | tropomyosin receptor kinase A |
References
- Allende, M.L.; Proia, R.L. Simplifying complexity: Genetically resculpting glycosphingolipid synthesis pathways in mice to reveal function. Glycoconj. J. 2014, 31, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Kalinovsky, D.V.; Doronin, I.; Deyev, S.M.; Kholodenko, R.V. Neuroblastoma origin and therapeutic targets for immunotherapy. J. Immunol. Res. 2018, 7394268. [Google Scholar] [CrossRef]
- Shimada, H.; Ambros, I.M.; Dehner, L.P.; Hata, J.; Joshi, V.V.; Roald, B.; Stram, D.O.; Gerbing, R.B.; Lukens, J.N.; Matthay, K.K.; et al. The International Neuroblastoma Pathology Classification (the Shimada System). Cancer 1999, 86, 364–372. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG task force report. J. Clin. Oncol. 2009, 27, 287–297. [Google Scholar] [CrossRef]
- Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, N. Biologic vagaries in neuroblastoma. In Neuroblastoma Clinical and Biological Manifestations; Pochedly, C., Ed.; Elsevier Sciences Publishing Co.: New York, NY, USA, 1982; pp. 293–309. [Google Scholar]
- Kawai, H.; Allende, M.L.; Wada, R.; Kono, M.; Sango, K.; Deng, C.; Miyakawa, T.; Crawley, J.N.; Werth, N.; Bierfreund, U.; et al. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J. Biol. Chem. 2001, 276, 6885–6888. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.A.; Cross, H.; Proukakis, C.; Priestman, D.A.; Neville, D.C.; Reinkensmeier, G.; Wang, H.; Wiznitzer, M.; Gurtz, K.; Verganelaki, A. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 2004, 36, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Bowser, L.E.; Young, M.; Wenger, O.K.; Ammous, Z.; Brigatti, K.W.; Carson, V.J.; Moser, T.; Deline, J.; Aoki, K.; Morlet, T.; et al. Recessive GM3 synthase deficiency: Natural history, biochemistry, and therapeutic frontier. Mol. Genet. Metab. 2019, 126, 475–488. [Google Scholar] [CrossRef]
- Harlalka, G.V.; Lehman, A.; Chioza, B.; Baple, E.L.; Maroofian, R.; Cross, H.; Sreekantan-Nair, A.; Priestman, D.A.; Al-Turki, S.; McEntagart, M.E.; et al. Mutations in B4GALNT1 (GM2 synthase) underlie a new disorder of ganglioside biosynthesis. Brain J. Neurol. 2013, 136, 3618–3624. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Svennerholm, L. Ganglioside designation. Adv. Exp. Med. Biol. 1980, 125, 11. [Google Scholar] [CrossRef]
- Kumagai, T.; Sato, T.; Natsuka, S.; Kobayashi, Y.; Zhou, D.; Shinkai, T.; Hayakawa, S.; Furukawa, K. Involvement of murine β-1,4-galactosyltransferase V in lactosylceramide biosynthesis. Glycoconj. J. 2010, 27, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.; Trauger, S.A.; Blain, M.; Nadeau, M.; Patel, B.; Alvarez, J.I.; Mascanfroni, I.D.; Yeste, A.; Kivisäkk, P.; Kallas, K.; et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 2014, 20, 1147–1156. [Google Scholar] [CrossRef]
- Sturgill, E.R.; Aoki, K.; Lopez, P.H.; Colacurcio, D.; Vajn, K.; Lorenzini, I.; Majić, S.; Yang, W.H.; Heffer, M.; Tiemeyer, M.; et al. Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology 2012, 22, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Indellicato, R.; Domenighini, R.; Malagolini, N.; Cereda, A.; Mamoli, D.; Pezzani, L.; Iascone, M.; dall’Olio, F.; Trinchera, M. A novel nonsense and inactivating variant of ST3GAL3 in two infant siblings suffering severe epilepsy and expressing circulating CA19.9. Glycobiology 2020, 30, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R. The biology of gangliosides. Adv. Carbohydr. Chem. Biochem. 2019, 76, 113–148. [Google Scholar] [CrossRef]
- Matsumoto, M.; Taki, T.; Samuelsson, B.; Pascher, I.; Hirabayashi, Y.; Li, S.C.; Li, Y.T. Further characterization of the structure of GM1b ganglioside from rat ascites hepatoma. J. Biol. Chem. 1981, 256, 9737–9741. [Google Scholar] [PubMed]
- Schengrund, C.-L.; Repman, M.A.; Shochat, S.J. Ganglioside composition of human neuroblastomas. Correlation with prognosis. A Pediatric Oncology Group study. Cancer 1985, 56, 2640–2646. [Google Scholar] [CrossRef]
- Hettmer, S.; Malott, C.; Woods, W.; Ladisch, S.; Kaucic, K. Biological stratification of human neuroblastoma by complex “B” pathway ganglioside expression. Cancer Res. 2003, 63, 7270–7276. [Google Scholar]
- Schulz, G.; Cheresh, D.A.; Varki, N.M.; Yu, A.; Staffileno, L.K.; Reisfeld, R.A. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984, 44, 5914–5920. [Google Scholar]
- Zhang, S.; Cordon-Cardo, C.; Zhang, H.S.; Reuter, V.E.; Adluri, S.; Hamilton, W.B.; Lloyd, K.O.; Livingston, P.O. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int. J. Cancer 1997, 73, 42–49. [Google Scholar] [CrossRef]
- Dong, L.; Liu, Y.; Colberg-Poley, A.M.; Kaucic, K.; Ladisch, S. Induction of GM1a/GD1b synthase triggers complex ganglioside expression and alters neuroblastoma cell behavior; a new tumor cell model of ganglioside function. Glycoconj. J. 2011, 28, 137–147. [Google Scholar] [CrossRef]
- Voeller, J.; Sondel, P.M. Advances in anti-GD2 immunotherapy for treatment of high-risk neuroblastoma. J. Pediatr. Hematol. Oncol. 2019, 41, 163–169. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809. [Google Scholar] [CrossRef]
- Fugmann, T.; Hausser, A.; Schöffler, P.; Schmid, S.; Pfizenmaier, K.; Olayioye, M. A Regulation of secretory transport by protein kinase D–mediated phosphorylation of the ceramide transfer protein. J. Cell Biol. 2007, 178, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Halter, D.; Neumann, S.; van Dijk, S.M.; Wolthoorn, J.; de Maziere, A.M.; Vieira, O.V.; Mattjus, P.; Klumperman, J.; van Meer, G.; Sprong, H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol. 2007, 179, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Kumagai, K.; Tomishige, N.; Yamaji, T.; Wakatsuki, S.; Nishijima, M.; Hanada, K.; Kato, R. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc. Natl. Acad. Sci. USA 2008, 105, 488–493. [Google Scholar] [CrossRef]
