Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation
Abstract
:1. Introduction
2. Results
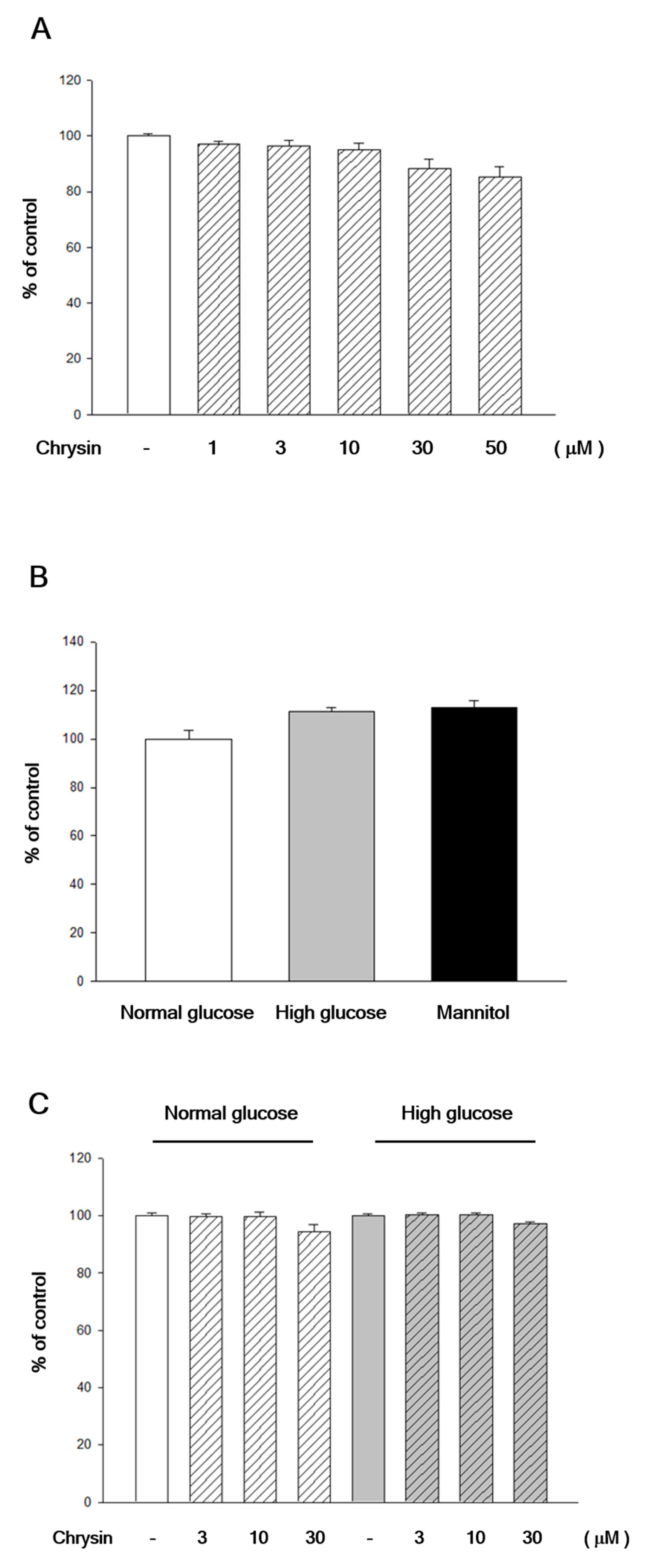
2.1. Chrysin Showed No Cytotoxicity to RF/6A Cells
2.2. Chrysin Inhibited High-Glucose Induced RF/6A Migration via Inhibiting AKT and ERK Phosphorylation and Decreasing MMP-2 Expression
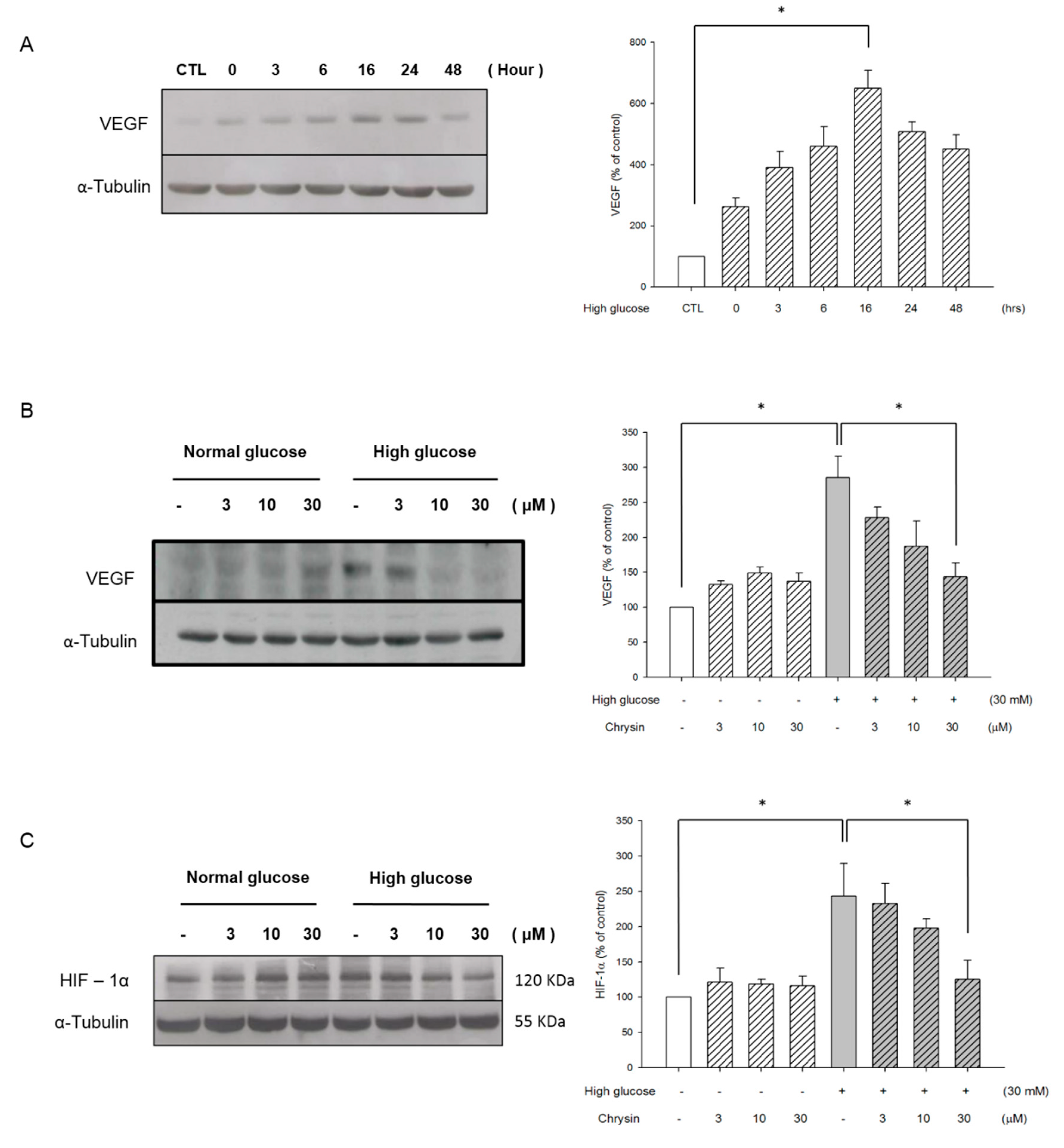
2.3. Chrysin Inhibits the HIF-1α and VEGF Expression
2.4. Chrysin Down-Regulates VEGF Receptor Proteins and mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chrysin Treatment and High-Glucose Induction
4.3. MTT Viability Assay
4.4. Transwell Assays
4.5. Scratch Wound Assay
4.6. Western Blotting Analysis
4.7. Real-Time Quantitative RT-PCR
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| DM | Diabetes mellitus |
| DR | Diabetic retinopathy |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| MMP-2 | Matrix metalloproteinase-2 |
| qRT-PCR | Quantitative real time polymerase chain reaction |
| AKT | Protein kinase b |
| ERK | Extracellular signal-regulated kinase |
| ROS | Reactive oxygen species |
| LPS | Lipopolysaccharide |
| EC | Endothelial cells |
| MAPK | Mitogen-activated protein kinase |
| JNK | C-Jun N-terminal kinase |
| RPE | Retinal pigmented epithelium |
| ECM | Extracellular matrix |
| CNV | Choroidal neovascularization |
| DMEM | Dulbecco’s modified Eagles medium |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| PFA | Paraformaldehyde |
| HPF | High power field |
| PBS | Phosphate buffered saline |
| BCA | Bicinchoninic acid |
| SDS | Sodium dodecyl sulfate |
| PVDF | Polyvinylidene fluoride |
| SE | Standard error |
References
- Metea, M.R.; Newman, E.A. Signaling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Green, W.R.; Wilson, D.J. Choroidal neovascularization. Ophthalmology 1986, 93, 1169–1176. [Google Scholar] [CrossRef]
- Chan, C.M.; Hsiao, C.Y.; Li, H.J.; Fang, J.Y.; Chang, D.C.; Hung, C.F. The Inhibitory Effects of Gold Nanoparticles on VEGF-A-Induced Cell Migration in Choroid-Retina Endothelial Cells. Int. J. Mol. Sci. 2019, 21, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubilewicz, A.; Hecquet, C.; Jeanny, J.; Soubrane, G.; Courtois, Y.; Mascarelli, F. Proliferation of CECs requires dual signaling through both MAPK/ERK and PI 3-K/Akt pathways. Investig. Ophthalmol. Vis. Sci. 2001, 42, 488–496. [Google Scholar]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Fu, B.; Xue, J.; Li, Z.; Shi, X.; Jiang, B.H.; Fang, J. Chrysin inhibits expression of