Use of Intravenous Immunoglobulins in Sepsis Therapy—A Clinical View
Abstract
1. Introduction
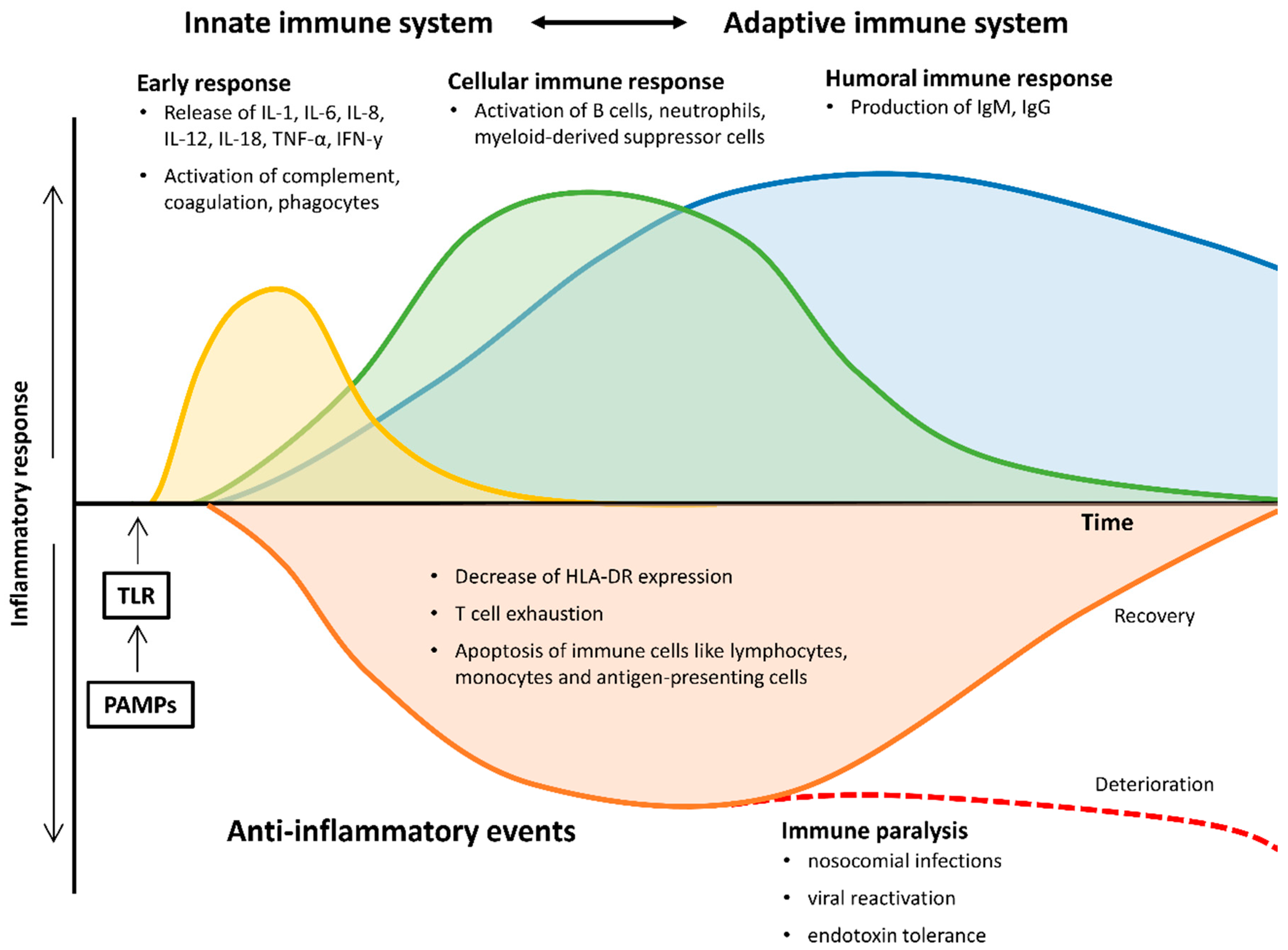
2. The Changing Immune System in Sepsis
2.1. Early Pro- and Anti-Inflammatory Responses
2.2. Late and Persistent Immunosuppressive Events
2.3. Monocytes and Antigen-Presenting Cells
2.4. Neutrophils
2.5. Myeloid-Derived Suppressor Cells
2.6. B-Lymphocytes
2.7. Immunoglobulins
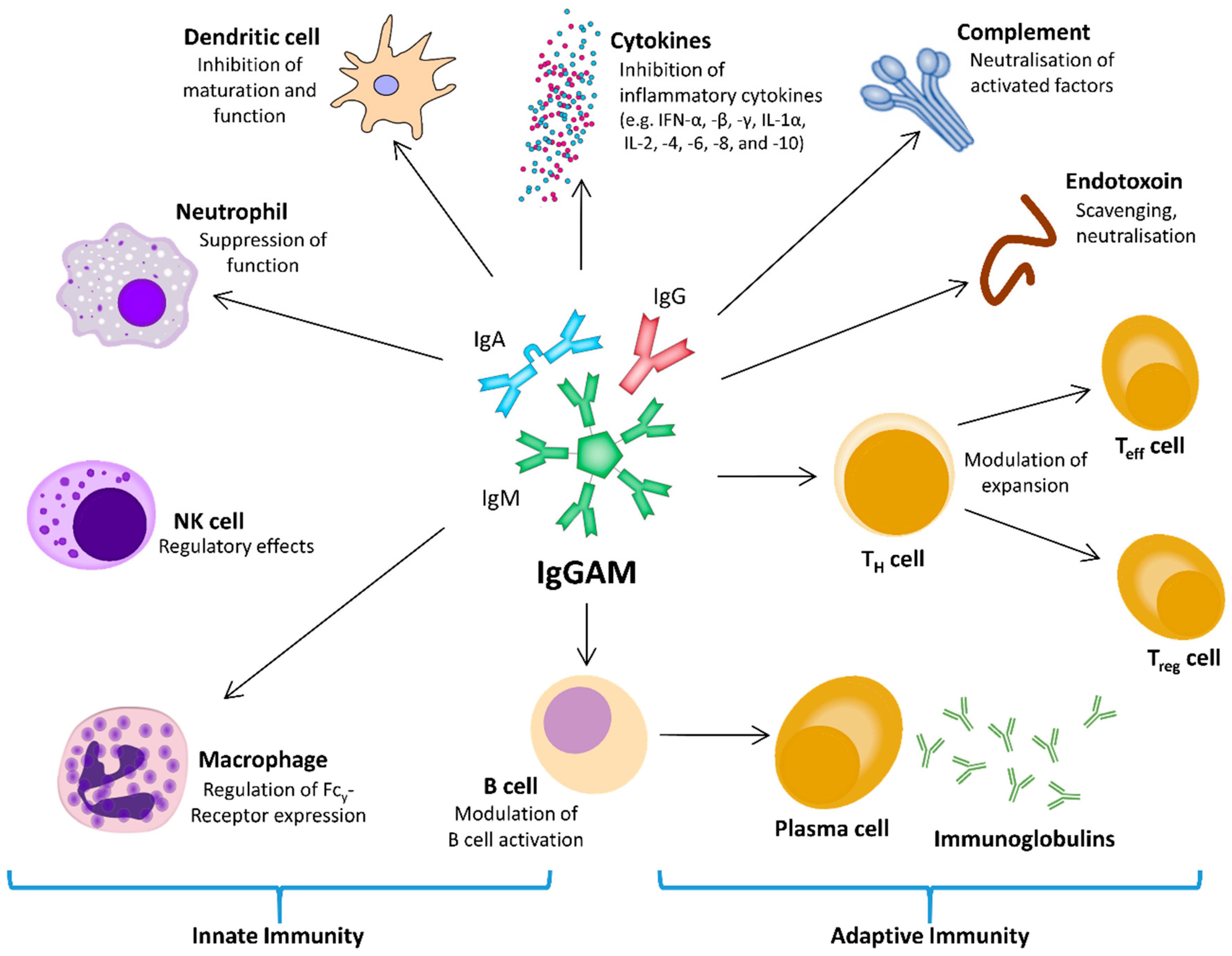
3. Considerations for the Therapeutic Use of Immunoglobulins
4. Immunoglobulins in Clinical Use
The Questions of “Who?”, “When?” and “How Much?”
5. A Novel Situation in COVID-19
6. Conclusions
- The use of IVIg in sepsis and septic shock appears to be safe.
- The use of IgGAM shows beneficial effects on sepsis-related inflammation and coagulopathy.
- For adjunctive sepsis therapy with IVIg, IgM-enriched formulations may be advantageous for specific patients.
