1. Introduction
Recent studies have shown that immune regulation in vivo is more diverse than previously appreciated. A unique antigen (Ag)-specificity of T cell immunosuppression was described previously in contact sensitivity (CS) induced by epicutaneous immunization of mice with reactive hapten [
1,
2,
3,
4,
5]. This form of Ag-specific T cell tolerance is systemically generated by intravenous (IV) administration of high doses of hapten-conjugated syngeneic erythrocytes, followed by skin sensitization with the same reactive hapten. This induces CD8
+ suppressor T cells (Ts) that are not FoxP3
+ regulatory T cells (Treg) [
4].
The Ts cells produce and release suppressive, Ag-specific extracellular vesicles (EVs), namely exosome-like nanovesicles [
4], that deliver inhibitory miRNA-150. Exosome-targeted antigen-presenting cells (APC), in turn, suppressed the CS effector T cells [
4,
6]. Exosome-mediated suppression was unequivocally confirmed by an in vivo experiment, showing that systemic administration of these exosomes to actively sensitized hosts at the peak of the hapten-specific effector T cell-mediated CS strongly reduced subsequent immune skin swelling responses measured for over four days [
4].
The Ag-specificity of this Ts cell-derived exosome-mediated suppression is due to a surface coating with antibody (Ab) free light chains (FLC) provided by B1a cells that also were activated during tolerogenesis [
4]. This Ag-specificity of suppressive exosomes was proved in dual Ag crisscross experiments using two non-cross reactive haptens, and also in similar dual crisscross Ag-affinity column chromatography [
4].
We showed previously that contact immunization rapidly activates a subpopulation of Ag-specific peritoneal B1a cells to migrate to the spleen, where they produce specific IgM Ab and their derived Ab FLC [
7]. By tolerizing µMT and JH
neg/neg antibody deficient mice, and coating the resulting exosomes with chosen FLC, we demonstrated that the Ag-specific Ab FLC bind to the surface of Ts cell-derived exosomes. This in turn is responsible for the Ag-specificity of the exosome targeting of CS effector cells [
8]. Furthermore, the B1a cells also release non-suppressive exosomes, that already express Ag-specific B cell receptor (BCR) and/or surface Ab FLC [
9]. Consequently, we have uniquely shown that in vitro association of these Ag-specific B1a cell-derived exosome-like nanovesicles with miRNA-150, but not control miRNAs, also renders them suppressive. Their effect is similar to the exosomes induced in the Ts-cells of Ag-tolerized mice, that endogenously acquired miRNA-150. This was termed “an alternate Ag-specific exosome-mediated suppression pathway” [
10].
Results of these prior studies on CS suppression led to the conclusion that in vivo these Ag-specific B1a cell-derived exosomes can associate with exogenous inhibitory non-exosomal miRNA, perhaps carried in vivo by RNA-binding argonaute proteins [
4]. Consequently, such miRNA-150-associated exosomes were strongly inhibitory against CS effector cells [
4]. Therefore, it seemed that this miRNA likely was acquired from the freely circulating, extracellular pool of non-exosomal RNAs protected from RNases by chaperones like Argonaut proteins [
4]. As an in vivo consequence, these B1a cell-derived exosomes seem able to act indirectly by similar Ag-specific binding to surface Ag of APCs, that then inhibit CS-effector T cells via transfer of their acquired inhibitory miRNA-150 [
10].
Given the demonstrated initial suppressive exosome targeting of the APC in CS [
6], it was postulated that the actual Ag bound by these FLC-coated suppressive exosomes could be hapten chemically conjugated to self-protein-peptides complexed with MHC on the APC surface, but that could not be determined in the hapten CS system. In the current study, we sought to determine if similar suppression mechanisms applied to classical cutaneous delayed-type hypersensitivity (DTH) induced by ovalbumin (OVA) protein Ag. In this case, it was postulated could show that that exosome-coating FLC might bind target OVA Ag peptide determinants complexed with MHC on the APC surface. The most definitive experiments were administering suppressive exosomes systemically to actively immunized mice at the peak of the skin responses and determining effects on elicited DTH over subsequent days [
4]. Surprisingly, the resulting long term systemic suppression resulting from oral administration of either the T or B cell-derived suppressive exosomes was superior to intravenous (IV) or intraperitoneal (IP) injections.
3. Discussion
3.1. Ag-Specific Ts Cell-Derived Exosomes Mediate Tolerance Induced by Ag High Dose Systemic Administration
The current study has contributed greatly to further understanding of our original discovery of Ag-specific immunosuppressive exosomes [
4]. These prior findings were from the hapten-specific effector T cell-mediated, in vivo system of cutaneous CS, where the Ag-specific tolerance was shown to be due to exosome-like nanovesicles coated with Ab FLC delivering miRNA-150 [
4,
8]. The central finding of this unique system was that systemic treatment of mice with such Ag-specific, immune suppressive exosome-like nanovesicles strongly inhibited established CS in actively sensitized hosts, when we systemically administered a single physiological dose of the vesicles at the 24-h peak of the cutaneous immune response. Those inhibitory exosome-like nanovesicles in the CS system were secreted by CD8
+ Ts cells not expressing the Treg cell marker FoxP3 [
4], and targeted Ag-presenting macrophages [
6]. After induction of tolerance to hapten in donor mice, their suppressive exosome-like nanovesicles were found in their immune sera, plasma, and in the supernatant of their cultured spleen and lymph node cells [
4]. Thus, suppressive exosomes were released and acted systemically to achieve Ag-specific immunological tolerance due to their suppression, and were able to act at local sites of CS elicitation in the ear skin after systemic administration.
This current new study was extended to OVA protein Ag-induced tolerance, that was similarly mediated by Ag-specific nanovesicles, determined to be common small EVs, most likely exosomes, coated with anti-OVA Ab FLC, and also again delivering inhibitory miRNA-150, acting on macrophage APC, likely by binding to their surface expressed OVA peptides in MHC to inhibit their companion peptide-Ag-MHC-specific DTH effector T cells.
3.2. Development of the OVA Protein Ag-Induced DTH System Regulated by Ag-Specific, Ts Cell-Derived Inhibitory Exosomes Delivering miRNA-150
Our findings in the hapten-induced CS system were new, unique, unconventional, and a bit controversial. The findings were made in a system of hapten chemical Ag-specificity, and indicated that the exosome-like nanovesicles targeted the APC, that guided the eventual suppression of their companion CS-effector T cells [
6]. However, the exact entity likely on the APC surface bound by the Ag-specific FLC coating these suppressive exosomes could not be determined in this CS system. This is because hapten immunization and challenge results in hapten binding over the whole APC surface as it becomes chemically covalently linked to diverse host proteins.
The current study was performed in the different, OVA protein Ag-induced DTH system, and allowed expansion of the previous findings by being able to determine the Ag entity targeted on the APC surface. This enabled exosome-mediated transfer of miRNA-150, that in turn activated suppressive function of these APC, that then inhibited companion OVA-specific DTH-effector T cells. Our current new findings indicate that peptides of the OVA Ag, likely in complex with MHC on the APC surface, are very likely bound by Ab FLC coating the surface of these Ts cell-derived suppressive exosomes.