hypoxia-inducible factor-1alpha through reducing hypoxia-inducible factor-1alpha stability and inhibiting its protein synthesis. Mol. Cancer Ther. 2007, 6, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Tanwar, H.; Jayashankar, B.; Sharma, J.; Murthy, S.; Chanda, S.; Singh, S.B.; Ganju, L. Quercetin exhibits adjuvant activity by enhancing Th2 immune response in ovalbumin immunized mice. Biomed. Pharmacother. 2017, 90, 354–360. [Google Scholar] [CrossRef]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin Induces Death of Prostate Cancer Cells by Inducing ROS and ER Stress. J. Cell. Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef]
- Salimi, A.; Roudkenar, M.H.; Seydi, E.; Sadeghi, L.; Mohseni, A.; Pirahmadi, N.; Pourahmad, J. Chrysin as an Anti-Cancer Agent Exerts Selective Toxicity by Directly Inhibiting Mitochondrial Complex II and V in CLL B-lymphocytes. Cancer Investig. 2017, 35, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.L.; Fang, J.Y.; Chen, M.; Wu, C.J.; Huang, C.C.; Hung, C.F. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J. Agric. Food Chem. 2011, 59, 8391–8400. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Wu, N.L.; Pu, C.M.; Hsiao, C.Y.; Chang, D.C.; Hung, C.F. Chrysin alleviates imiquimod-induced psoriasis-like skin inflammation and reduces the release of CCL20 and antimicrobial peptides. Sci. Rep. 2020, 10, 2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.M.; Chang, H.; Li, S.Y.; Wu, I.H.; Chiu, J.H. Chrysin inhibits lipopolysaccharide-induced angiogenesis via down-regulation of VEGF/VEGFR-2(KDR) and IL-6/IL-6R pathways. Planta Med. 2006, 72, 708–714. [Google Scholar] [CrossRef]
- Kalra, S.; Mukherjee, J.J.; Venkataraman, S.; Bantwal, G.; Shaikh, S.; Saboo, B.; Das, A.K.; Ramachandran, A. Hypoglycemia: The neglected complication. Indian J. Endocrinol. Metab. 2013, 17, 819–834. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol. Res. 2015, 99, 137–148. [Google Scholar] [CrossRef]
- Rodriguez, M.L.; Perez, S.; Mena-Molla, S.; Desco, M.C.; Ortega, A.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid Med. Cell. Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef] [Green Version]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Makin, R.D.; Apicella, I.; Nagasaka, Y.; Kaneko, H.; Turner, S.D.; Kerur, N.; Ambati, J.; Gelfand, B.D. RF/6A Chorioretinal Cells Do Not Display Key Endothelial Phenotypes. Invest. Ophthalmol. Vis. Sci. 2018, 59, 5795–5802. [Google Scholar] [CrossRef]
