Changes in Novel AKI Biomarkers after Exercise. A Systematic Review
Abstract
1. Introduction
2. Results: Studies Concerning Novel Biomarkers of AKI after Exercise
2.1. Functional Biomarker—Serum Cystatin C
2.1.1. Changes in sCyst-C after a Marathon
2.1.2. Changes in sCyst-C after Exercises Shorter than a Marathon
2.1.3. Changes in sCyst-C after Longer Exercise than a Marathon
2.1.4. sCyst-C is a Better Marker of eGFR than sCr
2.1.5. Summary of Changes in sCyst-C
2.2. Plasma and Serum Damage Markers
2.2.1. Changes in Plasma NGAL (pNGAL) after Short Exercises
2.2.2. Changes in pNGAL or Serum NGAL (sNGAL) Levels after Long Exercises
2.2.3. Changes in pNGAL after Work in Heat
2.2.4. Summary of Changes in s/pNGAL
2.3. Urinary Damage Markers
2.3.1. Urinary Cystatin C (uCyst-C)
2.3.2. Changes in uNGAL and uKIM-1 after a Marathon
2.3.3. Changes in uNGAL after Exercises Shorter than a Marathon
2.3.4. Changes in uNGAL after Exercises Longer than a Marathon
2.3.5. Changes in uNGAL after Exercise in Heat
2.3.6. Summary of Changes in uNGAL
2.3.7. Changes in Urinary KIM
2.3.8. Summary of Changes in uKIM-1
2.3.9. Changes in Urinary L-FABP after Exercise
2.3.10. Other Studies Concerning Changes in uL-FABP
2.3.11. Urinary Interleukins
2.4. Pre-Injury Phase Biomarkers IGFBP-7/TIMP-2
2.5. Other Promising Markers of AKI (YKL-40, MCP-1 and TNF-alfa, Trefoil Factor 3 (TTF3), Calbindin)
3. Discussion
3.1. Limitations of the Studies Presented
3.2. Serum or Plasma Markers
3.3. Urinary Markers
3.4. Interpretation
- Exercise-induced renal impairment is commonly present but temporary,
- Severe complications of exercise, like AKI requiring hemodialysis, are rare,
- Repeated episodes of AKI lead to CKD,
- There is no data showing that CKD could be related to sports activity,
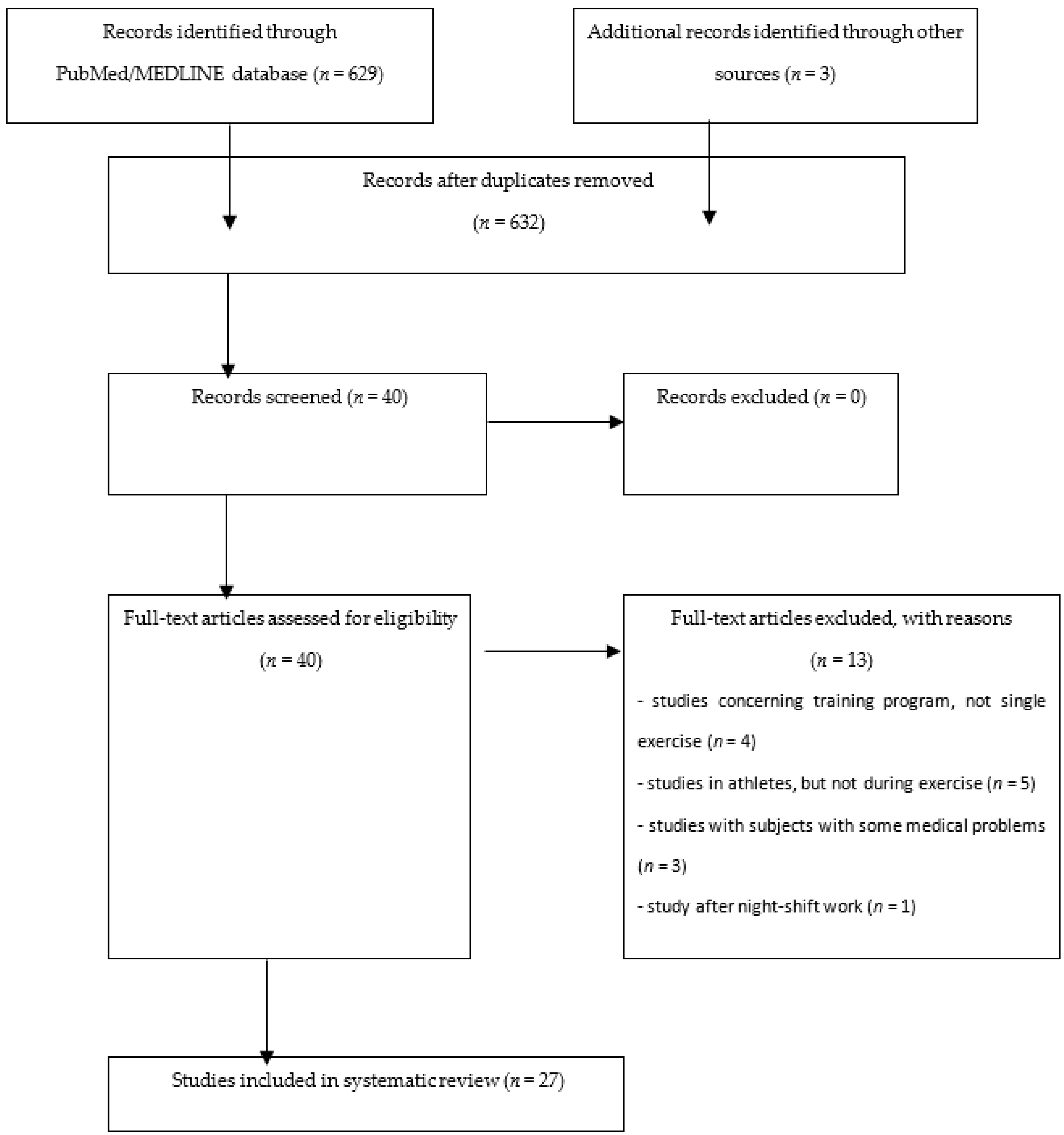
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| u | urinary |
| s | serum |
| p | plasma |
| NGAL | neutrophil gelatinase-associated lipocalin |
| KIM-1 | kidney injury molecule-1 |
| Cyst-C | cystatin C |
| Cr | creatinine |
| L-FABP | liver-type fatty-acid-binding protein |
| IL | interleukin |
| TTF3 | trefoil factor-3 |
| TNFα | tumor necrosis factor α |
| YKL-40 | chitinase 3-like protein 1 |
| MCP-1 | monocyte chemoattractant protein-1 |
| IGFBP7 | insulin-like growth factor binding protein 7 |
| TIMP-2 | tissue inhibitor of metalloproteinases-2 |
| uMarker/uCyst-C | urinary marker normalized to cystatin C |
| uMarker/uCr | urinary marker normalized to creatinine |
| uMarker/uOsm | urinary marker normalized to osmolality |
| uMarker/u.f. | urinary marker normalized to urine flow |
| eGFR | estimated glomerular filtration rate |