There was a further important property of treatment with the previously studied hapten Ag-specific exosomes, expanded here in the OVA protein Ag DTH system. In CS, in vivo suppression was mediated by physiological doses of the nanovesicle exosomes, that was effective even when a single systemic IP dose of suppressive exosome vesicles was administered at the in vivo maximum 24 h peak of the cutaneous response and lasted for several days [
4]. In the current study, suppression of OVA DTH was achieved by systemic administration of a physiological dose of exosomes from OVA-tolerized mice by the IP, IV, ID, and even the oral route; the latter producing the most powerful inhibition (
Figure 3E).
3.3. Significance of Administrating the Suppressive Exosomes Orally
A remarkable new finding was that the OVA-specific suppressive exosomes can even be given orally to actively immunized recipients in a single dose, again at the height of the T cell effector response to achieve strong inhibition of elicited DTH. In fact, this produced the strongest effect of all routes examined with prolonged suppression over four days of established DTH at the particular systemic site of the Ag-challenged ear skin (
Figure 3E). These findings are very important since in general they resemble the clinical situation of treating a patient with a pre-existing and ongoing immune-mediated reaction. Furthermore, successful and powerful suppressive treatment with exosomes via the oral route—if translated to the clinical situation—is a low impact and high effect treatment modality that obviously would be convenient to patients; especially children.
3.4. Hapten and Protein Ag-Specific Suppressive Exosomes
Our prior study was the first description of natural, Ag-specific exosome-like nanovesicles, uniquely composed of components from two different cell types; i.e., T cells and B cells. The T cell-derived component was miRNA-150 contained in exosome-like nanovesicles from hapten immune tolerance-mediating CD3
+ CD8
+ Ts cells induced by administration of high systemic doses of hapten Ag [
4]. These CD8
+ Ts cell-derived exosomes had Ag non-specific, suppressive activity due to their ability to deliver inhibitory miRNA-150 to targeted cells [
4]. Furthermore, anti-hapten B1a cells, also activated by the tolerogenic procedure, provided hapten-specific Ab FLC for coating the Ts cell-derived exosome-like nanovesicles to render them Ag-specificcally suppressive of elicited CS responses [
4,
8].
In the current study, OVA Ag-induced high dose tolerance was also found to be mediated by CD8
+ CD3
+ cells producing Ag-specific, suppressive exosomes, similarly coated with Ab FLC and particularly transferring miRNA-150 cargo. Furthermore, the OVA-Ag-specific suppressive exosomes similarly targeted the APC that were companions of the finally suppressed OVA-specific DTH effector T cells (
Figure 2C).
Other EV-mediated mechanisms of immune suppression have already been described and claimed to have Ag-specific activity. However, this has often been tested only in one-way Ag-activity system by simple comparison to challenge with another Ag or just media, or testing non-immune hosts with the involved Ag, as controls. Thus, the mechanisms in these studies should instead be considered only Ag-dependent [
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. Furthermore, the Ag-specificity of the function of other suppressive exosomes was not established in the important and meticulous study of RNAs involved in the suppression of Th1 cells by classical FoxP3
+ Treg cells [
25], and in another study on immunopathogenesis mediated by Treg cell-derived EVs [
29].
In great contrast, our prior studies on CS suppression definitively established the strict hapten Ag-specificity of exosomes by dual reciprocal testing [
4,
8,
10]. In the current DTH studies, we have also reciprocally tested the Ag-specificity of exosome-mediated tolerance to a pair of unrelated protein Ag; i.e., OVA and KLH. Accordingly, OVA-specific suppressive exosomes strongly inhibited effector DTH responses to OVA protein Ag in actively immunized mice. Reciprocally, KLH-specific suppressive exosomes failed to suppress OVA-induced DTH (
Figure 3D and
Figure 2B). This proved that protein-Ag-specific suppressive exosomes acted in a strictly Ag-specific manner.
3.5. Protein Ag-Specific, B1a Cell-Derived, Non-Suppressive Exosomes Can Be Rendered Suppressive by In Vitro Association with miRNA-150
A very important aspect of the hapten CS-inhibiting, Ts-cell-derived exosomes was carriage and then delivery of miRNA-150 to targeted cells, that alone compared to controls inhibited Ag presentation by these acceptor macrophages [
6]. Importantly, we confirmed this exact scenario in OVA protein Ag high dose tolerance induced by exosomes mediating suppression of OVA DTH, that were effective only in the presence of Ag-presenting macrophages. In both the CS and DTH systems, Ag-specific targeting of exosomes derived from CD8
+ CD3
+ Ts cells was mediated by their surface Ab FLC derived from cells other than those that produced the exosomes (i.e., by co-activated B1a cells) [
8].
Furthermore, we described another and equivalent complex biological process that was based on the recognition that the essential aspects of the suppressive exosomes in CS and DTH was exosome surface Ag-specificity and delivery of miRNA-150 [
4,
10]. To construct this with natural components, we employed exosomes derived from B1a cells generated at only two days after immunization ID with OVA alone [
11,
30]. Although these B1a cell-derived exosomes were OVA-specific due to the surface expression of OVA Ab FLC and/or anti-OVA BCR, they were not suppressive, since they lacked a miRNA-150 cargo, because the donors were not Ag-tolerized. Thus, of crucial significance—as in CS suppression [
10]—we found that in vitro association of these B1a cell-derived, OVA Ag-specific exosomes with miRNA-150 alone rendered them suppressive of the OVA-specific effector T cells by acting on their APC. Furthermore, these miRNA-150-associated, anti-OVA B1a cell-derived exosomes were strongly suppressive over subsequent days, when injected systemically at the 24-h peak of the OVA-specific DTH response (
Figure 5F), and this was comparable to suppression mediated by the OVA-specific Ts cell-derived exosomes (
Figure 3D,E) [
10]. In summary, we hold that achieving this suppression by simply combining the naturally produced B1a cell-derived exosomes from optimally immunized donors that express surface Ab—together with selection of carried miRNA-150 cargo—is the strongest proof of principal definitively verifying the ideas concerning the biologically functional Ag-specific suppressive exosomes. This was the main focus of our ongoing studies.
3.6. Dose Response Testing to Determine the Limiting Dose of Associating miRNA-150 to Mediate Suppression of OVA DTH by B1a Cell Exosomes
The in vitro procedure of associating miRNA-150 with anti-OVA B1a cell-derived exosomes also allowed for testing the limiting dose of associating miRNA-150 able to mediate suppression of OVA-induced DTH. Such a rarely done dose response experiment showed that the minute dose of only 300 ng or 1 × 10
−13 moles of miRNA-150 sufficed for suppression of an OVA-specific DTH effector cell mixture adoptively transferring DTH (
Figure 5E). This compares to the very unusual, ultra-low dose of 50 × 10
−15 moles, or 50 femtomoles of miRNA-150, associating with B1a cell-derived hapten Ag-specific exosomes for suppression of CS adoptive cell transfer [
10]. However, there is as yet no method allowing accurate determination of the percentage of miRNA-150 taken up by these exosomes and further exosome uptake by the targeted cells. Therefore, in actuality, much less miRNA-150 may be exosome-transferred in both cases to induce the biological effects.