- Further clinical data are urgently needed to be able to make definitive statements on the cost-benefit ratio.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APC | antigen-presenting cell |
| CRP | C-reactive protein |
| DAMP | damage associated molecular pattern |
| DNA | desoxyribonucleic acid |
| HLA-DR | human leukocyte antigen-D related |
| ICU | intensive care unit |
| IFN | interferon |
| IL | interleukin |
| IL-1ra | IL-1 receptor antagonist |
| Ig | immunoglobulin(s) |
| IVIg | intravenous immunoglobulin |
| IgGAM | immunoglobulin G/A/M |
| MDR | multi drug resistant |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility |
| NET | neutrophil extracellular trap |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK cell | natural killer cell |
| PAMP | pathogen-associated molecular pattern |
| PCT | procalcitonin |
| PRR | pathogen recognition receptor |
| qSOFA | quick sepsis-related organ failure |
| TCGF | T cell growth factor |
| Teff cell | effector T cell |
| TGF-β | transforming growth factor-β |
| TH cell | helper T cell |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
| Treg cell | regulatory T cell |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 19902–017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Rubio, I.; Osuchowski, M.F.; Shankar-Hari, M.; Skirecki, T.; Winkler, M.S.; Lachmann, G.; La Rosee, P.; Monneret, G.; Venet, F.; Bauer, M.; et al. Current gaps in sepsis immunology: New opportunities for translational research. Lancet Infect. Dis. 2019, 19, e422–e436. [Google Scholar] [CrossRef]
- Tang, B.M.; Huang, S.J.; McLean, A.S. Genome-wide transcription profiling of human sepsis: A systematic review. Crit. Care 2010, 14, R237. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Fernandez, A.; Almansa, R.; Carrasco, E.; Heredia, M.; Lajo, C.; Goncalves, L.; Gomez-Herreras, J.I.; de Lejarazu, R.O.; Bermejo-Martin, J.F. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 2011, 22, 82–87. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Andaluz-Ojeda, D.; Bobillo, F.; Iglesias, V.; Almansa, R.; Rico, L.; Gandia, F.; Resino, S.; Tamayo, E.; de Lejarazu, R.O.; Bermejo-Martin, J.F. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine 2012, 57, 332–336. [Google Scholar] [CrossRef]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Rauch, P.J.; Chudnovskiy, A.; Robbins, C.S.; Weber, G.F.; Etzrodt, M.; Hilgendorf, I.; Tiglao, E.; Figueiredo, J.L.; Iwamoto, Y.; Theurl, I.; et al. Innate response activator B cells protect against microbial sepsis. Science 2012, 335, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Scumpia, K.M.; Scumpia, P.O.; Weinstein, J.S.; Delano, M.J.; Cuenca, A.G.; Nacionales, D.C.; Wynn, J.L.; Lee, P.Y.; Kumagai, Y.; Efron, P.A.; et al. B cells enhance early innate immune responses during bacterial sepsis. J. Exp. Med. 2011, 208, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Mathur, P.; Kumar, S.; Gupta, A.; Gupta, D.; John, N.V.; Varghese, P.; Misra, M.C. Depressed Monocytic Activity may be a Predictor for Sepsis. J. Lab. Physicians 2015, 7, 26–31. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Jin, H.; Yan, J.; Liang, H.P. Alterations of dendritic cells in sepsis: Featured role in immunoparalysis. BioMed Res. Int. 2015, 2015, 903720. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Chang, K.C.; Cobb, J.P.; Buchman, T.G.; Korsmeyer, S.J.; Karl, I.E. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 14541–14546. [Google Scholar] [CrossRef]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.M.; Compton, S.M.; Hotchkiss, R.S. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef]
- Venet, F.; Rimmele, T.; Monneret, G. Management of Sepsis-Induced Immunosuppression. Crit. Care Clin. 2018, 34, 97–106. [Google Scholar] [CrossRef]
- Venet, F.; Davin, F.; Guignant, C.; Larue, A.; Cazalis, M.A.; Darbon, R.; Allombert, C.; Mougin, B.; Malcus, C.; Poitevin-Later, F.; et al. Early assessment of leukocyte alterations at diagnosis of septic shock. Shock 2010, 34, 358–363. [Google Scholar] [CrossRef]
- Cazalis, M.A.; Friggeri, A.; Cave, L.; Demaret, J.; Barbalat, V.; Cerrato, E.; Lepape, A.; Pachot, A.; Monneret, G.; Venet, F. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit. Care 2013, 17, R287. [Google Scholar] [CrossRef]