| CKD EPI | Chronic Kidney Disease Epidemiology Collaboration equation |
| HIIRT | high-intensity interval resistance training |
| VO2max | maximal oxygen consumption |
| HRmax | maximal heart rate |
| RH | relative humidity |
| OA | osteoarthiritis |
| MOF-VVPP | monomeric and oligomeric flavanols |
| VO2max | maximal oxygen consumption |
| MMP-9 | matrix metallopeptidase 9 |
References
- Armstrong, J.A. Urinalysis in Western culture: A brief history. Kidney Int. 2007, 71, 384–387. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.C. Dr Richard Bright—A man of many parts. His bicentenary year—1789–1858. Bristol Med. Chir. J. 1989, 104, 63–67. [Google Scholar] [PubMed]
- Gardner, K.D. Athletic pseudonephritis; alteration of urine sediment by athletic competition. J. Am. Med. Assoc. 1956, 161, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Feehally, J. Introduction to Glomerular Disease: Clinical Presetation. In Comprehensive Clinical Nephrology, 4th ed.; Floege, J., Johnson, R.J., Feehally, J., Eds.; Elsevier Inc.: New York, NY, USA, 2010; pp. 15–28. ISBN 978-0-323-05876-6. [Google Scholar]
- Oh, D.J. A long journey for acute kidney injury biomarkers. Ren. Fail. 2020, 42, 154–165. [Google Scholar] [CrossRef]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef]
- Rhee, E.P. How omics data can be used in nephrology. Am. J. Kidney Dis. 2018, 72, 129–135. [Google Scholar] [CrossRef]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017, 55, 1074–1089. [Google Scholar] [CrossRef]
- Liu, X.; Guan, Y.; Xu, S.; Li, Q.; Sun, Y.; Han, R.; Jiang, C. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney Blood Press Res. 2016, 41, 680–700. [Google Scholar] [CrossRef]
- Guzzi, L.M.; Bergler, T.; Binnall, B.; Engelman, D.T.; Forni, L.; Germain, M.J.; Gluck, E.; Göcze, I.; Joannidis, M.; Koyner, J.L.; et al. Clinical use of [TIMP-2]•[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: Guidance from an expert panel. Crit. Care 2019, 23, 225. [Google Scholar] [CrossRef]
- Jamieson, H.C. Some newer tests of renal function. Can. Med. Assoc. J. 1933, 29, 598–604. [Google Scholar]
- Narayanan, S.; Appleton, H.D. Creatinine: A review. Clin. Chem. 1980, 26, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Randers, E.; Kristensen, J.H.; Erlandsen, E.J.; Danielsen, H. Serum cystatin C as a marker of the renal function. Scand. J. Clin. Lab. Investig. 1998, 58, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, D.; Sud, K. Acute kidney injury and disease: Long-term consequences and management. BMJ Open Sport Exerc. Med. 2017, 3, e000093. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, L.E.; Walter, E.; Venn, R.M.