The apparent difference in limiting dose of miRNA-150 in CS and DTH may be due to the distinctive aspects of the involved mechanisms. In CS, the targeted cells are exposed to and linked with many molecules of reactive hapten applied to these macrophage APC, and thus will likely have great amounts of hapten covalently conjugated to multiple entities on their surface beyond those haptens conjugating to self-peptides complexed in MHC. This would allow for great diversity of Ab-linked suppressive exosome binding, uptake, and possible subsequent induction of intracellular mechanisms mediated by transferred miRNA-150. In contrast, in OVA-induced DTH, similar Ab-coated suppressive exosomes seem to just bind the foreign OVA Ag-derived peptides only expressed in complexes with limited numbers of MHC molecules on the APC surface, as the only possible Ag-specific targets for their surface Ab FLC.
3.7. Confirmation That Ag-Specific Exosomes Mediate miRNA Delivered Suppression in Two Different DTH Systems
Various findings of Ab-dependent, exosome-mediated suppression in hapten CS, were now repeated in protein Ag-induced DTH. As a first potential treatment scenario, this offered a unique situation of constructing functionally suppressive exosomes derived again from two cell types; i.e., T cells and B cells. Firstly, this has potential clinical significance, since there is the tolerogenic route of inducing Ts cell-derived endogenous miRNA-containing exosomes to be coated by either native or chosen B1a cell-derived, endogenously available Ab FLC.
In a second scenario, aimed at creating suppressive B cell-derived exosomes for treatment, immunization of patients with exogenous Ag or altered self-Ag might induce a B1a cell source of exosomes that are Ag-specific due to either a surface expression of BCR specific for that immunizing Ag, or via a coating of endogenous Ab FLC. Additionally, two exosome types—i.e., Ts cell- or B1 cell-derived, that could be, respectively, endogenously or in vitro associated with a chosen miRNA, or likely siRNA—would then mediate selected miRNA-dependent Ag-specific alteration of APC function. Indeed, when transferred to totally syngeneic recipients (
Figure 3E and
Figure 5E), they may induce desired epigenetic alterations in specifically targeted host APC, that bear Ag-peptide surface determinants of the given Ag to be bound by the Ab FLC or Ab-like BCR on the exosome surface. The in vitro miRNA association process would follow steps in the new alternate pathway for in vivo exosome-mediated transfer of extracellular RNA (exRNA) between cells that we described previously [
10]. Clinically, there are other conditions possibly applicable to regulation by the Ag-specific chosen miRNA-delivering exosomes, involving protein Ag DTH-like systems; possibly including protein immune reactivity in asthma, autoimmunity, transplantation, or cancer. Since exosomes seem to be universal nanoparticles of all of life, such natural nano particle constructs would constitute a new simple approach for simultaneous and mutually interacting chosen Ag and gene-specific therapy to curb excessive DTH or related T cell reactivity in such disease processes.
3.8. Potential Application of Dual Ag- and Gene-Specific Exosome to Clinical Conditions Like Anti-Cancer Therapies
Similar combined specific treatment of leukemia and cancers is becoming available in emerging chimeric T cell antigen receptor (CAR) therapy. This might be delivered instead by exosomes with advantages as cell-free carriers, a far less complicated modality for targeted therapy in cancers and possibly in other diseases [
31]. However, current CAR therapy is dependent on a single V-region gene encoded T cell reactivity to a single given peptide-Ag/MHC determinant. Unfortunately, this fixed property is subjected to the well-known ability of cancers to evolve resistance to a given therapy, in this case away from the vulnerable single tumor expressed monoclonal T cell receptor for Ag-peptide/MHC [
32,
33,
34]. This compares to the possibility of using exosomes coated with easily rotated Ab of differing specificity and also to the dual possibility to use readily selected, different therapeutic miRNA or siRNA. Together, this achieves varying Ag or oncogene specific exosome-mediated therapy, that would be much less prone to tumor escape.
Furthermore, in a variety of cancers, treatment with anti-PD-1 or anti-PD-L1 Ab, or other analogous negative co-stimulation specific Ab, that often is incomplete when used as Ab alone, could be used as an exosome coating for check point targeting to reverse tumor mediated suppression of host effector T cell anti-tumor reactivity. When armed with multiple check point-targeting mAb, such therapeutic, Ag-specific exosomes might not only cover multiple target check points, making tumor escape less likely, but also could simultaneously deliver relevant inhibitory RNAs to disable the pro-oncogenetic function of involved suppressive cells [
35]. Accordingly, exosomes could be constructed to inhibit cancer cell-induced immunosuppression by both, specific anti-PD1/L1 Ab coating and delivery of engineered hybridizing gene sequences encoded by selected DNA or RNA, or instead for CRISPR/cas−9 oncogene targeting [
36]. Additionally, for these various scenarios of dual Ag and gene specific exosome-mediated anti-cancer therapies, the prospect of efficacious oral therapy would undoubtedly have greater patient acceptance and comfort, especially when combined with existing drug and radiotherapy that might be reduced to achieve less toxicity in the patients.
3.9. Ab FLC Surface Binding Is Associated with ‘Activated’ Exosomes
A unique feature of both, the hapten-Ag and OVA protein-Ag systems, is that the generated exosomes are able to bind selected Ag-specific Ab FLC and associate with chosen miRNA-150. These unusual binding and associating features uniquely provide both highly Ag-specific binding to targeted cells and ability to transfer gene directed, functional alterations; such as epigenetic changes. Such dual-specific targeting, in part resulting from cargo transfer, is a unique property of the exosome subpopulation we have described, as no other laboratories have yet reported these combined properties. In contrast, when exosomes are similarly prepared from normal, unmanipulated animals, they do not have either ability to be significantly coated with Ab FLC nor miRNA-150 associating properties compared to those from animals stimulated by diverse modes of positive immunization or immune tolerization.
We refer to these exosomes having the combined Ab FLC binding and miRNA associating properties as ‘activated’ exosomes, as they are preferentially derived from activated lymphoid cells of immunized or tolerized mice, and not unmanipulated normal donors [
4,
10]. It is currently not known what steps in immunization or tolerization induce these activation properties, nor what are the stimuli that produce these changes, that are not found on exosomes from non-immune donors. Acquisition of activation characteristics is true for exosomes of mice undergoing strong and prolonged exposure to high doses of Ag in tolerogenesis, or even mere two day ID immunization with proteins without an adjuvant, and to exosomes from various Ag high dose-tolerized Ab-deficient or miRNA KO mice as well.
The association of the mAb FLC with the exosome surface was determined by the ability of mAb FLC to bind the surface of ‘activated’, but not normal, exosomes. This was verified by cytometric visualization of FLC on the exosome surface (
Figure 1C and
Figure 5B) [
4,
8]. Such surface and other activations seemed to account for acquisition of both, the ability to bind specific Ag via surface Ab FLC, that enabled mediation of Ag-specific suppressive function, and also to associate and then transfer selected miRNA for alteration of intracellular functional effects.
This Ag-specificity was demonstrated by OVA Ag-affinity column separation of the total suppressive exosome population that yielded two subpopulations (
Figure 2A) [
4]. These were specifically Ag-binding, Ab-coated, therefore considered ‘activated’, small exosomes that had the suppressive functional activity vs. Ag-non-binding and functionally inactive exosomes (
Figure 2A). This suggested that, in this case, employing Ag-affinity column separation suggested that only around 10% or less of the total exosomes from immunized or tolerized animals are actually ‘activated’ and able to bind the Ag [
4], or express the involved CD9 tetraspanin.