- Kjaergaard, A.G.; Nielsen, J.S.; Tonnesen, E.; Krog, J. Expression of NK cell and monocyte receptors in critically ill patients--potential biomarkers of sepsis. Scand. J. Immunol. 2015, 81, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Shalova, I.N.; Lim, J.Y.; Chittezhath, M.; Zinkernagel, A.S.; Beasley, F.; Hernandez-Jimenez, E.; Toledano, V.; Cubillos-Zapata, C.; Rapisarda, A.; Chen, J.; et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1alpha. Immunity 2015, 42, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E., Jr.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Scumpia, P.O.; Weinstein, J.S.; Coco, D.; Nagaraj, S.; Kelly-Scumpia, K.M.; O’Malley, K.A.; Wynn, J.L.; Antonenko, S.; Al-Quran, S.Z.; et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007, 204, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Taneja, R.; Sharma, A.P.; Hallett, M.B.; Findlay, G.P.; Morris, M.R. Immature circulating neutrophils in sepsis have impaired phagocytosis and calcium signaling. Shock 2008, 30, 618–622. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Lou, J.; Zhou, Y.; Bo, L.; Zhu, J.; Zhu, K.; Wan, X.; Cai, Z.; Deng, X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit. Care 2011, 15, R70. [Google Scholar] [CrossRef]
- De Pablo, R.; Monserrat, J.; Prieto, A.; Alvarez-Mon, M. Role of circulating lymphocytes in patients with sepsis. BioMed Res. Int. 2014, 2014, 671087. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Hynninen, M.; Pettila, V.; Takkunen, O.; Orko, R.; Jansson, S.E.; Kuusela, P.; Renkonen, R.; Valtonen, M. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis. Shock 2003, 20, 1–4. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Datta, D.; Wilson, J.; Assi, V.; Stephen, J.; Weir, C.J.; Rennie, J.; Antonelli, J.; Bateman, A.; Felton, J.M.; et al. Early PREdiction of sepsis using leukocyte surface biomarkers: The ExPRES-sepsis cohort study. Intensiv. Care Med. 2018, 44, 1836–1848. [Google Scholar] [CrossRef]
- Pena, O.M.; Hancock, D.G.; Lyle, N.H.; Linder, A.; Russell, J.A.; Xia, J.; Fjell, C.D.; Boyd, J.H.; Hancock, R.E. An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. EBioMedicine 2014, 1, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Drifte, G.; Dunn-Siegrist, I.; Tissieres, P.; Pugin, J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013, 41, 820–832. [Google Scholar] [CrossRef]
- Daix, T.; Guerin, E.; Tavernier, E.; Mercier, E.; Gissot, V.; Herault, O.; Mira, J.P.; Dumas, F.; Chapuis, N.; Guitton, C.; et al. Multicentric Standardized Flow Cytometry Routine Assessment of Patients With Sepsis to Predict Clinical Worsening. Chest 2018, 154, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.E.; Walstein, K.; Vollger, L.; Reuner, F.; Bick, A.; Dotsch, A.; Engler, A.; Peters, J.; von Kockritz-Blickwede, M.; Schafer, S.T. Neutrophil extracellular trap formation and nuclease activity in septic patients. BMC Anesthesiol. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.; Pittet, J.F. The coagulopathy of acute sepsis. Curr. Opin. Anaesthesiol. 2015, 28, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Lu, T.; Kobayashi, S.D.; Quinn, M.T.; Deleo, F.R. A NET Outcome. Front. Immunol. 2012, 3, 365. [Google Scholar] [CrossRef]
- Ortmann, W.; Kolaczkowska, E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018, 371, 473–488. [Google Scholar] [CrossRef]
- Camicia, G.; Pozner, R.; de Larranaga, G. Neutrophil extracellular traps in sepsis. Shock 2014, 42, 286–294. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Delano, M.J.; Kelly-Scumpia, K.M.; Moreno, C.; Scumpia, P.O.; Laface, D.M.; Heyworth, P.G.; Efron, P.A.; Moldawer, L.L. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 2011, 17, 281–292. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Durandy, A.; Kaveri, S.V.; Kuijpers, T.W.; Basta, M.; Miescher, S.; Ravetch, J.V.; Rieben, R. Intravenous immunoglobulins--understanding properties and mechanisms. Clin. Exp. Immunol. 2009, 158 (Suppl. 1), 2–13. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K. Innate response activator B cells: Origins and functions. Int. Immunol. 2015, 27, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Chousterman, B.G.; He, S.; Fenn, A.M.; Nairz, M.; Anzai, A.; Brenner, T.; Uhle, F.; Iwamoto, Y.; Robbins, C.S.; et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 2015, 347, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, B.G.; Park, C.J.; Kim, S.; Kim, D.H.; Jang, S.; Hong, S.K.; Chi, H.S. An extended leukocyte differential count (16 types of circulating leukocytes) using the cytodiff flow cytometric system can provide informations for the discrimination of sepsis severity and prediction of outcome in sepsis patients. Cytom. Part B Clin. Cytom. 2013, 86, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.; de Pablo, R.; Diaz-Martin, D.; Rodriguez-Zapata, M.; de la Hera, A.; Prieto, A.; Alvarez-Mon, M. Early alterations of B cells in patients with septic shock. Crit. Care 2013, 17, R105. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, Q.; Zheng, Q.; Liu, X.; Wang, Y.; Xie, Z.; Liu, T.; Yang, F.; Gao, W.; Bai, X.; et al. Alterations of B Cells in Immunosuppressive Phase of Septic Shock Patients. Crit. Care Med. 2020, 48, 815–821. [Google Scholar] [CrossRef]