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L.G. Acute kidney injury associated with endurance events-is it a cause for concern? A systematic review. Nephrology 2018, 23, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.S.A.; Junior, G.B.D.S.; Liborio, A.B.; Daher, E.D.F. Acute kidney injury due to rhabdomyolysis. Saudi J. Kidney Dis. Transplant. 2008, 19, 721–729. [Google Scholar]
- Lipman, G.S.; Shea, K.; Christensen, M.; Phillips, C.; Burns, P.; Higbee, R.; Koskenoja, V.; Eifling, K.; Krabak, B.J. Ibuprofen versus placebo effect on acute kidney injury in ultramarathons: A randomized controlled trial. Emerg. Med. J. 2017, 34, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.L.; Johnson, B.D.; Sackett, J.R.; Parker, M.D.; Schlader, Z.J. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R189–R198. [Google Scholar] [CrossRef]
- Kupferman, J.; Ramírez-Rubio, O.; Amador, J.J.; López-Pilarte, D.; Wilker, E.H.; Laws, R.L.; Sennett, C.; Robles, N.V.; Lau, J.L.; Salinas, A.J.; et al. Acute Kidney Injury in Sugarcane Workers at Risk for Mesoamerican Nephropathy. Am J Kidney Dis. 2018, 72, 475–482. [Google Scholar] [CrossRef]
- Mingels, A.; Jacobs, L.; Kleijnen, V.; Wodzig, W.; Dieijen-Visser, M. Cystatin C a marker for renal function after exercise. Int. J. Sports Med. 2009, 30, 668–671. [Google Scholar] [CrossRef]
- Odutayo, A.; Cherney, D. Cystatin C and acute changes in glomerular filtration rate. Clin. Nephrol. 2012, 78, 64–75. [Google Scholar] [CrossRef]
- Scherr, J.; Braun, S.; Schuster, T.; Hartmann, C.; Moehlenkamp, S.; Wolfarth, B.; Pressler, A.; Halle, M. 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med. Sci. Sports Exerc. 2011, 43, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Colar, J.M.; Geddes, T.; Gold, J.M.; Trivax, J.E. Changes in renal markers and acute kidney injury after marathon running. Nephrology 2011, 16, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Hewing, B.; Schattke, S.; Spethmann, S.; Sanad, W.; Schroeckh, S.; Schimke, I.; Halleck, F.; Peters, H.; Brechtel, L.; Lock, J.; et al. Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc. Ultrasound 2015, 13, 13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poortmans, J.R.; Gulbis, B.; De Bruyn, E.; Baudry, S.; Carpentier, A. Limitations of serum values to estimate glomerular filtration rate during exercise. Br. J. Sports Med. 2013, 47, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Bongers, C.C.W.G.; Alsady, M.; Nijenhuis, T.; Tulp, A.D.M.; Eijsvogels, T.M.H.; Deen, P.M.T.; Hopman, M.T.E. Impact of acute versus prolonged exercise and dehydration on kidney function and injury. Physiol. Rep. 2018, 6, e13734. [Google Scholar] [CrossRef] [PubMed]