Prior studies with mast cells originally determined binding of Ab FLC to the cell membrane, that, as shown here, also apply to the surface of ‘activated’ exosomes. The binding of Ab FLC to the mast cell surface enabled subsequent binding of specific Ag [
37]. This allowed for induction of mast cell degranulation and mediator release [
37,
38], and correlated with the activity of mast cells in several models of immunological diseases [
39]. Another connected and also unusual property of the ‘activated’ exosomes was that there was no bioactive exosome surface binding of Ab free heavy chains, nor whole Ag-specific IgG of the same Ab source, despite their ability to bind the Ab FLC [
4]. This was also true for the mast cell binding [
37,
38].
3.10. Possible Role of Membrane Lipids in EV Surface Activation
We have formed the hypothesis that these properties of exosome activation are due to an increased content of components in their membranes. This idea is based on our unpublished findings that these vesicles have greater size (150–225 nm) than the typical exosome-like nanovesicles (50–120 nm), and by flow cytometry showing that they can have lower expression of routine molecules present on the surface of most exosomes, like MHC class I (unpublished). This suggests that there has been a filling in of an expanded surface with membrane components, that we believe are likely lipids. This would be the most immediately manipulatable surface component during exosome donor cell stimulation [
40]. Furthermore, study of cellular uptake of exosomes showed that interactions with targeted cells can be modified by changing the lipid composition of their membranes [
41,
42].
Concerning membrane lipids and Ab FLC binding, our ideas also came from studies showing that binding of immunoglobulin FLC to human mononuclear cell subsets depends on surface lipids [
43,
44,
45]. These ideas were sustained by our preliminary unpublished studies examining lipid binding of FLC. Firstly, mAb FLC were shown to have stronger binding ability to certain individual lipid components, such a phosphoethanolamine. This was determined firstly with an ELISA-type assay of lipids adsorbed on plastic wells and then bound by mAb FLC, quantitated by a second binding with anti-light chain mAb linked with enzyme and detected enzymatically. Secondly, liposomes constructed at small EV size and dominantly composed of the best individual lipid binders, like phosphoethanolamine, and less of poor Ab FLC binders like sphingomyelin, were able to bind the Ab FLC, as detected by western blotting. Thirdly, these Ab FLC-associated liposomes had biological properties that routinely composed liposomes did not have (Hutchinson and Askenase, unpublished data).
Studies are underway to similarly examine miRNA interactions with activated vs. normal exosomes. The amounts of miRNA involved in association with ‘activated’ OVA suppressive exosomes are likely minute, as we showed before in CS [
10], and here in DTH (
Figure 5E). It was hoped that biotinylation of miRNA-150 would be helpful for such analysis, since it could aid detection of the binding of miRNA to the exosome surface or associated within the ‘activated’ exosomes by pull-down techniques [
46]. Unfortunately, this biotinylation caused inactivation of miRNA-150 biological properties (
Figure 5E, Group G); as predicted in the literature [
47].
3.11. Similar ‘Activated’ Exosomes May Mediate RNA Transfers in Other Systems
Lipid changes of ‘activated’ exosome membranes may account for their other exceptional exosoe subpopulation properties, like our unique finding that oral systemic suppressive therapy with Ag-specific exosomes can affect a strong local immune reaction at a specific cutaneous anatomical site. This may relate to the physiologically transferred functions of numerous exosomes particularly contained in mothers’ milk and delivered to neonates [
48,
49,
50]. The special property of these exosomes is resistance to harsh conditions in the neonatal stomach, like very low pH plus digestive enzymes [
51,
52], that also could be properties of ‘activated’ exosomes in the immunological systems we described [
53]. RNA cargo of milk exosomes may transfer epigenetic information to neonates after intestinal absorption [
54,
55], perhaps via intestinal epithelial cell endocytosis [
56,
57], benefitting the neonatal intestine [
58,
59], and allowing postulated subsequent systemic transfer [
60], that may influence neonatal immunity [
48,
49,
61,
62,
63,
64], bone formation [
65,
66], and development of the nervous and endocrine systems in the neonates. However, there are as yet few studies actually showing beneficial systemic effects in neonates of orally-delivered milk EV-carried miRNAs, and there is some contrary data [
67]. Thus, our findings of systemic oral exosome transfer of miRNA mediated immune suppression strengthens the idea that milk exosomes might orally transfer systemic effects to the neonates.
Of further great relevance, there are numerous claims that among miRNAs taken orally in foods, in some cases these mixtures contain exosomes that also can pass stomach digestion for uptake systemically to affect host functional processes [
68,
69,
70]. Again, there is controversy with some compelling data against this concept [
67,
71,
72,
73,
74]. Note, however, that these are complex issues with many technical barriers to be overcome for definitive determinations before these issues can be settled [
75,
76,
77,
78]. Herein however, oral treatment with claimed ‘activated’ subsets of exosomes carrying miRNA-150 in our non-dietary system clearly showed strong and prolonged in vivo immune suppression at a particular systemic skin site. Thus, suppressive effects of orally administered, Ts cell-derived, Ag-specific exosomes (
Figure 3E), delivering a particular inhibitory miRNA-150, favor the idea of transferred functionality of dietary food miRNAs, under some circumstances.
Recently, casein-specific Ts cell-derived exosomes have been shown to suppress casein-induced DTH in actively immunized mice when delivered per os in a system aimed at study of milk allergy [
79]. This implies that biological functionality after oral administration is a general feature of Ts cell-derived exosomes. One can speculate that these suppressive exosomes are firstly endocytosed by intestinl epithelial or vascular endothelial cells in the small intestine and then transmitted to circulation, as observed in the case of cow’s milk exosomes [
56,
60]. Otherwise, they may be transmitted to mucosal lymphatic tissue to induce Treg lymphocytes. However, this aspect requires much further investigation.
3.12. Demonstration That Ag-Derived Peptides Are the Binding Targets of FLC on Ag-Specific Suppressive Exosomes
Our prior study in the CS hapten-system showed that macrophage APC, and not the finally suppressed effector T cells, were the direct target of the Ag-specific suppressive exosomes [
6]. We hypothesized that Ab FLC on the surface of the inhibitory exosomes bound to Ag peptides in MHC complexes on the APC surface to lead to eventual suppression by the miRNA-150-influenced APC of the effector T cells that mediate CS [
30].
Our success in generation of OVA protein Ag-specific suppressive exosomes made testing of these ideas possible. Firstly, we showed binding of Ts cell-derived exosomes to OVA-coupled Sepharose on an Ag-affinity column only by the effectively suppressive minor exosome subpopulation (
Figure 2A), and to an anti-CD9-coupled Ab-affinity column (
Figure 1F), that strongly support our hypotheses. In addition, cytometric analysis revealed the co-expression of CD9 and Ab FLC by exosomes from both Ts and B1a cells (
Figure 1C and
Figure 5B). To further extend these findings, we set up an ELISA with native OVA absorbed on plastic wells, and determined the binding of four different anti-OVA mAb rendered into FLC, as well as the isolated heavy chains, compared to whole anti-OVA IgGs. We found that binding of OVA by IgG was the strongest, heavy chains next and FLC last, but definitely detectable (
Figure 6A,B). Comparing the four mAb, there was a consistent hierarchy of binding for all chains and the whole IgG among each mAb type. Thus, binding native OVA by each mAb and its chains was different; ranging from excellent to much less in some, and to nil or nearly nil in others (
Figure 6). The similar binding within a mAb IgG and its heavy and light chains likely was due to identical immunoglobulin V-region DNA sequence mutations in the producing B cells, and thus identical specificity of the translated mAb. Conversely, the different binding between the four mAb likely was due to different V-region mutations resulting in different derived mAb affinities for native OVA Ag determinants adsorbed on plastic.