- Yamaji, T.; Kumaga, K.; Tomishige, N.; Hanada, K. Two sphingolipid transfer proteins, CERT and FAPP2: Their roles in sphingolipid metabolism. IUBMB Life 2008, 60, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, C.G.; Daniotti, J.L.; Maccioni, H.J. Physical and functional association of glycolipid N-acetyl-galactosaminyl and galactosyl transferases in the Golgi apparatus. Proc. Natl. Acad. Sci. USA 2001, 98, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Uliana, A.S.; Crespo, P.M.; Martina, J.A.; Daniotti, J.L.; Maccioni, H.J. Modulation of GalT1 and SialT1 sub-Golgi localization by SialT2 expression reveals an organellar level of glycolipid synthesis control. J. Biol. Chem. 2006, 281, 32852–32860. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Radin, N.S. Determination of brain gangliosides by determination of ganglioside stearic acid. J. Lipid Res. 1966, 7, 141–145. [Google Scholar] [PubMed]
- Schengrund, C.-L.; Garrigan, O.W. A comparative study of gangliosides from the brains of various species. Lipids 1969, 4, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G. Glycosignaling: A general review. Adv. Neurobiol. 2014, 9, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Murata, S.; Schmuth, M.; Behne, M.J.; Lee, J.D.; Ichikawa, S.; Elias, P.M.; Hirabayashi, Y.; Holleran, W.M. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J. Lipid Res. 2002, 43, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Guan, Y.; Chen, W.; Shi, C.; Yao, D.; Wang, F.; Lam, S.M.; Shui, G.; Cao, X. ACBD3 is required for FAPP2 transferring glucosylceramide through maintaining the Golgi integrity. J. Mol. Cell Biol. 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kolmakova, A.; Rajesh, M. Regulation of lactosylceramide synthase (glucosylceramide b1-4 galactosyltransferase); implication as a drug target. Curr. Drug Targets 2008, 9, 272–281. [Google Scholar] [CrossRef]
- Chung, T.-W.; Choi, H.-J.; Lee, Y.-C.; Kim, C.-H. Molecular mechanism for transcriptional activation of ganglioside GM3 synthase and its function in differentiation of HL-60 cells. Glycobiology 2005, 15, 233–244. [Google Scholar] [CrossRef]
- Xia, T.; Zeng, G.; Gao, L.; Yu, R.K. Sp1 and AP2 enhance promoter activity of the mouse GM3-synthase gene. Gene 2005, 351, 109–118. [Google Scholar] [CrossRef]
- Uemura, S.; Yoshida, S.; Shishido, F.; Inokuchi, J. The cytoplasmic tail of GM3 synthase defines its subcellular localization, stability, and in vivo activity. Mol. Biol. Cell 2009, 20, 3088–3100. [Google Scholar] [CrossRef]
- Martina, J.A.; Daniotti, J.L.; Maccioni, H.J. Influence of N-glycosylation and N-glycan trimming on the activity and intracellular traffic of GD3 synthase. J. Biol. Chem. 1998, 273, 3725–3731. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.-S.; Kim, K.-S.; Moon, H.-I.; An, H.-K.; Park, S.-J.; Kim, C.-H.; Lee, Y.-C. Cordycepin-mediated transcriptional regulation of human GD3 synthase (hST8Sia I) in human neuroblastoma SK-N-BE(2)-C cells. Acta Biochim. Biophys. Sin. 2014, 46, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Itokazu, Y.; Tsai, Y.T.; Yu, R.K. Epigenetic regulation of ganglioside expression in neural stem cells and neuronal cells. Glycoconj. J. 2017, 34, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Shishido, F.; Uemura, S.; Kashimura, M.; Inokuchi, J.I. Identification of a new b4GalNAcT1 (GM2/GD2/GA2 synthase) isoform, and regulation of enzyme stability and intracellular transport by arginine-based motif. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Mahata, B.; Dhir, A.; Mandal, T.K.; Biswas, K. Elevated histone H3 acetylation and loss of the Sp1-HDAC1 complex de-repress the GM2-synthase gene in renal cell carcinoma. J. Biol. Chem. 2019, 294, 1005–1018. [Google Scholar] [CrossRef]
- Woronowicz, A.; Amith, S.R.; De Vusser, K.; Laroy, W.; Contreras, R.; Basta, S.; Szewczuk, M.R. Dependence of neurotrophic factor activation of Trk tyrosine kinase receptors on cellular sialidase. Glycobiology 2007, 17, 10–24. [Google Scholar] [CrossRef]
- Olsson, M.; Beck, S.; Kogner, P.; Martinsson, T.; Carén, H. Genome-wide methylation profiling identifies novel methylated genes in neuroblastoma tumors. Epigenetics 2016, 11, 74–84. [Google Scholar] [CrossRef]
- Daniotti, J.L.; Iglesias-Bartolome, R. Metabolic pathways and intracellular trafficking of gangliosides. IUBMB Life 2011, 63, 513–520. [Google Scholar] [CrossRef]
- Miyagi, T.; Wada, T.; Iwamatsu, A.; Hata, K.; Yoshikawa, Y.; Tokuyama, S.; Sawada, M. Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J. Biol. Chem. 1999, 274, 5004–5011. [Google Scholar] [CrossRef]
- Kalka, D.; von Reitzenstein, C.; Kopitz, J.; Cantz, M. The plasma membrane ganglioside sialidase cofractionates with markers of lipid rafts. Biochem. Biophys. Res. Commun. 2001, 283, 989–993. [Google Scholar] [CrossRef]