- Poussel, M.; Touzé, C.; Allado, E.; Frimat, L.; Hily, O.; Thilly, N.; Rousseau, H.; Vauthier, J.C.; Chenuel, B. Ultramarathon and Renal Function: Does Exercise-Induced Acute Kidney Injury Really Exist in Common Conditions? Front. Sports Act. Living 2020, 1, 17. [Google Scholar] [CrossRef]
- Colombini, A.; Corsetti, R.; Machado, M.; Graziani, R.; Lombardi, G.; Lanteri, P.; Banfi, G. Serum creatine kinase activity and its relationship with renal function indices in professional cyclists during the Giro d’Italia 3-week stage race. Clin. J. Sport Med. 2012, 22, 408–413. [Google Scholar] [CrossRef]
- Colombini, A.; Corsetti, R.; Graziani, R.; Lombardi, G.; Lanteri, P.; Banfi, G. Evaluation of creatinine, cystatin C and eGFR by different equations in professional cyclists during the Giro d’Italia 3-weeks stage race. Scand. J. Clin. Lab. Investig. 2012, 72, 114–120. [Google Scholar] [CrossRef]
- Banfi, G.; Del Fabbro, M.; D’Eril, G.M.; Melegati, G. Reliability of cystatin C in estimating renal function in rugby players. Ann. Clin. Biochem. 2009, 46, 428. [Google Scholar] [CrossRef]
- Lousa, I.; Nascimento, H.; Rocha, S.; Catarino, C.; Reis, F.; Rêgo, C.; Santos-Silva, A.; Seabra, A.; Ribeiro, S.; Belo, L. Influence of the 6-month physical activity programs on renal function in obese boys. Pediatr. Res. 2018, 83, 1011–1015. [Google Scholar] [CrossRef]
- Beetham, K.S.; Howden, E.J.; Isbel, N.M.; Coombes, J.S. Agreement between cystatin-C and creatinine based eGFR estimates after a 12-month exercise intervention in patients with chronic kidney disease. BMC Nephrol. 2018, 18–19, 366. [Google Scholar] [CrossRef] [PubMed]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Junglee, N.A.; Lemmey, A.B.; Burton, M.; Searell, C.; Jones, D.; Lawley, J.S.; Jibani, M.M.; Macdonald, J.H. Does proteinuria-inducing physical activity increase biomarkers of acute kidney injury? Kidney Blood Press Res. 2012, 36, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Junglee, N.A.; Di Felice, U.; Dolci, A.; Fortes, M.B.; Jibani, M.M.; Lemmey, A.B.; Walsh, N.P.; Macdonald, J.H. Exercising in a hot environment with muscle damage: Effects on acute kidney injury biomarkers and kidney function. Am. J. Physiol. Ren. Physiol. 2013, 305, F813–F820. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Kaesser, U.; Eichner, G.; Bachmann, G.; Steinmeyer, J. Biomarkers of Hand Osteoarthritis Are Detectable after Mechanical Exercise. J. Clin. Med. 2019, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Sugama, K.; Sakuma, J.; Kawakami, Y.; Suzuki, K. Evaluation of serum leaking enzymes and investigation into new biomarkers for exercise-induced muscle damage. Exerc. Immunol. Rev. 2014, 20, 39–54. [Google Scholar] [PubMed]