Knowing that Ag-presenting macrophages were the target of the Ab FLC on the exosomes for mediating the final Ts cell suppression of the effector T cells [
4,
6], we further tested suppression by the four mAb FLC separately coating the same exosomes from Ag-tolerized, Ab-deficient JH
neg/neg mice, that gave a remarkable result. We observed a very different hierarchy in the strength of mAb dependent exosome-mediated suppression (
Figure 7B) compared to binding of native OVA (
Figure 6A,B). This was consistent with the idea that the Ab FLC on the suppressive exosomes bound to Ag-peptides on the APC and not to native OVA.
To investigate this further, we made a tryptic digested peptide mixture from native OVA and tested its ability to block suppression mediated by these exosomes, using different protocols. In the first experiments, the OVA peptides were incubated in vitro with the exosomes coated with two different anti-OVA mAb FLC that had mediated intermediate strength exosome suppression. In both cases we found strong reversal of suppression, when FLC-coated exosomes were blocked by OVA tryptic peptides (
Figure 7E). Additionally, from past studies, we knew that the two day sera of OVA-immunized mice contain both anti-OVA IgM and also Ab FLC, derived from activated B1a cells [
9,
16,
80]. Therefore, we also coated JH
neg/neg mouse Ts cell-derived exosomes with this sera, presumably containing polyclonal immune anti-OVA Ab FLC (
Figure 6E), that in fact produced strong suppression (
Figure 7D). Inhibitory blocking of suppression by these polyclonal Ab FLC with OVA tryptic peptides resulted in the best reversal of suppression (
Figure 7E, Groups G and H); likely due to the polyclonal nature of the serum Ab vs. the mAb FLC. This overall result contributed significant evidence suggesting that Ab FLC coating the Ag-specific exosomes bound to OVA Ag peptides on the APC surface. This would confer the Ag-specificity of Ts cell-mediated immune tolerance [
8]. We have recently reported that, in the particular circumstances, self-tolerant exosomes can be redirected toward suppression of allergen-induced DTH responses by coating with allergen-specific Ab FLC [
81].
In a second protocol, the OVA peptide mixture was used to construct peptide Ag-affinity columns. Passage of the Ab FLC down this column enabled testing those that actually had bound peptide on the column in the Ag-specific exosome-mediated suppression of DTH. The same two mAb FLC, intermediately active when coating exosomes for Ag-specific suppression, and serum polyclonal anti-OVA Ab FLC, were applied to OVA peptide columns. The OVA peptide binding Ab FLC fractions were eluted from each column and then used to coat exosomes from Ag tolerized JH
neg/neg mice in the suppression assay. These eluted FLC, that had bound the peptide mixture, when subsequently coating these exosomes, led to strong suppression of DTH (
Figure 7D). This result confirmed that those Ab FLC that can bind peptides on the column, may mediate the Ag-specificity of the suppressive exosomes (
Figure 7D, Groups C, D, and E) [
8].
This unanticipated result can be seen as further evidence that the binding of the anti-OVA Ab FLC on the suppressive exosomes is likely to OVA peptides complexed in MHC on the APC surface. Thus, an explanation may be due to the manner of binding peptides by Ab FLC in the ELISA compared to FLC on the exosome surface that bind peptides in MHC. In the ELISA, there is only binding of low-affinity, isolated Ab FLC dispersed in solution to the peptides adsorbed on the artificial plastic surfaces in non-physiological conformations. In comparison, the in vivo measured, Ag-specific exosome-mediated suppression depends on FLC that are not free but linked to the exosome surface. Here, the mAb FLC are not dispersed but in multiples closely together on the exosome surface, turning a series of low affinity bindings into far greater avidity for binding Ag determinant of physiological conformation when complexed with MHC, which was confirmed in vitro in ELISA-based assay (
Figure 7F). However, more direct study of mAb FLC binding to the peptide-MHC complex will be needed to prove this point in vivo.
3.13. Characteristics of Ts Cell-Derived Exosomes
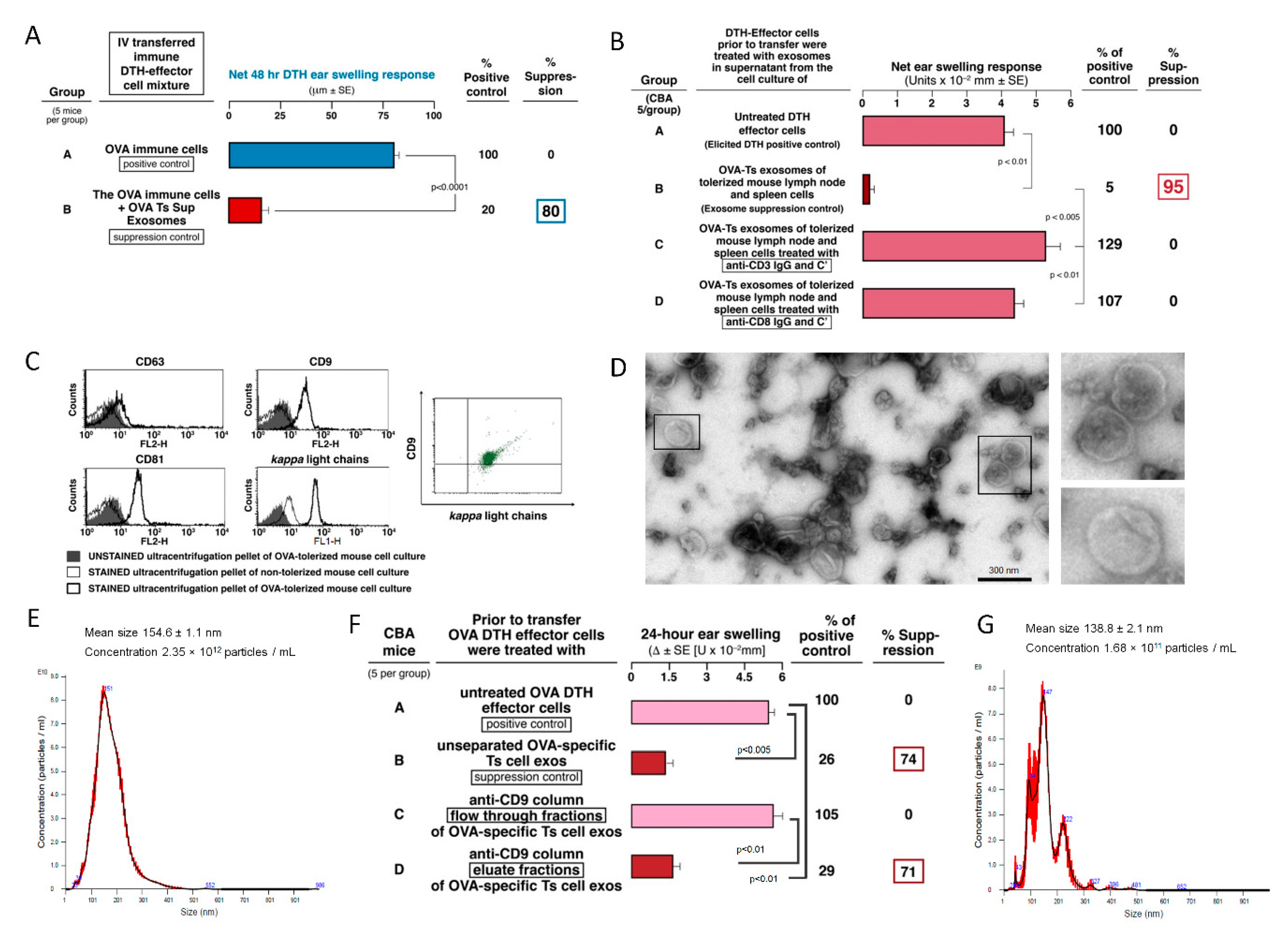
The current study revealed that OVA-induced DTH effector cells are suppressed by Ts cell-derived EVs of a small size (around 150 nm). These bilamellar vesicles are strongly positive for CD9 and CD81 tetraspanins but weakly express CD63 marker (
Figure 1). Additionally, our previous reports demonstrated that the buoyant fraction of Ts cell-derived EVs is responsible for the observed suppressive activity [
4,
81]. They are concentrated by ultracentrifugation from the cell culture supernatant earlier deprived of cellular debris and filtered down to 0.22 µm [
82]. Thus, the light small EV subpopulation were called exosomes. However, it should be stressed that each cell produces very heterogeneous populations of EVs differing in size and origin [
13]. Specific subtypes of small EVs can be classified according to displayed pattern of markers, including variability in tetraspanin expression [
82]. Besides, the complexity of EVs makes that yielded subpopulations of EVs vary across the methods used for their purification prior to functional studies. Thus, more detailed characteristics of Ts cell-derived exosomes remains a subject of our further investigation.