- Krautz, C.; Maier, S.L.; Brunner, M.; Langheinrich, M.; Giamarellos-Bourboulis, E.J.; Gogos, C.; Armaganidis, A.; Kunath, F.; Grutzmann, R.; Weber, G.F. Reduced circulating B cells and plasma IgM levels are associated with decreased survival in sepsis—A meta-analysis. J. Crit. Care 2018, 45, 71–75. [Google Scholar] [CrossRef]
- Andaluz-Ojeda, D.; Iglesias, V.; Bobillo, F.; Nocito, M.; Loma, A.M.; Nieto, C.; Ramos, E.; Gandia, F.; Rico, L.; Bermejo-Martin, J.F. Early levels in blood of immunoglobulin M and natural killer cells predict outcome in nonseptic critically ill patients. J. Crit. Care 2013, 28, 1110-e7–1110-e10. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Spencer, J.; Sewell, W.A.; Rowan, K.M.; Singer, M. Bench-to-bedside review: Immunoglobulin therapy for sepsis-biological plausibility from a critical care perspective. Crit. Care 2012, 16, 206. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Singer, M.; Spencer, J. Can Concurrent Abnormalities in Free Light Chains and Immunoglobulin Concentrations Identify a Target Population for Immunoglobulin Trials in Sepsis? Crit. Care Med. 2017, 45, 1829–1836. [Google Scholar] [CrossRef]
- Sjoberg, A.P.; Trouw, L.A.; Blom, A.M. Complement activation and inhibition: A delicate balance. Trends Immunol. 2009, 30, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J.; Naud, J.F.; Campisi, G.; Giannias, B.; Liu, S.; DiCarlo, A.; Ferri, L.E.; Pascual, J.L.; Tchervenkov, J.; Christou, N.V. Alteration of chemoattractant receptor expression regulates human neutrophil chemotaxis in vivo. Ann. Surg. 2002, 235, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Seely, A.J.; Pascual, J.L.; Christou, N.V. Science review: Cell membrane expression (connectivity) regulates neutrophil delivery, function and clearance. Crit. Care 2003, 7, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Arora, Y.K.; Walker, S.M. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens [see comment]. J. Clin. Investig. 1993, 91, 602–607. [Google Scholar] [CrossRef]
- Darville, T.; Milligan, L.B.; Laffoon, K.K. Intravenous immunoglobulin inhibits staphylococcal toxin-induced human mononuclear phagocyte tumor necrosis factor alpha production. Infect. Immun. 1997, 65, 366–372. [Google Scholar] [CrossRef]
- Bueno, C.; Criado, G.; McCormick, J.K.; Madrenas, J. T cell signalling induced by bacterial superantigens. Chem. Immunol. Allergy 2007, 93, 161–180. [Google Scholar] [CrossRef]
- Cinel, I.; Opal, S.M. Molecular biology of inflammation and sepsis: A primer. Crit. Care Med. 2009, 37, 291–304. [Google Scholar] [CrossRef]
- Aukrust, P.; Muller, F.; Svenson, M.; Nordoy, I.; Bendtzen, K.; Froland, S.S. Administration of intravenous immunoglobulin (IVIG) in vivo--down-regulatory effects on the IL-1 system. Clin. Exp. Immunol. 1999, 115, 136–143. [Google Scholar] [CrossRef]
- Sewell, W.A.; Jolles, S. Immunomodulatory action of intravenous immunoglobulin. Immunology 2002, 107, 387–393. [Google Scholar] [CrossRef]
- Bendtzen, K.; Hansen, M.B.; Ross, C.; Svenson, M. Detection of autoantibodies to cytokines. Mol. Biotechnol. 2000, 14, 251–261. [Google Scholar] [CrossRef]
- Menezes, M.C.; Benard, G.; Sato, M.N.; Hong, M.A.; Duarte, A.J. In vitro inhibitory activity of tumor necrosis factor alpha and interleukin-2 of human immunoglobulin preparations. Int. Arch. Allergy Immunol. 1997, 114, 323–328. [Google Scholar] [CrossRef]
- Ross, C.; Svenson, M.; Nielsen, H.; Lundsgaard, C.; Hansen, M.B.; Bendtzen, K. Increased in vivo antibody activity against interferon alpha, interleukin-1alpha, and interleukin-6 after high-dose Ig therapy. Blood 1997, 90, 2376–2380. [Google Scholar] [CrossRef] [PubMed]
- Werdan, K.; Pilz, G.; Bujdoso, O.; Fraunberger, P.; Neeser, G.; Schmieder, R.E.; Viell, B.; Marget, W.; Seewald, M.; Walger, P.; et al. Score-based immunoglobulin G therapy of patients with sepsis: The SBITS study. Crit. Care Med. 2007, 35, 2693–2701. [Google Scholar] [PubMed]
- Werdan, K.; Pilz, G.; Muller-Werdan, U.; Maas Enriquez, M.; Schmitt, D.V.; Mohr, F.W.; Neeser, G.; Schondube, F.; Schafers, H.J.; Haverich, A.; et al. Immunoglobulin G treatment of postcardiac surgery patients with score-identified severe systemic inflammatory response syndrome—The ESSICS study. Crit. Care Med. 2008, 36, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Group, I.C.; Brocklehurst, P.; Farrell, B.; King, A.; Juszczak, E.; Darlow, B.; Haque, K.; Salt, A.; Stenson, B.; Tarnow-Mordi, W. Treatment of neonatal sepsis with intravenous immune globulin. N. Engl. J. Med. 2011, 365, 1201–1211. [Google Scholar] [CrossRef]
- Cui, J.; Wei, X.; Lv, H.; Li, Y.; Li, P.; Chen, Z.; Liu, G. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: A meta-analysis with trial sequential analysis. Ann. Intensiv. Care 2019, 9, 27. [Google Scholar] [CrossRef]