- Rullman, E.; Olsson, K.; Wågsäter, D.; Gustafsson, T. Circulating MMP-9 during exercise in humans. Eur. J. Appl. Physiol. 2013, 113, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.P.; Smith, C.R.; Butts, C.L.; Caldwell, A.R.; Lee, E.C.; Vingren, J.L.; Munoz, C.X.; Kunces, L.J.; Williamson, K.; Ganio, M.S.; et al. Renal stress and kidney injury biomarkers in response to endurance cycling in the heat with and without ibuprofen. J. Sci. Med. Sport 2018, 21, 1180–1184. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Salvagno, G.L.; Aloe, R.; Schena, F.; Guidi, G.C. Variation of serum and urinary neutrophil gelatinase associated lipocalin (NGAL) after strenuous physical exercise. Clin. Chem. Lab. Med. 2012, 50, 1585–1589. [Google Scholar] [CrossRef]
- Andreazzoli, A.; Fossati, C.; Spaccamiglio, A.; Salvo, R.; Quaranta, F.; Minganti, C.; Di Luigi, L.; Borrione, P. Assessment of pN-GAL as a marker of renal function in elite cyclists during professional competitions. J. Biol. Regul. Homeost. Agents 2017, 31, 829–835. [Google Scholar]
- Mellor, A.; Boos, C.; Stacey, M.; Hooper, T.; Smith, C.; Begley, J.; Yarker, J.; Piper, R.; O’Hara, J.; King, R.; et al. Neutrophil gelatinase-associated lipocalin: Its response to hypoxia and association with acute mountain sickness. Dis. Markers 2013, 35, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.L.; Johnson, B.D.; Vargas, N.T.; Hostler, D.; Parker, M.D.; Schlader, Z.J. Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. J. Appl. Physiol. 2020, 128, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Bongers, C.C.W.G.; Alsady, M.; Nijenhuis, T.; Hartman, Y.A.W.; Eijsvogels, T.M.H.; Deen, P.M.T.; Hopman, M.T.E. Impact of acute versus repetitive moderate intensity endurance exercise on kidney injury markers. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Wołyniec, W.; Kasprowicz, K.; Giebułtowicz, J.; Korytowska, N.; Zorena, K.; Bartoszewicz, M.; Rita-Tkachenko, P.; Renke, M.; Ratkowski, W. Changes in Water Soluble Uremic Toxins and Urinary Acute Kidney Injury Biomarkers After 10- and 100-km Runs. Int. J. Environ. Res. Public Health 2019, 16, 4153. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.G.; Verma, G.; Pata, R.W.; Martin, T.G.; Perazella, M.A.; Parikh, C.R. Kidney Injury and Repair Biomarkers in Marathon Runners. Am. J. Kidney Dis. 2017, 70, 252–261. [Google Scholar] [CrossRef]
- Wołyniec, W.; Ratkowski, W.; Urbański, R.; Bartoszewicz, M.; Siluk, D.; Wołyniec, Z.; Kasprowicz, K.; Zorena, K.; Renke, M. Urinary Kidney Injury Molecule-1 but Not Urinary Neutrophil Gelatinase Associated Lipocalin Is Increased after Short Maximal Exercise. Nephron 2018, 138, 29–34. [Google Scholar] [CrossRef]