3.14. Summary of the New Findings of Biological and Clinical Significance of the Ag- and miRNA-Specific Exosomes That Suppress OVA Protein-Induced DTH
New findings brought by our current studies could be summarized into several main points. (i) Uniquely, suppressive function depends on exosomes consisting of components from two lymphocyte types (T and B cells). (ii) Ag-specificity in DTH suppression is due to exosome surface coating with anti-OVA Ab FLC. (iii) This Ag-specificity was definitively demonstrated by reciprocal functional suppression testing of two distinct protein-specific suppressive exosomes. (iv) Also, there was definitive demonstration that T or B cell exosome-mediated suppression in DTH is mediated by delivered inhibitory miRNA-150. (v) Systemic suppression of DTH could be achieved in vivo by administration of single physiological doses of exosomes; remarkably including oral administration. (vi) Strong, systemic suppression of maximal in vivo DTH responses at a given peripheral tissue site was observed over several days after administration of a single physiological dose of Ab FLC coated and miRNa-150 delivering exosomes. (vii) Exosomes suppressing DTH could be constructed with chosen dual Ag-specific targeting and the transfer of selected miRNA-encoded epigenetic functional effects by mixing and matching chosen surface Ab FLC and selected miRNA. (viii) These exosomes mediated suppression by very low miRNA-150 associating doses. (ix) Determination that the Ab FLC coating the suppressive exosomes likely binds OVA peptides that are complexed in MHC on the APC surface was shown firstly by comparing in vitro binding of the Ab FLC to OVA, compared to strength of suppression when coating exosomes from Ag-tolerized, Ab-deficient JHneg/neg mice; and secondly by different peptide inhibition protocols.
To summarize, Ag-specific, Ab FLC-dependent, ‘activated’ exosome delivery of suppressive miRNA-150 via targeted binding of Ag-peptides in MHC on APC provides a new mechanism of immune regulation with great clinical significance, since it employs natural physiological exosomes that can be given orally and fashioned for both chosen Ag-specific targeting and selected miRNA-specific functional alterations of targeted cells that can last for several days.
4. Materials and Methods
4.1. Mice
Nine to 10-week-old CBA/J, BALB/c, JH knock out (JHneg/neg), C57BL/6 or miRNA-150 knock out (miRNA-150neg/neg) male mice from Jackson Laboratories (Bar Harbor, ME, USA) or CBA mice from the Breeding Unit of the Jagiellonian University Medical College, Faculty of Medicine (Krakow, Poland) were fed autoclaved food and water ad libitum. All experiments were performed according to the guidelines of Ethics Committees of Yale (approval no. 07381) and Jagiellonian (approval no. 122/2013 and 88/2017) Universities.
4.2. Antigens, Reagents, and Culture Media
The following reagents were used: ovalbumin (OVA), serum-free Mishell–Dutton medium, RPMI−1640, minimal essential medium with amino acids, HEPES, 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO, USA), keyhole limpet hemocyanin (KLH, Stellar Biotechnologies, Port Hueneme, CA, USA), Dulbecco’s phosphate-buffered saline (DPBS), penicillin/streptomycin, sodium pyruvate, L-glutamine (Gibco Life Technologies, Grand Island, NY), acetone, ethanol, glucose (P.O.Ch., Gliwice, Poland), EDC (1-ethyl−3-(3-dimethylaminopropyl)-carbodiamide, Pierce, Thermo Fisher Scientific, Waltham, MA, USA), EDTA (BDH, Poole, UK), extra virgin olive oil (Basso Fedelee Figli, San Michele di Serino, Italy).
4.3. Generation of Ts cell-Derived, Protein Ag-Specific, Suppressive Exosomes
Ts cells from OVA Ag-tolerized mice releasing suppressive exosomes were induced in an analogous manner to the tolerogenic procedures described in CS [
4]. Thus, mice were injected IV on days 0 and 4 with 0.2 mL of a freshly prepared 10% DPBS suspension of syngeneic erythrocytes conjugated with OVA or, in some instances, with KLH protein Ag. We determined in preliminary experiments that EDC is an efficient activating agent for the coupling of protein Ag to erythrocyte membrane proteins. For full tolerogenesis, these IV treatments were followed by intradermal (ID) immunization on two consecutive days 8 and 9 with a total of 0.2 mL of a 0.5 mg/mL OVA each time (100 μg, injected into four sites in the abdominal areas at 25 μg per site), or similarly KLH, both used as a 0.9% NaCl-solution and without adjuvant. After lymph node and spleen collection on day 11, single cell suspensions were cultured at 37 °C in protein-free Mishell-Dutton medium at a concentration of 2 × 10
7 cells/mL for 48 h. To enrich exosomes, the resulting culture supernatant was subsequently centrifuged at 300×
g and then 3000×
g for 10 min, then filtered through 0.45- and then 0.22-micrometer molecular filters (Miltenyi Biotec, Waltham, MA, USA) and finally ultracentrifuged twice at 100,000×
g for 70 min at 4 °C [
4]. The resulting pellet was resuspended in DPBS and used as OVA Ag-specific (or KLH Ag-specific) exosomes [
4]. This pellet containing OVA-specific Ts cell-derived exosomes was absorbed onto copper grids and negatively stained with 3% uranyl acetate, and then visualized under transmission electron microscope (JEOL JEM2100, Tokyo, Japan). Furthermore, it was subjected to nanoparticle tracking analysis (NTA, Nanosight, Malvern, UK) [
4].