- Rodriguez-Walker, M.; Daniotti, J.L. Human Sialidase Neu3 is S-Acylated and behaves like an integral membrane protein. Sci. Rep. 2017, 7, 4167. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Takahashi, K.; Yamamoto, K.; Shiozaki, K.; Yamaguchi, K. Biological and pathological roles of ganglioside sialidases. Prog. Mol. Biol. Transl. Sci. 2018, 156, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Sandbhor, M.S.; Soya, N.; Albohy, A.; Zheng, R.B.; Cartmell, J.; Bundle, D.R.; Klassen, J.S.; Cairo, C.W. Substrate recognition of the membrane-associated sialidase NEU3 requires a hydrophobic aglycone. Biochemistry 2011, 50, 6753–6762. [Google Scholar] [CrossRef]
- Pshezhetsky, A.V.; Ashmarina, M. Keeping it trim: Roles of neuraminidases in CNS function. Glycoconj. J 2018, 35, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Morosi, L.; Pozzi, C.; Forcella, M.; Tettamanti, G.; Venerando, B.; Monti, E.; Fusi, P. Human sialidase NEU4 long and short are extrinsic proteins bound to outer mitochondrial membrane and the endoplasmic reticulum, respectively. Glycobiology 2010, 20, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Seyrantepe, V.; Landry, K.; Trudel, S.; Hassan, J.A.; Morales, C.R.; Pshezhetsky, A.V. Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J. Biol. Chem. 2004, 279, 37021–37029. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Hata, K.; Koseki, K.; Shiozaki, K.; Akita, H.; Wada, T.; Moriya, S.; Miyagi, T. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem. J. 2005, 390, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Schengrund, C.-L.; Duff, R.; Rosenberg, A. Sialidase activity of oncogenic cells transformed by Herpes Simplex virus. Virology 1974, 58, 595–599. [Google Scholar] [CrossRef]
- Schengrund, C.-L.; Rosenberg, A.; Repman, M.A. Ecto-ganglioside-sialidase activity of Herpes Simplex virus-transformed hamster embryo fibroblasts. J. Cell. Biol. 1976, 70, 555–561. [Google Scholar] [CrossRef]
- Hata, K.; Wada, T.; Hasegawa, A.; Kiso, M.; Miyagi, T. Purification and characterization of a membrane-associated ganglioside sialidase from bovine brain. J. Biochem. 1998, 123, 899–905. [Google Scholar] [CrossRef]
- Proshin, S.; Yamaguchi, K.; Wada, T.; Miyagi, T. Modulation of neuritogenesis by ganglioside-specific sialidase (Neu 3) in human neuroblastoma NB-1 cells. Neurochem. Res. 2002, 27, 841–846. [Google Scholar] [CrossRef]
- Schengund, C.-L.; Repman, M.A. Association of endogenous substrate with solubilized bovine brain sialidase. J. Neurosci. Res. 1986, 15, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 2015, 40, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Fukumot, S.; Furukawa, K.; Ichimura, A.; Miyazaki, H.; Kusunoki, S.; Urano, T.; Furukawa, K. Overepression of GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells. J. Biol. Chem. 2004, 279, 33368–33378. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takahashi, K.; Shiozaki, K.; Yamaguchi, K.; Moriya, S.; Hosono, M.; Shima, H.; Miyagi, T. Potentiation of epidermal growth factor-mediated oncogenic transformation by sialidase NEU3 leading to Src activation. PLoS ONE 2015, 10, e0120578. [Google Scholar] [CrossRef]
- Alam, S.; Fedier, A.; Kohler, R.S.; Jacob, F. Glucosylceramide synthase inhibitors differentially affect expression of glycosphingolipids. Glycobiology 2015, 25, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Wada, R.; Sasaki, T.; Deng, C.; Bierfreund, U.; Sandhoff, K.; Proia, R.L. A vital role for glycosphingolipid synthesis during development and differentiation. Proc. Natl. Acad. Sci. USA 1999, 96, 9142–9147. [Google Scholar] [CrossRef]
- Yu, R.K.; Itokazu, Y. Glycolipid and glycoprotein expression during neural development. Adv. Neurobiol. 2014, 9, 185–222. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Yoshimura, S.; Yu, R.K. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro. 2011, 3, 69–74. [Google Scholar] [CrossRef]
- Wang, J.; Yu, R.K. Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 19137–19142. [Google Scholar] [CrossRef]
- Nguyen, K.; Yan, Y.; Yuan, B.; Dasgupta, A.; Sun, J.; Mu, H.; Do, K.A.; Ueno, N.T.; Andreeff, M.; Battula, V.L. ST8SIA1 regulates tumor growth and metastasis in TNBC by activating the FAK-AKT-mTOR signaling pathway. Mol. Cancer Ther. 2018, 17, 2689–2701. [Google Scholar] [CrossRef]
- Ruan, S.; Lloyd, K.O. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells: Relative glycosyltransferase levels determine ganglioside patterns. Cancer Res. 1992, 52, 5725–5735. [Google Scholar] [PubMed]
- Fleurence, J.; Fougeray, S.; Bahri, M.; Cochonneau, D.; Clémenceau, B.; Paris, F.; Heczey, A.; Birklé, S. Targeting O-Acetyl-GD2 ganglioside for cancer immunotherapy. J. Immunol. Res. 2017, 5604891. [Google Scholar] [CrossRef]
- Shibuya, H.; Hamamura, K.; Hotta, H.; Matsumoto, Y.; Nishida, Y.; Hattori, H.; Furukawa, K.; Ueda, M.; Furukawa, K. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci. 2012, 103, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Linkowski, M.; Tarim, J.; Piperdi, S.; Sowers, R.; Geller, D.; Gill, J.; Gorlick, R. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 2014, 120, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Giussani, P.; Tringali, C.; Riboni, L.; Viani, P.; Venerando, B. Sphingolipids: Key regulators of apoptosis and pivotal players in cancer drug resistance. Int. J. Mol. Sci. 2014, 15, 4356–4392. [Google Scholar] [CrossRef]
- Batta, G.; Soltész, L.; Kovács, T.; Bozó, T.; Mészár, Z.; Kellermayer, M.; Szöllősi, J.; Nagy, P. Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease. Sci. Rep. 2018, 8, 157. [Google Scholar] [CrossRef]
- Kolmakova, A.; Rajesh, M.; Zang, D.; Pili, R.; Chatterjee, S. VEGF recruits lactosylceramide to induce endothelial cell adhesion molecule expression and angiogenesis in vitro and in vivo. Glycoconj. J. 2009, 26, 547–558. [Google Scholar] [CrossRef]