- Kreymann, K.G.; de Heer, G.; Nierhaus, A.; Kluge, S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit. Care Med. 2007, 35, 2677–2685. [Google Scholar]
- Alejandria, M.M.; Lansang, M.A.; Dans, L.F.; Mantaring, J.B., III. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Tziolos, N.; Routsi, C.; Katsenos, C.; Tsangaris, I.; Pneumatikos, I.; Vlachogiannis, G.; Theodorou, V.; Prekates, A.; Antypa, E.; et al. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin. Microbiol. Infect. 2016, 22, 499–506. [Google Scholar] [CrossRef]
- Cavazzuti, I.; Serafini, G.; Busani, S.; Rinaldi, L.; Biagioni, E.; Buoncristiano, M.; Girardis, M. Early therapy with IgM-enriched polyclonal immunoglobulin in patients with septic shock. Intensiv. Care Med. 2014, 40, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Dellinger, R.P.; Ebelt, H.; Ferrer, M.; Opal, S.M.; Singer, M.; Vincent, J.L.; Werdan, K.; Martin-Loeches, I.; Almirall, J.; et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: A randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensiv. Care Med. 2018, 44, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Domizi, R.; Adrario, E.; Damiani, E.; Scorcella, C.; Carsetti, A.; Giaccaglia, P.; Casarotta, E.; Gabbanelli, V.; Pantanetti, S.; Lamura, E.; et al. IgM-enriched immunoglobulins (Pentaglobin) may improve the microcirculation in sepsis: A pilot randomized trial. Ann. Intensiv. Care 2019, 9, 135. [Google Scholar] [CrossRef]
- Hoffman, J.N.; Fertmann, J.M.; Vollmar, B.; Laschke, M.W.; Jauch, K.W.; Menger, M.D. Immunoglobulin M-enriched human intravenous immunoglobulins reduce leukocyte-endothelial cell interactions and attenuate microvascular perfusion failure in normotensive endotoxemia. Shock 2008, 29, 133–139. [Google Scholar] [CrossRef]
- Esen, F.; Orhun, G.; Ozcan, P.E.; Senturk, E.; Kucukerden, M.; Giris, M.; Akcan, U.; Yilmaz, C.U.; Orhan, N.; Arican, N.; et al. Neuroprotective effects of intravenous immunoglobulin are mediated through inhibition of complement activation and apoptosis in a rat model of sepsis. Intensiv. Care Med. Exp. 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Esen, F.; Senturk, E.; Ozcan, P.E.; Ahishali, B.; Arican, N.; Orhan, N.; Ekizoglu, O.; Kucuk, M.; Kaya, M. Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit. Care Med. 2012, 40, 1214–1220. [Google Scholar] [CrossRef]
- Behre, G.; Schedel, I.; Nentwig, B.; Wormann, B.; Essink, M.; Hiddemann, W. Endotoxin concentration in neutropenic patients with suspected gram-negative sepsis: Correlation with clinical outcome and determination of anti-endotoxin core antibodies during therapy with polyclonal immunoglobulin M-enriched immunoglobulins. Antimicrob. Agents Chemother. 1992, 36, 2139–2146. [Google Scholar] [CrossRef]
- El-Nawawy, A.; El-Kinany, H.; Hamdy El-Sayed, M.; Boshra, N. Intravenous polyclonal immunoglobulin administration to sepsis syndrome patients: A prospective study in a pediatric intensive care unit. J. Trop. Pediatr. 2005, 51, 271–278. [Google Scholar] [CrossRef]
- Capasso, L.; Borrelli, A.C.; Parrella, C.; Lama, S.; Ferrara, T.; Coppola, C.; Catania, M.R.; Iula, V.D.; Raimondi, F. Are IgM-enriched immunoglobulins an effective adjuvant in septic VLBW infants? Ital. J. Pediatr. 2013, 39, 63. [Google Scholar] [CrossRef][Green Version]
- Wand, S.; Klages, M.; Kirbach, C.; Warszawska, J.; Meybohm, P.; Zacharowski, K.; Koch, A. IgM-Enriched Immunoglobulin Attenuates Systemic Endotoxin Activity in Early Severe Sepsis: A Before-After Cohort Study. PLoS ONE 2016, 11, e0160907. [Google Scholar] [CrossRef]
- Stehr, S.N.; Knels, L.; Weissflog, C.; Schober, J.; Haufe, D.; Lupp, A.; Koch, T.; Heller, A.R. Effects of IGM-enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemia. Shock 2008, 29, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Rieben, R.; Roos, A.; Muizert, Y.; Tinguely, C.; Gerritsen, A.F.; Daha, M.R. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation. Blood 1999, 93, 942–951. [Google Scholar] [CrossRef]
- Walpen, A.J.; Laumonier, T.; Aebi, C.; Mohacsi, P.J.; Rieben, R. Immunoglobulin M-enriched intravenous immunoglobulin inhibits classical pathway complement activation, but not bactericidal activity of human serum. Xenotransplantation 2004, 11, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Nachbaur, D.; Herold, M.; Gachter, A.; Niederwieser, D. Modulation of alloimmune response in vitro by an IgM-enriched immunoglobulin preparation (Pentaglobin). Immunology 1998, 94, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef]
- Ghelani, A.; Bates, D.; Conner, K.; Wu, M.Z.; Lu, J.; Hu, Y.L.; Li, C.M.; Chaudhry, A.; Sohn, S.J. Defining the Threshold IL-2 Signal Required for Induction of Selective Treg Cell Responses Using Engineered IL-2 Muteins. Front. Immunol. 2020, 11, 1106. [Google Scholar] [CrossRef]
- Paul, W.E.; Zhu, J. How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef]