- Spada, T.C.; Silva, J.M.R.D.; Francisco, L.S.; Marçal, L.J.; Antonangelo, L.; Zanetta, D.M.T.; Yu, L.; Burdmann, E.A. High intensity resistance training causes muscle damage and increases biomarkers of acute kidney injury in healthy individuals. PLoS ONE 2018, 13, e0205791. [Google Scholar] [CrossRef]
- Semen, K.O.; van der Doelen, R.H.A.; van der Lugt, M.; van Dam, D.; Reimer, J.; Stassen, F.R.M.; Janssen, L.; Janssen, P.; Janssen, M.J.W.; Bast, A.; et al. Non- steroidal anti-inflammatory drugs increase urinary neutrophil gelatinase-associated lipocalin in recreational runners. Scand. J. Med. Sci. Sports 2020. [Google Scholar] [CrossRef]
- Semen, K.O.; Weseler, A.R.; Janssen, M.J.W.; Drittij-Reijnders, M.J.; le Noble, J.L.M.L.; Bast, A. Effects of Monomeric and Oligomeric Flavanols on Kidney Function, Inflammation and Oxidative Stress in Runners: A Randomized Double-Blind Pilot Study. Nutrients 2020, 12, 1634. [Google Scholar] [CrossRef]
- Jouffroy, R.; Lebreton, X.; Mansencal, N.; Anglicheau, D. Acute kidney injury during an ultra-distance race. PLoS ONE 2019, 14, e0222544. [Google Scholar] [CrossRef]
- Machado, J.C.Q.; Volpe, C.M.O.; Vasconcellos, L.S.; Nogueira-Machado, J.A. Quantification of NGAL in Urine of Endurance Cycling Athletes. J. Phys. Act. Health 2018, 15, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, K.; Kamijo-Ikemori, A.; Sugaya, T.; Kumamoto, S.; Tanahashi, K.; Kumagai, H.; Kimura, K.; Shibagaki, Y.; Maeda, S. Incremental short maximal exercise increases urinary liver-type fatty acid-binding protein in adults without CKD. Scand. J. Med. Sci. Sports 2020, 30, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Noiri, E.; Ono, Y.; Doi, K.; Negishi, K.; Kamijo, A.; Kimura, K.; Fujita, T.; Kinukawa, T.; Taniguchi, H.; et al. Renal L-type fatty acid—Binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 2007, 18, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, K.; Kamijo-Ikemori, A.; Yasuda, T.; Hotta, C.; Izawa, K.P.; Watanabe, S.; Sugaya, T.; Kimura, K. Moderate-intensity single exercise session does not induce renal damage. J. Clin. Lab. Anal. 2013, 27, 177–180. [Google Scholar] [CrossRef]
- Kosaki, K.; Kamijo-Ikemori, A.; Sugaya, T.; Tanahashi, K.; Kumagai, H.; Sawano, Y.; Akazawa, N.; Ra, S.G.; Kimura, K.; Shibagaki, Y.; et al. Relationship between exercise capacity and urinary liver-type fatty acid-binding protein in middle-aged and older individuals. Clin. Exp. Nephrol. 2017, 21, 810–817. [Google Scholar] [CrossRef]