- Park, J.; Kwak, C.H.; Ha, S.H.; Kwon, K.M.; Abekura, F.; Cho, S.H.; Chang, Y.C.; Lee, Y.C.; Ha, K.T.; Chung, T.W.; et al. Ganglioside GM3 suppresses lipopolysaccharide-induced inflammatory responses in rAW 264.7 macrophage cells through NF-κB, AP-1, and MAPKs signaling. J. Cell Biochem. 2018, 119, 1173–1182. [Google Scholar] [CrossRef]
- Šmíd, V.; Šuk, J.; Kachamakova-Trojanowska, N.; Jašprová, J.; Valášková, P.; Józkowicz, A.; Dulak, J.; Šmíd, F.; Vítek, L.; Muchová, L. Heme oxygenase-1 may affect cell signalling via modulation of ganglioside composition. Oxid. Med. Cell Longev. 2018, 2018, 3845027. [Google Scholar] [CrossRef]
- Zhuo, D.; Guan, F. Ganglioside GM1 promotes contact inhibition of growth by regulating the localization of epidermal growth factor receptor from glycosphingolipid-enriched microdomain to caveolae. Cell Prolif. 2019, 52, e12639. [Google Scholar] [CrossRef]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Gangliosides contribute to vascular insulin resistance. Int. J. Mol. Sci. 2019, 20, 1819. [Google Scholar] [CrossRef]
- Juhola, H.; Postila, P.A.; Rissanen, S.; Lolicato, F.; Vattulainen, I.; Róg, T. Negatively charged gangliosides promote membrane association of amphipathic neurotransmitters. Neuroscience 2018, 384, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Yin, L.; Yuan, L.; Sui, D.; Sun, Y.; Fu, H.; Chen, L.; Wang, X. Ganglioside GM1 protects against high altitude cerebral edema in rats by suppressing the oxidative stress and inflammatory response via the PI3K/AKT-Nrf2 pathway. Mol. Immunol. 2018, 95, 91–98. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Pomè, D.Y.; Maggioni, M.; Di Biase, E.; Parravicini, C.; Palazzolo, L.; Loberto, N.; Eberini, I.; Sonnino, S. Role of the GM1 ganglioside oligosaccharide portion in the TrkA-dependent neurite sprouting in neuroblastoma cells. J. Neurochem. 2017, 143, 645–659. [Google Scholar] [CrossRef]
- Chung, T.W.; Choi, H.J.; Park, M.J.; Choi, H.J.; Lee, S.O.; Kim, K.J.; Kim, C.H.; Hong, C.; Kim, K.H.; Joo, M.; et al. The function of cancer-shed gangliosides in macrophage phenotype: Involvement with angiogenesis. Oncotarget 2017, 8, 4436–4448. [Google Scholar] [CrossRef]
- Ni, Y.F.; Zhang, W.; Bao, X.F.; Wang, W.; Song, L.; Jiang, B. GM1 ganglioside reverses the cognitive deficits induced by MK801 in mice. Behav. Pharmacol. 2016, 27, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tian, J.; Long, M.K.; Chen, Y.; Lu, J.; Zhou, C.; Wang, T. Protection against experimental stroke by ganglioside GM1 Is associated with the inhibition of autophagy. PLoS ONE 2016, 11, e0144219. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; André, S.; Gabius, H.J.; Ledeen, R.W. Functional interplay between ganglioside GM1 and cross-linking galectin-1 induces axon-like neuritogenesis via integrin-based signaling and TRPC5-dependent Ca2+ influx. J. Neurochem. 2016, 136, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Doronin, I.I.; Vishnyakova, P.A.; Kholodenko, I.V.; Ponomarev, E.D.; Ryazantsev, D.Y.; Molotkovskaya, I.M.; Kholodenko, R.V. Ganglioside GD2 in reception and transduction of cell death signal in tumor cell. BMC Cancer 2014, 14, 295. [Google Scholar] [CrossRef]
- Tong, W.; Maira, M.; Gagnon, M.; Saragovi, H.U. Ligands binding to cell surface ganglioside GD2 cause Src-dependent activation of N-Methyl-D-Aspartate receptor signaling and changes in cellular morphology. PLoS ONE 2015, 10, e0134255. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Koodie, L.; Jacobsen, K.; Hanzawa, K.; Miyamoto, Y.; Yamamoto, M. b4GALNT1 induces angiogenesis, anchorage independence growth and motility, and promotes tumorigenesis in melanoma by induction of ganglioside GM2/GD2. Sci. Rep. 2020, 10, 1199. [Google Scholar] [CrossRef]
- Lim, H.; Lee, J.; You, B.; Oh, J.H.; Mok, H.J.; Kim, Y.S.; Yoon, B.E.; Kim, B.G.; Back, S.K.; Park, J.S.; et al. GT1b functions as a novel endogenous agonist of toll-like receptor 2 inducing neuropathic pain. EMBO J. 2020, 39, e102214. [Google Scholar] [CrossRef] [PubMed]
- Schengrund, C.-L. Gangliosides: Glycosphingolipids essential for normal neural development and function. Trends Biochem. Sci. 2015, 40, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Berois, N.; Osinaga, E. Glycobiology of neuroblastoma: Impact on tumor behavior, prognosis, and therapeutic strategies. Front. Oncol. 2014, 4, 114. [Google Scholar] [CrossRef]
- Russo, D.; Capolupo, L.; Loomba, J.S.; Sticco, L.; D’Angelo, G. Glycosphingolipid metabolism in cell fate specification. J. Cell Sci. 2018, 131, jcs219204. [Google Scholar] [CrossRef]
- Itokazu, Y.; Wang, J.; Yu, R.K. Gangliosides in nerve cell specification. Prog. Mol. Biol. Transl. Sci. 2018, 156, 241–263. [Google Scholar] [CrossRef]
- Tsai, Y.; Itokazu, Y.; Yu, R.K. GM1 Ganglioside is involved in epigenetic activation loci of neuronal cells. Neurochem. Res. 2016, 41, 107–115. [Google Scholar] [CrossRef]
- Okada, Y.; Mugnai, G.; Bremer, E.G.; Hakomori, S. Glycosphingolipids in detergent-insoluble substrate attachment matrix (DISAM) prepared from substrate attachment material (SAM). Their possible role in regulating cell adhesion. Exp. Cell. Res. 1984, 155, 448–456. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 2007, 17, 1R–13R. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, A.R.; Hakomori, S.-I. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta 2008, 1780, 421–433. [Google Scholar] [CrossRef]