- Dantal, J. Intravenous immunoglobulins: In-depth review of excipients and acute kidney injury risk. Am. J. Nephrol. 2013, 38, 275–284. [Google Scholar] [CrossRef]
- Katz, U.; Achiron, A.; Sherer, Y.; Shoenfeld, Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun. Rev. 2007, 6, 257–259. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensiv. Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Azevedo, L.C.; Park, M.; Schettino, G.P. Novel potential therapies for septic shock. Shock 2008, 30 (Suppl. 1), 60–66. [Google Scholar] [CrossRef] [PubMed]
- Vaschetto, R.; Clemente, N.; Pagni, A.; Esposito, T.; Longhini, F.; Mercalli, F.; Boggio, E.; Boldorini, R.; Chiocchetti, A.; Dianzani, U.; et al. A double blind randomized experimental study on the use of IgM-enriched polyclonal immunoglobulins in an animal model of pneumonia developing shock. Immunobiology 2017, 222, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Barratt-Due, A.; Sokolov, A.; Gustavsen, A.; Hellerud, B.C.; Egge, K.; Pischke, S.E.; Lindstad, J.K.; Pharo, A.; Castellheim, A.; Thorgersen, E.B.; et al. Polyvalent immunoglobulin significantly attenuated the formation of IL-1beta in Escherichia coli-induced sepsis in pigs. Immunobiology 2013, 218, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Pantzaris, N.D.; Platanaki, C.; Lagadinou, M.; Papachristodoulou, E.; Velissaris, D. The use of IgM-enriched immunoglobulin in adult patients with sepsis. J. Crit. Care 2018, 47, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef]
- Cohen, J.; Vincent, J.L.; Adhikari, N.K.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A roadmap for future research. Lancet Infect. Dis. 2015, 15, 581–614. [Google Scholar] [CrossRef]
- Delano, M.J.; Ward, P.A. Sepsis-induced immune dysfunction: Can immune therapies reduce mortality? J. Clin. Investig. 2016, 126, 23–31. [Google Scholar] [CrossRef]
- Santacruz, C.A.; Pereira, A.J.; Celis, E.; Vincent, J.L. Which Multicenter Randomized Controlled Trials in Critical Care Medicine Have Shown Reduced Mortality? A Systematic Review. Crit. Care Med. 2019, 47, 1680–1691. [Google Scholar] [CrossRef]
- Geier, C.; Schroder, J.; Tamm, A.; Dietz, S.; Nuding, S.; Holder, K.; Khandanpour, O.; Werdan, K.; Ebelt, H. Influence of the serum levels of immunoglobulins on clinical outcomes in medical intensive-care patients. Med. Klin. Intensivmed. Notfallmed. 2017, 112, 30–37. [Google Scholar] [CrossRef]
- Soares, M.O.; Welton, N.J.; Harrison, D.A.; Peura, P.; Shankar-Hari, M.; Harvey, S.E.; Madan, J.J.; Ades, A.E.; Palmer, S.J.; Rowan, K.M. An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): Incorporating a systematic review, meta-analysis and value of information analysis. Health Technol. Assess. 2012, 16, 1–186. [Google Scholar] [CrossRef]
- Turgeon, A.F.; Hutton, B.; Fergusson, D.A.; McIntyre, L.; Tinmouth, A.A.; Cameron, D.W.; Hebert, P.C. Meta-analysis: Intravenous immunoglobulin in critically ill adult patients with sepsis. Ann. Intern. Med. 2007, 146, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Antcliffe, D.B.; Gordon, A.C. Why Understanding Sepsis Endotypes Is Important for Steroid Trials in Septic Shock. Crit. Care Med. 2019, 47, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro da Silva, F.; Cesar Machado, M.C. Personalized Medicine for Sepsis. Am. J. Med. Sci. 2015, 350, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Giamarellos-Bourboulis, E.J. The beginning of personalized medicine in sepsis: Small steps to a bright future. Clin. Genet. 2014, 86, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.R.; Burchardi, H.; Schneider, H. Cost-effectiveness of immunoglobulin M-enriched immunoglobulin (Pentaglobin) in the treatment of severe sepsis and septic shock. J. Crit. Care 2005, 20, 239–249. [Google Scholar] [CrossRef]
- Grossmann, S.; Schroll, S.; Pfeifer, M. [Procalcitonin in the intensive care unit: Differential diagnostic and differential therapeutic possibilities]. Med. Klin. Intensivmed. Notfallmed. 2020. [Google Scholar] [CrossRef]
- Tseng, J.; Nugent, K. Utility of the shock index in patients with sepsis. Am. J. Med. Sci. 2015, 349, 531–535. [Google Scholar] [CrossRef]
- Berger, T.; Green, J.; Horeczko, T.; Hagar, Y.; Garg, N.; Suarez, A.; Panacek, E.; Shapiro, N. Shock index and early recognition of sepsis in the emergency department: Pilot study. West. J. Emerg. Med. 2013, 14, 168–174. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Culshaw, N.; Post, B.; Tamayo, E.; Andaluz-Ojeda, D.; Bermejo-Martin, J.F.; Dietz, S.; Werdan, K.; Beale, R.; Spencer, J.; et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: Systematic review and meta-analysis. Intensiv. Care Med. 2015, 41, 1393–1401. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Rodriguez-Fernandez, A.; Herran-Monge, R.; Andaluz-Ojeda, D.; Muriel-Bombin, A.; Merino, P.; Garcia-Garcia, M.M.; Citores, R.; Gandia, F.; Almansa, R.; et al. Immunoglobulins IgG1, IgM and IgA: A synergistic team influencing survival in sepsis. J. Intern. Med. 2014, 276, 404–412. [Google Scholar] [CrossRef]