- Sardar Pasha, S.P.B.; Sishtla, K.; Sulaiman, R.S.; Park, B.; Shetty, T.; Shah, F.; Fishel, M.L.; Wikel, J.H.; Kelley, M.R.; Corson, T.W. Ref-1/APE1 Inhibition with Novel Small Molecules Blocks Ocular Neovascularization. J. Pharmacol. Exp. Ther. 2018, 367, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.; Zhou, L.; Zhang, T.; Lu, B.; Sheng, Y.; Ji, L. Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vasc. Pharmacol. 2018, 101, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, S.; Angayarkanni, N. Chebulagic acid Chebulinic acid and Gallic acid, the active principles of Triphala, inhibit TNFalpha induced pro-angiogenic and pro-inflammatory activities in retinal capillary endothelial cells by inhibiting p38, ERK and NFkB phosphorylation. Vasc. Pharmacol. 2018, 108, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Du, J.; Yao, Y.; Yao, G.; Wang, X. Adiponectin inhibits high glucose-induced angiogenesis via inhibiting autophagy in RF/6A cells. J. Cell. Physiol. 2019, 234, 20566–20576. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bai, Y.; Zhao, M.; Huang, L.; Li, S.; Li, X.; Chen, Y. Quercetin inhibits vascular endothelial growth factor-induced choroidal and retinal angiogenesis in vitro. Ophthalmic Res. 2015, 53, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Jiang, F.; Tang, Y.T.; Si, M.Y.; Jiao, X.Y. The association of serum vascular endothelial growth factor and ferritin in diabetic microvascular disease. Diabetes Technol. Ther. 2014, 16, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Tilton, R.G.; Kawamura, T.; Chang, K.C.; Ido, Y.; Bjercke, R.J.; Stephan, C.C.; Brock, T.A.; Williamson, J.R. Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J. Clin. Investig. 1997, 99, 2192–2202. [Google Scholar] [CrossRef]
- Lin, C.M.; Shyu, K.G.; Wang, B.W.; Chang, H.; Chen, Y.H.; Chiu, J.H. Chrysin suppresses IL-6-induced angiogenesis via down-regulation of JAK1/STAT3 and VEGF: An in vitro and in ovo approach. J. Agric. Food Chem. 2010, 58, 7082–7087. [Google Scholar] [CrossRef]
- Rauf, A.; Khan, R.; Raza, M.; Khan, H.; Pervez, S.; De Feo, V.; Maione, F.; Mascolo, N. Suppression of inflammatory response by chrysin, a flavone isolated from Potentilla evestita Th. Wolf. In silico predictive study on its mechanistic effect. Fitoterapia 2015, 103, 129–135. [Google Scholar] [CrossRef]
- Ha, S.K.; Moon, E.; Kim, S.Y. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-kappaB and JNK activations in microglia cells. Neurosci. Lett. 2010, 485, 143–147. [Google Scholar] [CrossRef]