- Ohmi, Y.; Ohkawa, Y.; Yamauchi, Y.; Tajima, O.; Furukawa, K.; Furukawa, K. Essential roles of gangliosides in the formation and maintenance of membrane microdomains in brain tissues. Neurochem. Res. 2012, 37, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S.; Handa, K.; Iwabuchi, K.; Yamamura, S.; Prinetti, A. New insights in glycosphingolipid function: “Glycosignaling domain”, a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology 1998, 8, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Rodríguez-Walker, M.; Dewald, J.H.; Daniotti, J.L.; Delannoy, P. Gangliosides in cancer cell signaling. Prog. Mol. Biol. Transl. Sci. 2018, 156, 197–227. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate-protein interactions: Basis of glycobiology. Acc. Chem Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Mauri, L.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Maggioni, M.; Valsecchi, M.; Prioni, S.; Loberto, N.; Pomè, D.Y.; et al. Parkinson’s disease recovery by GM1 oligosaccharide treatment in the B4galnt1+/- mouse model. Sci. Rep. 2019, 9, 19330. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Kopitz, J.; Abad-Rodríguez, J.; Gabius, H.J. Glycan chains of gangliosides: Functional ligands for tissue lectins (Siglecs/Galectins). Prog. Mol. Biol. Transl. Sci. 2018, 156, 289–324. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, S.; Wondimu, A.; Bob, D.; Weiss, M.; Sliwinski, K.; Villar, J.; Notario, V.; Sutherland, M.; Colberg-Poley, A.M.; et al. Ganglioside synthase knockout in oncogene-transformed fibroblasts depletes gangliosides and impairs tumor growth. Oncogene 2010, 29, 3297–3306. [Google Scholar] [CrossRef]
- Nguyen, R.; Houston, J.; Chan, W.K.; Finkelstein, D.; Dyer, M.A. The role of interleukin-2, all-trans retinoic acid, and natural killer cells: Surveillance mechanisms in anti-GD2 antibody therapy in neuroblastoma. Cancer Immunol. Immunother. 2018, 67, 615–626. [Google Scholar] [CrossRef]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of disialoganglioside (GD2) in neuroblastic tumors: A prognostic value for patients treated with anti-GD2 immunotherapy. Pediatr. Dev. Pathol. 2018, 21, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Balis, F.M.; Busch, C.M.; Desai, A.V.; Hibbitts, E.; Naranjo, A.; Bagatell, R.; Irwin, M.; Fox, E. The ganglioside GD2 as a circulating tumor biomarker for neuroblastoma. Pediatr. Blood Cancer 2020, 67, e28031. [Google Scholar] [CrossRef]
- Beiske, K.; Burchill, S.A.; Cheung, I.Y.; Hiyama, E.; Seeger, R.C.; Cohn, S.L.; Pearson, A.D.J.; Matthay, K.K. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRT-PCR: Recommendations by the International Neuroblastoma Risk Group Task Force. Br. J. Cancer 2009, 100, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Seeger, R.C.; Reynolds, C.P.; Gallego, R.; Stram, D.O.; Gerbing, R.B.; Matthay, K.K. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: A Children’s Cancer Group Study. J. Clin. Oncol. 2000, 18, 4067–4076. [Google Scholar] [CrossRef]
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr. Blood Cancer 2017, 64, 46–56. [Google Scholar] [CrossRef]
- Hoon, D.S.; Kuo, C.T.; Wen, S.; Wang, H.; Metelitsa, L.; Reynolds, C.P.; Seeger, R.C. Ganglioside GM2/GD2 synthetase mRNA is a marker for detection of infrequent neuroblastoma cells in bone marrow. Am. J. Pathol. 2001, 159, 493–500. [Google Scholar] [CrossRef][Green Version]
- Szanto, C.L.; Cornel, A.M.; Vijver, S.V.; Nierkens, S. Monitoring immune responses in neuroblastoma patients during therapy. Cancers 2020, 12, 519. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Otto, M.; Baldwin, W.M., 3rd; Vail, E.; Gillies, S.D.; Handgretinger, R.; Barfield, R.C.; Ming Yu, H.; Yu, A.L. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 2010, 149, 135–142. [Google Scholar] [CrossRef]
- Kholodenko, R.V.; Kalinovsky, D.V.; Doronin, I.I.; Ponomarev, E.D.; Kholodenko, I.V. Antibody fragments as potential biopharmaceuticals for cancer therapy: Success and limitations. Curr. Med. Chem. 2019, 26, 396–426. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kalinovsky, D.V.; Svirshchevskaya, E.V.; Doronin, I.I.; Konovalova, M.V.; Kibardin, A.V.; Shamanskaya, T.V.; Larin, S.S.; Deyev, S.M.; Kholodenko, R.V. Multimerization through pegylation improves pharmacokinetic properties of scFv fragments of GD2-specific antibodies. Molecules 2019, 24, 3835. [Google Scholar] [CrossRef]
- Thompson, J.P.; Schengrund, C.-L. Oligosaccharide-derivatized dendrimers: Defined multivalent inhibitors of the adherence of the cholera toxin B subunit and the heat labile enterotoxin of E. coli to GM1. Glycoconj. J. 1997, 14, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kitov, P.I.; Sadowska, J.M.; Mulvey, G.; Armstrong, G.D.; Ling, H.; Pannu, N.S.; Read, R.J.; Bundle, D.R. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 2000, 403, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Kroesen, M.; Büll, C.; Gielen, P.R.; Brok, I.C.; Armandari, I.; Wassink, M.; Looman, M.W.G.; Boon, L.; den Brok, M.H.L.; Hoogerbrugge, P.M.; et al. Anti-GD2 mAb and Vorinostat synergize in the treatment of neuroblastoma. Oncoimmunology 2016, 5, e1164919. [Google Scholar] [CrossRef]
- Van den Bijgaart, R.J.E.; Kroesen, M.; Wassink, M.; Brok, I.C.; Kers-Rebel, E.D.; Boon, L.; Heise, T.; van Scherpenzeel, M.; Lefeber, D.J.; Boltje, T.J.; et al. Combined sialic acid and histone deacetylase (HDAC) inhibitor treatment up-regulates the neuroblastoma antigen GD2. J. Biol. Chem. 2019, 294, 4437–4449. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, C.; Ruuth, K.; Kamaraj, S.; Wang, C.-L.; Yang, H.-L.; Combaret, V.; Djos, A.; Martinsson, T.; Christensen, J.G.; Palmer, R.H.; et al. Anaplastic Lymphoma Kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells. Oncogene 2012, 31, 5193–5200. [Google Scholar] [CrossRef]