- Berlot, G.; Vassallo, M.C.; Busetto, N.; Bianchi, M.; Zornada, F.; Rosato, I.; Tartamella, F.; Prisco, L.; Bigotto, F.; Bigolin, T.; et al. Relationship between the timing of administration of IgM and IgA enriched immunoglobulins in patients with severe sepsis and septic shock and the outcome: A retrospective analysis. J. Crit. Care 2012, 27, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Peters van Ton, A.M.; Kox, M.; Abdo, W.F.; Pickkers, P. Precision Immunotherapy for Sepsis. Front. Immunol. 2018, 9, 1926. [Google Scholar] [CrossRef]
- Winters, B.D.; Eberlein, M.; Leung, J.; Needham, D.M.; Pronovost, P.J.; Sevransky, J.E. Long-term mortality and quality of life in sepsis: A systematic review. Crit. Care Med. 2010, 38, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Derhovanessian, A.; De Cruz, S.; Belperio, J.A.; Deng, J.C.; Hoo, G.S. Subsequent infections in survivors of sepsis: Epidemiology and outcomes. J. Intensiv. Care Med. 2014, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Efron, P.A.; Mohr, A.M.; Bihorac, A.; Horiguchi, H.; Hollen, M.K.; Segal, M.S.; Baker, H.V.; Leeuwenburgh, C.; Moldawer, L.L.; Moore, F.A.; et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery 2018, 164, 178–184. [Google Scholar] [CrossRef]
- Schefold, J.C. Measurement of monocytic HLA-DR (mHLA-DR) expression in patients with severe sepsis and septic shock: Assessment of immune organ failure. Intensiv. Care Med. 2010, 36, 1810–1812. [Google Scholar] [CrossRef]
- Drewry, A.M.; Ablordeppey, E.A.; Murray, E.T.; Beiter, E.R.; Walton, A.H.; Hall, M.W.; Hotchkiss, R.S. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: A prospective observational study. Crit. Care 2016, 20, 334. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Meisel, C.; Fux, M.; Schefold, J.C. Assessment of immune organ dysfunction in critical illness: Utility of innate immune response markers. Intensiv. Care Med. Exp. 2017, 5, 49. [Google Scholar] [CrossRef]
- Berlot, G.; Vassallo, M.C.; Busetto, N.; Nieto Yabar, M.; Istrati, T.; Baronio, S.; Quarantotto, G.; Bixio, M.; Barbati, G.; Dattola, R.; et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann. Intensiv. Care 2018, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Molnar, Z.; Giamarellos-Bourboulis, E.J.; Kumar, A.; Nierhaus, A. Sepsis: Diagnostic and Therapeutic Challenges. BioMed Res. Int. 2016, 2016, 5786182. [Google Scholar] [CrossRef]
- Zumla, A.; Hui, D.S.; Azhar, E.I.; Memish, Z.A.; Maeurer, M. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 2020, 395, e35–e36. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Wei, Y.; Yue, H.; Wang, Y.; Hu, M.; Zhang, S.; Cao, T.; Yang, C.; Li, M.; et al. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann. Intern. Med. 2020, 172, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin. Immunol. 2020, 146, 119–127.e114. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J.; Luo, W.; Chen, T.; Qin, Q.; Deng, P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef]
- Vaninov, N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lutgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schroder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.M.; Wan, L.; Xiang, T.X.; Le, A.; Liu, J.M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet. Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef]
- Rello, J.; Valenzuela-Sanchez, F.; Ruiz-Rodriguez, M.; Moyano, S. Sepsis: A Review of Advances in Management. Adv. Ther. 2017, 34, 2393–2411. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.D.; Natanson, C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin. Investig. Drugs 2000, 9, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarczak, D.; Kluge, S.; Nierhaus, A. Use of Intravenous Immunoglobulins in Sepsis Therapy—A Clinical View. Int. J. Mol. Sci. 2020, 21, 5543. https://doi.org/10.3390/ijms21155543
Jarczak D, Kluge S, Nierhaus A. Use of Intravenous Immunoglobulins in Sepsis Therapy—A Clinical View. International Journal of Molecular Sciences. 2020; 21(15):5543. https://doi.org/10.3390/ijms21155543
Chicago/Turabian StyleJarczak, Dominik, Stefan Kluge, and Axel Nierhaus. 2020. "Use of Intravenous Immunoglobulins in Sepsis Therapy—A Clinical View" International Journal of Molecular Sciences 21, no. 15: 5543. https://doi.org/10.3390/ijms21155543
APA StyleJarczak, D., Kluge, S., & Nierhaus, A. (2020). Use of Intravenous Immunoglobulins in Sepsis Therapy—A Clinical View. International Journal of Molecular Sciences, 21(15), 5543. https://doi.org/10.3390/ijms21155543