- Kosaki, K.; Kamijo-Ikemori, A.; Sugaya, T.; Tanahashi, K.; Sawano, Y.; Akazawa, N.; Ra, S.G.; Kimura, K.; Shibagaki, Y.; Maeda, S. Effect of habitual exercise on urinary liver-type fatty acid-binding protein levels in middle-aged and older adults. Scand. J. Med. Sci. Sports 2018, 28, 152–160. [Google Scholar] [CrossRef]
- Uchiyama, K.; Washida, N.; Morimoto, K.; Muraoka, K.; Nakayama, T.; Adachi, K.; Kasai, T.; Miyashita, K.; Wakino, S.; Itoh, H. Effects of exercise on residual renal function in patients undergoing peritoneal dialysis: A post-hoc analysis of a randomized controlled trial. Ther. Apher. Dial. 2020. [Google Scholar] [CrossRef]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc. Immunol. Rev. 2013, 19, 29–48. [Google Scholar]
- Dutheil, F.; Trousselard, M.; Perrier, C.; Lac, G.; Chamoux, A.; Duclos, M.; Naughton, G.; Mnatzaganian, G.; Schmidt, J. Urinary interleukin-8 is a biomarker of stress in emergency physicians, especially with advancing age—The JOBSTRESS* randomized trial. PLoS ONE 2013, 8, e71658. [Google Scholar] [CrossRef]
- Pryor, R.R.; Pryor, J.L.; Vandermark, L.W.; Adams, E.L.; Brodeur, R.M.; Schlader, Z.J.; Armstrong, L.E.; Lee, E.C.; Maresh, C.M.; Casa, D.J. Acute Kidney Injury Biomarker Responses to Short-Term Heat Acclimation. Int. J. Environ. Res. Public Health 2020, 17, 1325. [Google Scholar] [CrossRef]
- Apeland, T.; Danielsen, T.; Staal, E.M.; Åsberg, A.; Thorsen, I.S.; Dalsrud, T.O.; Ørn, S. Risk factors for exertional rhabdomyolysis with renal stress. BMJ Open Sport Exerc. Med. 2017, 3, e000241. [Google Scholar] [CrossRef] [PubMed]
- Matomäki, P.; Kainulainen, H.; Kyröläinen, H. Corrected whole blood biomarkers—The equation of Dill and Costill revisited. Physiol. Rep. 2018, 6, e13749. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, H.; Li, H. Dehydration-associated chronic kidney disease: A novel case of kidney failure in China. BMC Nephrol. 2020, 21, 159. [Google Scholar] [CrossRef] [PubMed]
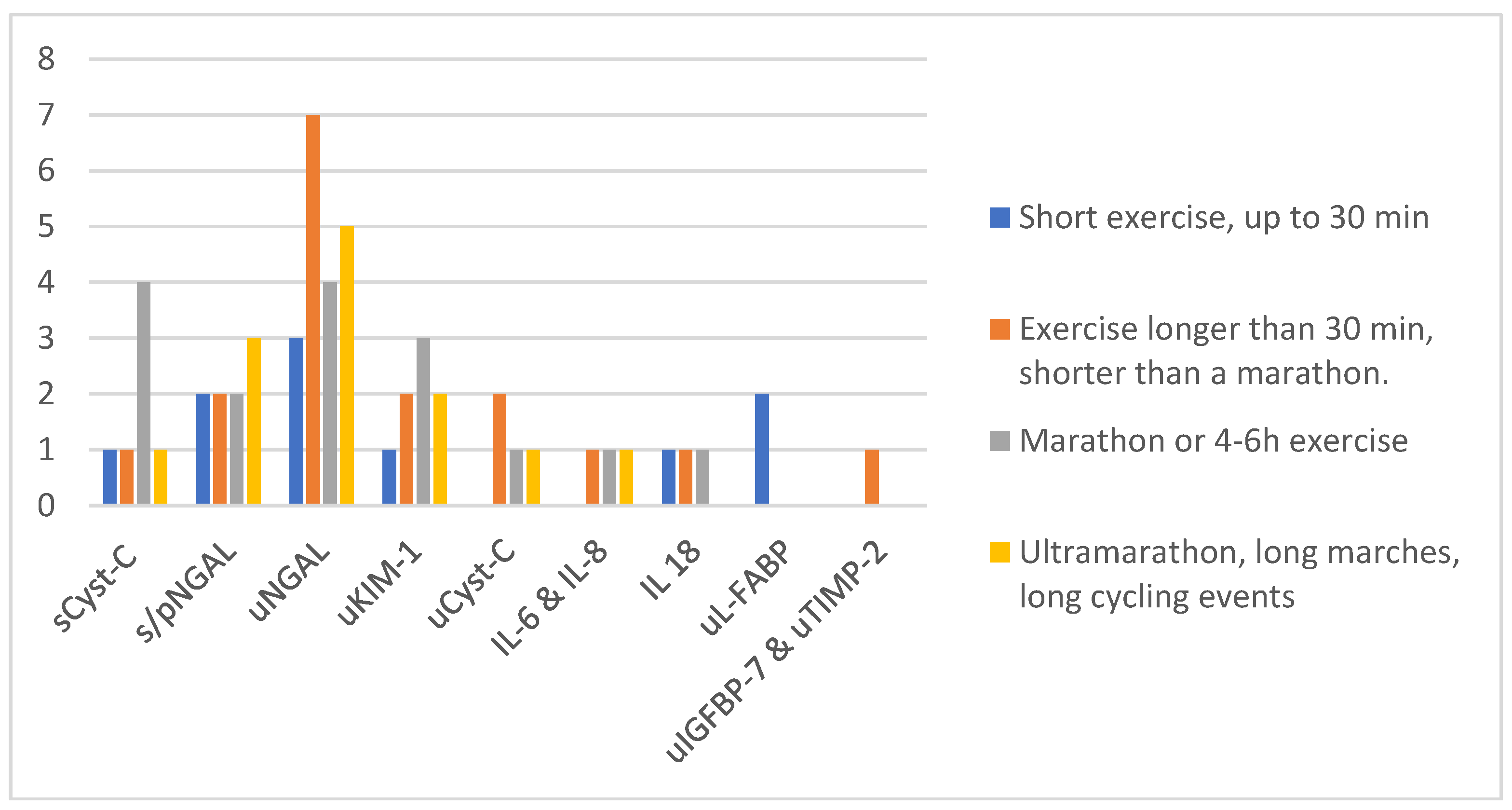
| Functional Biomarkers | Damage Biomarkers | Pre-Injury Phase Biomarkers |
|---|---|---|
| sCyst-C | uCyst-C uNGAL, sNGAL, pNGAL uKIM-1 uL-FABP uIL-6, uIL-8, uIL-18, uTTF uCalbindin uTNFα uYKL-40 uMCP-1 | uIGFBP-7 uTIMP-2 |
| Study | sCyst-C Before a Marathon (mg/L) | sCyst-C after a Marathon (mg/L) | The Relative Increase in sCyst-C (%) | sCyst-C in Follow-Up (mg/L) |
|---|---|---|---|---|
| Mingels et al. [20] | 0.71 (0.56–0.95) | 0.95 (0.63–1.45) | 34% (21% after correction of effect of dehydration) | 0.73 (0.6–0.93) (day after, measured only in 18/70 subjects) |
| Scherr et al. [22] | 0.77 (0.71–0.85) | 0.94 (0.86–1.01) | 22% | 0.9 (0.81–1.00) (24 h after the race) 0.81 (0.72–0.86) (72 h after the race) |