- Tucker, E.R.; Poon, E.; Chesler, L. Targeting MYCN and ALK in resistant and relapsing neuroblastoma. Cancer Drug Resist. 2019, 2, 803–812. [Google Scholar] [CrossRef]
- Bagatell, R.; Beck-Popovic, M.; London, W.B.; Zhang, Y.; Pearson, A.D.J.; Matthay, K.K.; Monclair, T.; Ambros, P.F.; Cohn, S.L. Significance of MYCN Amplification in International Neuroblastoma Staging System Stage 1 and 2 Neuroblastoma: A Report From the International Neuroblastoma Risk Group Database. J. Clin. Oncol. 2009, 27, 365–370. [Google Scholar] [CrossRef]
- Pastor, E.R.; Mousa, S.A. Current management of neuroblastoma and future direction. Crit. Rev. Oncol. Hematol. 2019, 138, 38–43. [Google Scholar] [CrossRef]
- Valentijn, L.J.; Koster, J.; Haneveld, F.; Aissa, R.A.; van Sluis, P.; Broekmans, M.E.; Molenaar, J.J.; van Nes, J.; Versteeg, R. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc. Natl. Acad. Sci. USA 2012, 109, 19190–19195. [Google Scholar] [CrossRef]
- Wang, L.L.; Teshiba, R.; Ikegaki, N.; Tang, X.X.; Naranjo, A.; London, W.B.; Hogarty, M.D.; Gastier-Foster, J.M.; Look, A.T.; Park, J.R.; et al. Augmented expression of MYC and/or MYCN protein defines highly aggressive MYC-driven neuroblastoma: A Children’s Oncology Group study. Br. J. Cancer 2015, 113, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Shyr, D.; Bagatell, R.; Fischer, M.; Nakagawara, A.; Nieto, A.C.; Brodeur, G.M.; Matthay, K.K.; London, W.B.; DuBois, S.G. Comprehensive evaluation of context dependence of the prognostic impact of MYCN amplification in neuroblastoma: A report from the International Neuroblastoma Risk Group (INRG) project. Pediatr. Blood Cancer 2019, 66, e27819. [Google Scholar] [CrossRef] [PubMed]
- Valter, K.; Zhivotovsky, B.; Gogvadze, V. Cell death-based treatment of neuroblastoma. Cell Death Dis. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Schwab, M. The mycN/max protein complex in neuroblastoma. Short Rev. Eur. J. Cancer 1995, 31, 516–519. [Google Scholar] [CrossRef]
- Costa, R.A.; Seuanez, H.N. Investigation of major genetic alterations in neuroblastoma. Mol. Biol. Rep. 2018, 45, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Oberthuer, A.; Hero, B.; Berthold, F.; Juraeva, D.; Faldum, A.; Kahlert, Y.; Asgharzadeh, S.; Seeger, R.; Scaruffi, P.; Tonini, G.P.; et al. Prognostic impact of gene expression-based classification for neuroblastoma. J. Clin. Oncol. 2010, 28, 3506–3515. [Google Scholar] [CrossRef]
- Tonini, G. Growth, progression and chromosome instability of neuroblastoma: A new scenario of tumorigenesis? BMC Cancer 2017, 17, 20. [Google Scholar] [CrossRef]
- He, X.; Qin, C.; Zhao, Y.; Zou, L.; Zhao, H.; Cheng, C. Gene signatures associated with genomic aberrations predict prognosis in neuroblastoma. Cancer Commun. 2020, 40, 105–118. [Google Scholar] [CrossRef]
- Thwin, K.K.M.; Ishida, T.; Uemura, S.; Yamamoto, N.; Lin, K.S.; Tamura, A.; Kozaki, A.; Saito, A.; Kishimoto, K.; Mori, T.; et al. Level of seven neuroblastoma-associated mRNAs detected by droplet digital PCR is associated with tumor relapse/regrowth of high-risk neuroblastoma patients. J. Mol. Diagn. 2020, 22, 236–246. [Google Scholar] [CrossRef]
- Thudichum, J.L.W. A Treatise on the Chemical Constitution of the Brain; Based throughout upon Original Researches; Baillière, Tindall, and Cox: London, UK, 1884. [Google Scholar]
- Bartels, T.; Kim, N.C.; Luth, E.S.; Selkoe, D.J. N-alpha-acetylation of a-synuclein increases its helical folding propensity, gm1 binding specificity and resistance to aggregation. PLoS ONE 2014, 9, e103727. [Google Scholar] [CrossRef]
 —glucose;
—glucose;  —galactose;
—galactose;  —N-acetylgalactosamine; and
—N-acetylgalactosamine; and  —sialic acid [11]. GA1, GA2, and GA3 indicate asialylated gangliosides. Nomenclature used for ganglioside series gangliosides was developed by Svennerholm [12]. Brackets indicate gangliosides that are in the a-series (one sialic residue linked ±2–3 to the galactose linked β1–4 to glucose) or in the b-series (two sialosyl residues linked to the galactose as shown). Gene abbreviations are those of the Human Genome Organization (HUGO) gene nomenclature committee (https://www.genenames.org/tools/multi-symbol-checker/). Enzymes indicated are: B4GALT5/B4GALT6 [13,14], UDP-galactose: glucosyceramide β1–4 galactosyl transferase (lactosylceramide synthase); B4GALNT1, UDP-GalNAc:LacCer/GM3/GD3/GT3 β1–4 N-acetylgalactosaminyl transferase (ganglioside GA2, GM2, GD2, synthase); B3GALT4, UDP-galactose:GA2/GM2/GD2/GT2 β1–3 galactosyl transferase (ganglioside GA1, GM1a, GD1b, and GT1c synthase); CerS, ceramide synthase; DES, dihydroceramide desaturase; UGCG, UDP-glucose:ceramide β1-1′-glucosyl transferase; KSpR, 3-ketosphinganine reductase; SPT, serine-palmitoyl transferase; ST3GAL5, CMP-sialic acid:lactosylceramide α2–3 sialyltransferase (GM3 synthase); ST8SIA1, CMP-sialic acid:GM3 α2–8-sialyltransferase (GD3 synthase); ST8SIA5, CMP-sialic acid:GD3 α2–8-sialyltransferase (GT3 synthase). * ST3GAL2/3 are needed in mice for synthesis of D1a and T1b [15], but the specificity of these enzymes in humans is still under study [16]. For a discussion of similarities and differences in genes needed for ganglioside synthesis in mice and humans see Schnaar [17]. For characterization of GM1b see [18].