In some instances prior to culture, mouse lymph node and spleen cells from Ag-tolerized donors, were depleted of either CD3+ or CD8+ cells by incubation with, respectively, anti-CD3 or anti-CD8 monoclonal IgG antibodies, and rabbit complement for 60 min in 37 °C water-bath. Afterwards, dead cells were removed by discontinuous gradient centrifugation on Ficoll (1.077 g/mL, GE Healthcare, Chicago, IL, USA).
In some experiments, actively tolerized mice were ear challenged on day 11, and subsequent ear swelling responses were measured as described below. In some instances, tolerized and actively immunized mice were depleted of clodronate-sensitive cells (i.e., macrophages) by IP injection of 0.2 mL of clodronate liposomes (Department of Molecular Cell Biology, Vrije University, Amsterdam, The Netherlands) in PBS, at 24 h before ear challenge [
6].
4.4. In Vivo Treatment of Mice Actively Immunized with OVA with Suppressive Exosomes Injected at the Peak of DTH Response
Mice were immunized ID with a total of 100 μg of OVA, as described above, and 5 days later DTH ear swelling was elicited by ID injection of 10 μL of OVA 0.9% NaCl solution (0.5 mg/mL, thus 5 μg per ear) into both ears. The pellet containing OVA Ag- or KLH Ag-specific suppressive exosomes was used for treatment of the mice actively ID immunized for elicitation of OVA-specific DTH either at immunization, or just before ear challenge or at the peak of the response 24 h after ear challenge with OVA Ag, at a dose of 1 × 10
10 EVs per mouse [
11]. These mice were systemically IP injected with the DPBS suspension of exosomes at a dose of about 1 × 10
10 nanovesicles per recipient. This model allowed testing of Ag-specificity of exosome action. Subsequent ear thickness was measured with an engineer’s micrometer daily up to 120 h after challenge by a blinded observer [
4,
12].
To calculate the increase in ear thickness indicating strength of DTH, values of background ear thickness, measured before ear challenge, were subtracted from values of ear thickness at particular time points after ear challenge. Furthermore, to evaluate the net swelling response, mean ear thickness increase in non-immunized but similarly OVA Ag challenged littermate control mice was subtracted from the ear thickness increase measured in each ear of mouse of each control or experimental group. In general, groups consisted of 5 mice and experiments were repeated 2–4 times. The average ear swelling was expressed as the delta ± standard error (SE), after subtraction of the negative control value. The two-tailed Student t-test or one-way analysis of variance (ANOVA) with post hoc RIR Tukey test were used to assess the significance of differences between groups, with p values of less than 0.05 taken as the minimum level of statistical significance.
Exosome counts were estimated by nanoparticle tracking analysis (Nanosite, Malvern, UK). The dose used for treatment of actively immunized mice was considered physiological, since this is the average number of exosomes per ml of blood in normal unmanipulated mice.
Where indicated, exosomes either derived from cultures of Ts-cells or derived from early two day OVA immune B1 a cell-derived supernatants and then associated in vitro with inhibitory miRNA-150, were systemically administered, at the same doses, but in different volumes of DPBS (0.2–0.5 mL), directly to actively immunized mice via IV, IP, or ID routes. Furthermore and most importantly, the same suppressive exosomes at the same dose were also administered orally (per os, PO) using a gastric feeding tube. For all of the compared systemic exosome treatments, suppressive and control exosomes were administered just after measurement of the 24-h peak of active ear swelling response. In the case of PO delivery of the exosomes, mice were kept fasting for 2 h before and 1 h after administration of the suppressive exosomes.
4.5. In Vitro Treatment of OVA DTH Effector Cell Mixture with Exosomes, Prior to Their Adoptive Transfer to Naive Recipients
OVA DTH-effector cells were obtained from mixed spleen and lymph node cells of mice actively sensitized by ID multiple injections of a total of 100 μg OVA in plain 0.9% NaCl (as described above) and harvested at day 4 after immunization [
4,
11]. OVA Ag-specific Ts-cell-derived, suppressive exosomes were used at the same dose of 1 × 10
10 EVs for in vitro pulsing of a mixture of DTH-effector T cells and APC, at 7 × 10
7 total lymphoid cells to be transferred per eventual recipient. Then, the exosome pulsed DTH effector cells were adoptively transferred into naive recipients, in which 24 h later DTH ear swelling responses were elicited by ID injection of 10 μL of OVA 0.9% NaCl solution (0.5 mg/mL, thus 5 μg per ear). Ears were measured for thickness with an engineer’s micrometer (Mitutoyo, Kawasaki, Japan), at 24 h after challenge. Results were statistically analyzed as described above.
4.6. Polyribonucleotide Treatments to Induce Suppressive Activity of Non-Suppressive Exosomes, or to Block Their Suppressive Activity, Prior to Incubation with DTH Effector Cell Mixture
In some instances, Ts-cell-derived, OVA-specific exosomes were pretreated with a polynucleotide antagonist of miRNA-150 (anti-miRNA-150, Dharmacon, Lafayette, CO, USA), at a dose of 3 μg per total exosomes (1010) per each of eventual five recipients of exosome-pulsed DTH-effector cells (7 × 107, the average number of cells transferred IV to each recipient). After washing away of unbound anti-miR-150 molecules by ultracentrifugation, the mixture of DTH effector cells and miRNA antagonist associated exosomes were incubated in a 37 °C water bath for 30 min. Then, the exosome treated and control DTH effector cells were adoptively transferred into naive recipients, as described above.
In other experiments, exosomes from Ag-tolerized miRNA-150 neg/neg mice were treated in a 37 °C water-bath for 30 min with synthetic miRNA-150 in a dose of 3 μg per 1010 exosomes to be used subsequently to treat the DTH-effector cell mixture (7 × 107), per single eventual recipient. Then, through ultracentrifugation, we removed excessive, exosome-non-associated miRNA-150 molecules and used the resulting miRNA-150 pulsed exosomes to treat the DTH effector cells in vitro prior to washing away the exosomes and then IV adoptive transfer of the cells.
4.7. Ab FLC Coating, OVA Peptide Blocking, and OVA Ag or Anti-CD9 Affinity Column Chromatography of Ts-Cell-Derived Exosomes
Ts cell-derived exosomes from Ag tolerized JHneg/neg mice were non-suppressive due to the lack of surface FLC, but nonetheless carried miRNA-150. These were converted for Ag-specific suppression by surface coating with various anti-OVA FLC (derived from either four different monoclonal Ab or immune serum of 2 day OVA-immunized mice) by incubation overnight on ice. This was followed by ultracentrifugation at 100,000× g to remove unbound FLC into the supernatant from the Ab FLC-coated exosomes in the pellet.
In other instances, OVA-specific Ts cell-derived exosomes endogenously coated with B1 a cell-derived Ab FLC, were initially blocked with tryptic digested OVA peptides by a preliminary 2-h incubation in 37 °C water-bath. This was followed by ultracentrifugation to remove excessive OVA peptides into the supernatant from peptide-blocked exosomes in the pellet. In yet other cases, these OVA-specific, Ts-cell-derived exosomes were separated by affinity chromatography on columns filled with Sepharose coupled with either native OVA Ag or with purified anti-CD9 monoclonal antibodies [
4].