- Yao, J.; Jiang, M.; Zhang, Y.; Liu, X.; Du, Q.; Feng, G. Chrysin alleviates allergic inflammation and airway remodeling in a murine model of chronic asthma. Int. Immunopharmacol. 2016, 32, 24–31. [Google Scholar] [CrossRef]
- Rashid, S.; Ali, N.; Nafees, S.; Hasan, S.K.; Sultana, S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem. Toxicol. 2014, 66, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Anandhi, R.; Annadurai, T.; Anitha, T.S.; Muralidharan, A.R.; Najmunnisha, K.; Nachiappan, V.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and antioxidative effects of an extract of the oyster mushroom, Pleurotus ostreatus, and its major constituent, chrysin, in Triton WR-1339-induced hypercholesterolemic rats. J. Physiol. Biochem. 2013, 69, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gong, L.; Jin, H.; Pi, J.; Bai, H.; Wang, H.; Cai, H.; Yang, P.; Cai, J. Chrysin-organogermanium (IV) complex induced Colo205 cell apoptosis-associated mitochondrial function and anti-angiogenesis. Scanning 2015, 37, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Moon, K.Y.; Lee, S.C.; Kim, S.S. Inhibition of Hypoxia-Inducible Factor-1 alpha and Vascular Endothelial Growth Factor by Chrysin in a Rat Model of Choroidal Neovascularization. Int. J. Mol. Sci. 2020, 21, 2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetze, S.; Bungenstock, A.; Czupalla, C.; Eilers, F.; Stawowy, P.; Kintscher, U.; Spencer-Hansch, C.; Graf, K.; Nurnberg, B.; Law, R.E.; et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension 2002, 40, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Wang, B. Nutrient distributions and their limitation on phytoplankton in the Yellow Sea and the East China Sea. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2003, 14, 1122–1126. [Google Scholar]
- Crean, J.K.; Finlay, D.; Murphy, M.; Moss, C.; Godson, C.; Martin, F.; Brady, H.R. The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J. Biol. Chem. 2002, 277, 44187–44194. [Google Scholar] [CrossRef] [Green Version]
- Goetze, S.; Eilers, F.; Bungenstock, A.; Kintscher, U.; Stawowy, P.; Blaschke, F.; Graf, K.; Law, R.E.; Fleck, E.; Grafe, M. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochem. Biophys. Res. Commun. 2002, 293, 1431–1437. [Google Scholar] [CrossRef]
- Cobb, M.H. MAP kinase pathways. Prog. Biophys. Mol. Biol. 1999, 71, 479–500. [Google Scholar] [CrossRef]
- Shi, Y.H.; Wang, Y.X.; Bingle, L.; Gong, L.H.; Heng, W.J.; Li, Y.; Fang, W.G. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: Involvement of PI-3K/Akt and MEK1/ERK pathways. J. Pathol. 2005, 205, 530–536. [Google Scholar] [CrossRef]