—sialic acid [11]. GA1, GA2, and GA3 indicate asialylated gangliosides. Nomenclature used for ganglioside series gangliosides was developed by Svennerholm [12]. Brackets indicate gangliosides that are in the a-series (one sialic residue linked ±2–3 to the galactose linked β1–4 to glucose) or in the b-series (two sialosyl residues linked to the galactose as shown). Gene abbreviations are those of the Human Genome Organization (HUGO) gene nomenclature committee (https://www.genenames.org/tools/multi-symbol-checker/). Enzymes indicated are: B4GALT5/B4GALT6 [13,14], UDP-galactose: glucosyceramide β1–4 galactosyl transferase (lactosylceramide synthase); B4GALNT1, UDP-GalNAc:LacCer/GM3/GD3/GT3 β1–4 N-acetylgalactosaminyl transferase (ganglioside GA2, GM2, GD2, synthase); B3GALT4, UDP-galactose:GA2/GM2/GD2/GT2 β1–3 galactosyl transferase (ganglioside GA1, GM1a, GD1b, and GT1c synthase); CerS, ceramide synthase; DES, dihydroceramide desaturase; UGCG, UDP-glucose:ceramide β1-1′-glucosyl transferase; KSpR, 3-ketosphinganine reductase; SPT, serine-palmitoyl transferase; ST3GAL5, CMP-sialic acid:lactosylceramide α2–3 sialyltransferase (GM3 synthase); ST8SIA1, CMP-sialic acid:GM3 α2–8-sialyltransferase (GD3 synthase); ST8SIA5, CMP-sialic acid:GD3 α2–8-sialyltransferase (GT3 synthase). * ST3GAL2/3 are needed in mice for synthesis of D1a and T1b [15], but the specificity of these enzymes in humans is still under study [16]. For a discussion of similarities and differences in genes needed for ganglioside synthesis in mice and humans see Schnaar [17]. For characterization of GM1b see [18].
 —glucose;
—glucose;  —galactose;
—galactose;  —N-acetylgalactosamine; and
—N-acetylgalactosamine; and  —sialic acid [11]. GA1, GA2, and GA3 indicate asialylated gangliosides. Nomenclature used for ganglioside series gangliosides was developed by Svennerholm [12]. Brackets indicate gangliosides that are in the a-series (one sialic residue linked ±2–3 to the galactose linked β1–4 to glucose) or in the b-series (two sialosyl residues linked to the galactose as shown). Gene abbreviations are those of the Human Genome Organization (HUGO) gene nomenclature committee (https://www.genenames.org/tools/multi-symbol-checker/). Enzymes indicated are: B4GALT5/B4GALT6 [13,14], UDP-galactose: glucosyceramide β1–4 galactosyl transferase (lactosylceramide synthase); B4GALNT1, UDP-GalNAc:LacCer/GM3/GD3/GT3 β1–4 N-acetylgalactosaminyl transferase (ganglioside GA2, GM2, GD2, synthase); B3GALT4, UDP-galactose:GA2/GM2/GD2/GT2 β1–3 galactosyl transferase (ganglioside GA1, GM1a, GD1b, and GT1c synthase); CerS, ceramide synthase; DES, dihydroceramide desaturase; UGCG, UDP-glucose:ceramide β1-1′-glucosyl transferase; KSpR, 3-ketosphinganine reductase; SPT, serine-palmitoyl transferase; ST3GAL5, CMP-sialic acid:lactosylceramide α2–3 sialyltransferase (GM3 synthase); ST8SIA1, CMP-sialic acid:GM3 α2–8-sialyltransferase (GD3 synthase); ST8SIA5, CMP-sialic acid:GD3 α2–8-sialyltransferase (GT3 synthase). * ST3GAL2/3 are needed in mice for synthesis of D1a and T1b [15], but the specificity of these enzymes in humans is still under study [16]. For a discussion of similarities and differences in genes needed for ganglioside synthesis in mice and humans see Schnaar [17]. For characterization of GM1b see [18].
—sialic acid [11]. GA1, GA2, and GA3 indicate asialylated gangliosides. Nomenclature used for ganglioside series gangliosides was developed by Svennerholm [12]. Brackets indicate gangliosides that are in the a-series (one sialic residue linked ±2–3 to the galactose linked β1–4 to glucose) or in the b-series (two sialosyl residues linked to the galactose as shown). Gene abbreviations are those of the Human Genome Organization (HUGO) gene nomenclature committee (https://www.genenames.org/tools/multi-symbol-checker/). Enzymes indicated are: B4GALT5/B4GALT6 [13,14], UDP-galactose: glucosyceramide β1–4 galactosyl transferase (lactosylceramide synthase); B4GALNT1, UDP-GalNAc:LacCer/GM3/GD3/GT3 β1–4 N-acetylgalactosaminyl transferase (ganglioside GA2, GM2, GD2, synthase); B3GALT4, UDP-galactose:GA2/GM2/GD2/GT2 β1–3 galactosyl transferase (ganglioside GA1, GM1a, GD1b, and GT1c synthase); CerS, ceramide synthase; DES, dihydroceramide desaturase; UGCG, UDP-glucose:ceramide β1-1′-glucosyl transferase; KSpR, 3-ketosphinganine reductase; SPT, serine-palmitoyl transferase; ST3GAL5, CMP-sialic acid:lactosylceramide α2–3 sialyltransferase (GM3 synthase); ST8SIA1, CMP-sialic acid:GM3 α2–8-sialyltransferase (GD3 synthase); ST8SIA5, CMP-sialic acid:GD3 α2–8-sialyltransferase (GT3 synthase). * ST3GAL2/3 are needed in mice for synthesis of D1a and T1b [15], but the specificity of these enzymes in humans is still under study [16]. For a discussion of similarities and differences in genes needed for ganglioside synthesis in mice and humans see Schnaar [17]. For characterization of GM1b see [18].