4.8. Generation of B1 a Cell-Derived Exosomes and Their In Vitro Association with miRNA-150
To induce very early activated Ag-specific B1 a cells, mice were immunized with OVA protein Ag by ID injections of total volume of 0.2 mL for four separate simultaneous intradermal injections of a 0.5 mg/mL OVA solution in 0.9% NaCl (total ID immunizing dose of 100 μg OVA) without adjuvants on days 0 and 1 [
11]. Then, on day 3, lymph nodes and spleens containing the immune B1 a cells were collected and single cell suspensions were cultured in protein-free Mishell-Dutton medium at a concentration of 2 × 10
7 cells/mL for 48 h [
9]. The resulting culture supernatant was subsequently centrifuged at 300×
g and then 3000×
g for 10 min, filtered through 0.45- and then 0.22-micrometer molecular filters and then ultracentrifuged twice at 100,000×
g for 70 min at 4 °C. The pellet presumably containing B1 a cell-derived exosomes was absorbed onto copper grids and negatively stained with osmium tetroxide, and then visualized under transmission electron microscope (FEI Tecnai T12, LaB6, Thermo Fisher Scientific, Waltham, MA, USA).
The resulting pellet containing enriched Ag-specific B1 a cell-derived exosomes was resuspended in DPBS. Then, where indicated, the Ag-specific B1 a cell-derived, non-suppressive exosomes that received no endogenous miRNA-150, since they were from immunized and not tolerized mice, were associated in vitro with synthetic miRNA-150. This was achieved by incubation in 37 °C water-bath for 30 min with miRNA-150 at a dose of 3µg per 1010 nanovesicles. These exogenously miRNA-150-associated exosomes were subsequently used to pulse 7 × 107 DTH-effector cells per single recipient. Prior to incubation with the DTH-effector cells, the Ag-specific, B1 a cell-derived exosomes that had been incubated with free miRNA-150 for association, were washed by a single ultracentrifugation to remove excessive, exosome-non-associated miRNA-150 molecules.
4.9. Cytometric Analysis of Exosomes and Evaluating the Presence of Antibody Light Chains in Two-Day Serum of OVA-Immunized Mice
Aldehyde/sulphate latex beads (4 µm, Life Technologies, Thermo-Fisher Scientific, Carlsbad, CA, USA) were incubated in DPBS with OVA-specific exosomes derived from Ts cells or B1 a cells at room temperature for 2 h with gentle agitation. Afterwards, exosome-coated beads were blocked with 100 mM glycine, washed, resuspended in DPBS, and stained with fluorescein isothiocyanate (FITC)-conjugated mAb against mouse kappa light chains (BD Biosciences, San Diego, CA, USA) and/or phycoerythrin (PE)-conjugated mAb against mouse CD9, CD63 or CD81 tetraspanins (BD Biosciences, San Diego, CA, USA). Alternatively, the aldehyde/sulphate latex beads were coated with native OVA Ag by overnight incubation at 4 °C with gentle agitation, and then, after washing, were incubated with 2 day immune serum from the ID OVA-immunized mice. After washing, these OVA-coupled beads were stained against mouse antibody kappa light chains to evaluate the eventual presence of anti-OVA FLC in the serum.
4.10. ELISA for Assessment of Binding to Native OVA, OVA Tryptic Peptides, or OVA 323-339 Peptide by Various Anti-OVA mAb FLC
Isolated OVA-specific mAb FLC and isolated mAb free heavy chains were made from four different OVA-specific mAb IgGs (clones F2 3.58, 7 E9.17, 7 H7.E1, and 11 B.1) from the Frank Fitch collection, University of Chicago, that were kindly provided by Anne Sperling. The whole IgGs were reduced and alkylated to separate heavy and light chains in the laboratory of Frank Redegeld at the University of Utrecht, The Netherlands. Proteins were eluted from columns with 6 M guanidine and subsequently loaded onto a gel filtration column (HiLoad 16/60 Superdex 200 pg, GE Healthcare, Chicago, IL, USA) to separate anti-OVA mAb heavy and light chains (FLC). Subsequently, samples were dialyzed versus PBS to remove guanidine and then concentrated.
Then, OVA Ag-specific mAb FLC, heavy chains, and whole IgGs were added to quadruplicate microwells of high binding ELISA plates that previously were coated with native OVA (25 µg/mL). After Ag coating, the plates were blocked with PBS/10% FBS/0.05% Tween-20 for 1 h at room temperature, washed and finally incubated with the four separate OVA-Ag-specific Ab FLC, heavy chains, or whole IgG1, all diluted in PBS/1%FBS/0.05% Tween-20 (assay buffer) at multiple serial dilutions for 1 h at room temperature. For detection of bound mAb FLC, heavy chains and whole IgGs, plates were subsequently incubated with 0.1 μg/mL HRP-labeled goat anti-mouse κ light chain Ab (Southern Biotech, Birmingham, AL 35260, UK), diluted in assay buffer for 1 h at room temperature. For detection of mAb heavy chains, 0.1 μg/mL HRP-labeled goat anti-mouse IgG/IgM H+L Ab (Jackson Labs, Bar Harbor, ME, USA) was used. Finally, TMB was used as a substrate for the enzymatic reaction, terminated by adding 1 M H2 SO4 after 23 min in the case of Ab FLC assaying or after 3 min in all other cases. Between incubation steps, wells were washed three times with PBS/0.05% Tween-20. To evaluate the binding of FLC to OVA 323-339 peptide, plate wells were coated with OVA 323-339 peptide at a concentration of 10 μg/mL, and assay was developed as above. To compare binding of FLC to native OVA and OVA tryptic peptides, plate wells were coated with OVA preparations at a concentration of 100 μg/mL, and the assay was performed as above, but the reaction was stopped by adding 1 M H3 PO4 after 8 min.
4.11. ELISA for Assessment of Binding to Native OVA, OVA Tryptic Peptides, or OVA 323-339 Peptide by Either JHneg/neg Mouse Exosomes Coated with Anti-OVA mAb FLC or Wild Type Mouse Exosomes
Plate wells were coated with either native OVA, OVA tryptic peptides or OVA 323-339 peptide, all at a concentration of 100 μg/mL overnight at 4 °C and then blocked with 2% BSA for 2 h at room temperature. Then exosome samples (approximately 5 × 109 exosomes per well) were added to particular wells and incubated overnight at 4 °C. After washing with 0.1% BSA in PBS, 100 μL/well of rat monoclonal IgG antibody anti-mouse CD9 (BD Biosciences, Franklin Lakes, NJ, USA) was added at a concentration of 4 μg/mL and plate was incubated for 2 h at room temperature. After washing, 100 μL/well of HRP-conjugated rabbit anti-rat IgG antibody (Invitrogen, ThermoFisher, Waltham, MA, USA) was added at a concentration of 2 μg/mL and plate was incubated for 1 h at room temperature. After extensive washing, 100 μL/well of TMB substrate was added and the reaction was terminated after 8 min by adding 50 μL/well of 1 M H3 PO4.