- Du, J.; Xu, R.; Hu, Z.; Tian, Y.; Zhu, Y.; Gu, L.; Zhou, L. PI3K and ERK-induced Rac1 activation mediates hypoxia-induced HIF-1alpha expression in MCF-7 breast cancer cells. PLoS ONE 2011, 6, e25213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Itagaki, T.; Yamagishi, K.; Ohkuma, H.; Uyama, M. A role of the retinal pigment epithelium in the involution of subretinal neovascularization. Nippon Ganka Gakkai Zasshi 1990, 94, 340–351. [Google Scholar] [PubMed]
- Huber, R.J.; O’Day, D.H. Extracellular matrix dynamics and functions in the social amoeba Dictyostelium: A critical review. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Prekeris, R. The regulation of MMP targeting to invadopodia during cancer metastasis. Front. Cell Dev. Biol. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baharivand, N.; Zarghami, N.; Panahi, F.; Dokht Ghafari, M.Y.; Mahdavi Fard, A.; Mohajeri, A. Relationship between vitreous and serum vascular endothelial growth factor levels, control of diabetes and microalbuminuria in proliferative diabetic retinopathy. Clin. Ophthalmol. 2012, 6, 185–191. [Google Scholar] [PubMed] [Green Version]
- Amin, R.H.; Frank, R.N.; Kennedy, A.; Eliott, D.; Puklin, J.E.; Abrams, G.W. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 1997, 38, 36–47. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Z.-Y.; Liang, I.-C.; Li, H.-J.; Wu, C.-C.; Lo, H.-M.; Chang, D.-C.; Hung, C.-F. Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation. Int. J. Mol. Sci. 2020, 21, 5541. https://doi.org/10.3390/ijms21155541
Liao Z-Y, Liang I-C, Li H-J, Wu C-C, Lo H-M, Chang D-C, Hung C-F. Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation. International Journal of Molecular Sciences. 2020; 21(15):5541. https://doi.org/10.3390/ijms21155541
Chicago/Turabian StyleLiao, Zhen-Yu, I-Chia Liang, Hsin-Ju Li, Chia-Chun Wu, Huey-Ming Lo, Der-Chen Chang, and Chi-Feng Hung. 2020. "Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation" International Journal of Molecular Sciences 21, no. 15: 5541. https://doi.org/10.3390/ijms21155541
APA StyleLiao, Z.-Y., Liang, I.-C., Li, H.-J., Wu, C.-C., Lo, H.-M., Chang, D.-C., & Hung, C.-F. (2020). Chrysin Inhibits High Glucose-Induced Migration on Chorioretinal Endothelial Cells via VEGF and VEGFR Down-Regulation. International Journal of Molecular Sciences, 21(15), 5541. https://doi.org/10.3390/ijms21155541