| INSS * (Uses Surgical Samples) [3] | INRGSS ** (Uses Imaging, Exams and Biopsies) [4] |
|---|---|
|
|
|
|
| |
| |
|
|
|
|
| Transport Protein/Enzyme | Factor(s) Affecting Activity | Species * |
|---|---|---|
| CERT (ceramide transporter) | Phosphorylation by protein kinase D  activity [26,27,28] activity [26,27,28] | Human |
| Glc-cer synthase | Ceramide  activity [36] activity [36] | Human |
| FAPP2 (four–phosphate adaptor protein 2) | Failure to interact with acyl-coenzyme A binding domain 3 (ACBD3) [37] | Human |
| Lac-cer synthase (B4GalT5/6) | Sp1 transcription factor  synthesis [38] synthesis [38] | Human |
| GM3 synthase (ST3GAL5) | PKC  CREB-mediated transcription CREB-mediated transcription   GM3 [39] GM3 [39]  Specificity promotor 1 and activating protein 2 promote expression [40] Specificity promotor 1 and activating protein 2 promote expression [40]Enzyme’s cytoplasmic tail determines activity, subcellular localization and stability [41] | Human Mouse Mouse |
| GD3 synthase (ST8SIA1) | N-glycosylation affects location and activity [42] NF-kB upregulates transcription [43] | Chicken Human |
| GA2/GM2/GD2 synthase (B4GALNT1) |  During neuronal differentiation [44] During neuronal differentiation [44] Coexistence of multiple isoforms [45]  Sp1 or HDAC1 Sp1 or HDAC1   transcription [46] transcription [46] | Mouse Hamster Human |
| Sialidase 3 (NEU3) | BDNF  its activity [47] its activity [47] | Rat |
| GA1, GM1a, GD1b, and GT1c synthase (B3GalT4) | Gene hypermethylation  expression [48] expression [48] | Human |
| Ganglioside | Effect | Cell Type |
|---|---|---|
| Glc-Cer | Anti-apoptotic, pro-survival  Endocytosis of transferrin receptor Endocytosis of transferrin receptor | Cancer cells [77] THP1 monocytes induced to become macrophage [78] |
| Lac-Cer | Lipid 2nd messenger   angiogenesis angiogenesis | Human endothelium [79] |
| GM3 |  Lipopolysaccharide-induced inflammation by Lipopolysaccharide-induced inflammation by  NF-κB, AP-1, and MAPKs signaling NF-κB, AP-1, and MAPKs signalingGM3 expression  during oxidative stress during oxidative stress due to  sialyltransferase activity sialyltransferase activity | rAW 264.7 macrophage [80] Human neuroblastoma (NB) cells [81] |
| GM2 | GM2, GM1, GD1a expression  during oxidative stress due to during oxidative stress due to  sialyltransferase activity sialyltransferase activity | Human NB cells [81] |
| GM1 |  Movement of EGFR to caveolae Movement of EGFR to caveolae  EGFR activity EGFR activity contact inhibition contact inhibition Insulin resistance in aging/senescence and Insulin resistance in aging/senescence and inflammation  Dopamine and histamine post-synaptic binding Dopamine and histamine post-synaptic binding  PI3K/AKT-Nrf2 pathway protects against high altitude-induced cerebral edema PI3K/AKT-Nrf2 pathway protects against high altitude-induced cerebral edema Oligosaccharide portion binds TrkA receptor   neurite outgrowth neurite outgrowthTumor shed GM1 acts on macrophages   angiogenesis angiogenesisReversed MK801 induced cognitive defects  Autophagy following experimental stroke Autophagy following experimental strokeBinding by galectin 1    axon growth axon growth | Human mammary epithelial cells [82] Human endothelial cells [83] Model lipid bilayers [84] Rat brains [85] Murine NB cells [86] Macrophages [87] C57BL/6 J mice [88] Rats [89] Neurons and NB cells [90] |
| GD3 |  EGFR signaling to maintain cell self-renewal EGFR signaling to maintain cell self-renewal | Mouse neural stem cells [71] |
| GD2 | Anti-GD2 antibodies induce nonclassical cell death Ab binding to GD2  Src kinases Src kinases  phosphorylation of NMDA receptor NR2B subunits, phosphorylation of NMDA receptor NR2B subunits,  cAMP cAMP FAK-AKT-ERK-mTOR signaling   growth and invasion of cells growth and invasion of cells  Angiogenesis Angiogenesis | Tumor [91] NB cells [92] Breast cancer stem like cells [72] Melanoma and NB cells [93] |
| GD1a |  Expression during oxidative stress due to Expression during oxidative stress due to  sialyltransferase activity | Human NB cells [81] |
| GT1b |  TLR2 TLR2   neuropathic pain neuropathic pain | Spinal cord [94] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schengrund, C.-L. Gangliosides and Neuroblastomas. Int. J. Mol. Sci. 2020, 21, 5313. https://doi.org/10.3390/ijms21155313
Schengrund C-L. Gangliosides and Neuroblastomas. International Journal of Molecular Sciences. 2020; 21(15):5313. https://doi.org/10.3390/ijms21155313
Chicago/Turabian StyleSchengrund, Cara-Lynne. 2020. "Gangliosides and Neuroblastomas" International Journal of Molecular Sciences 21, no. 15: 5313. https://doi.org/10.3390/ijms21155313
APA StyleSchengrund, C.-L. (2020). Gangliosides and Neuroblastomas. International Journal of Molecular Sciences, 21(15), 5313. https://doi.org/10.3390/ijms21155313