| McCullough et al. [23] | 0.8 ± 0.1 | 1.0 ± 0.2 | 25% | 0.8 ± 0.1 (24 h after the race) |
| Hewing et al. [24] | 0.68 (0.75–0.93) | 0.85 (0.69–0.99) | 25% | 0.66 (0.59–0.78) (14 days after the race) |
| Study | uNGAL before a Marathon (ng/mL) | uNGAL after a Marathon | Fold Increase | KIM-1 before a Marathon | uKIM-1 after a Marathon | Fold Increase |
|---|---|---|---|---|---|---|
| McCullough et al. [23] | 8.2 ± 4.0 | 47.0 ± 28.6 (10.6 ± 7.2 after 24 h) | 5.73× (1.29×) | 2.6 ± 1.6 ng/mL | 3.5 ± 1.6 (2.7 ± 1.6 after 24 h) ng/mL | 1.35× (1.03×) |
| Mansour et al. [46] | 8.00 (4.15–30.48) | 37.64 (19.03–84.61) (day 2: 18.49 (9.25–33.69)) | 4.71× (2.31×) | 132.59 (67.61–219.98) pg/mL | 723.32 (459.36–1970.64) (day 2: 702.42 (123.27–1098.67)) pg/mL | 5.46× (5.3×) |
| Author (Year of Publication) | Study Group | Exercise/Study Design | Markers |
|---|---|---|---|
| Mingels et al. (2009) [20] | 70 recreational male runners age 47 (range 30–68) years | marathon | sCyst-C |
| Scherr et al. (2011) [22] | 102 healthy male runners age 42 ± 9.5 y | Marathon | sCyst-C |
| McCullough et al. (2011) [23] | 25 healthy runners age 38.7 ± 9.0 years (13 females, 12 males) | Marathon | sCyst-C uNGAL uKIM-1 |
| Poortmans et al. (2012) [25] | 12 male physical educators age 25 ± 5 years | 30-min treadmill exercise at 80% of VO2max | sCyst-C |
| Junglee et al. (2012) [34] | 20 healthy active adults age 24 ± 4 years (7 females, 13 males) | 800-m sprint | pNGAL uNGAL uNGAL/uCr |
| Rullman et al. (2012) [38] | 10 healthy men age 25 (range 18–37) years | 60-min cycle ergometer test (20 min at 50% of VO2max; +40 min at 65% of VO2max) | pNGAL |
| Lippi et al. (2012) [40] | 16 trained male athletes age 42 (range 34–52) years | 60-km ultramarathon | sNGAL, uNGAL, uNGAl/uCr |
| Junglee et al. (2013) [35] | 10 active healthy men age 20 ± 2 years | 1. 60-min running downhill at a −10% gradient + 40-min run on the treadmill at a 1% gradient at 65% VO2max in a temp. of 33 °C with 50% RH 2. 60-min flat run + 40-min run on the treadmill at a 1% gradient at 65% VO2max in a temp. of 33 °C with 50% RH | pNGAL uNGAL uNGLA/u.f. |
| Mellor et al. (2013) [42] | 22 subjects age 36 ± 2.4 years (7 females, 15 males) | ascent from sea level to 1085 m over 6 h | pNGAL |
| Sugama et al. (2013) [59] | 14 male triathletes age 28.7 ± 7.9 years | duathlon race: 5 km of running + 40 km of cycling + 5 km of running | uIL-6 uIL-8 |
| Kanda et al. (2014) [37] | 9 untrained healthy men age 24.8 ± 1.3 years | One leg calf-raise exercise 10 sets of 40 repetitions of exercise at 0.5 Hz with 3 min rest between sets | pNGAL uNGAL uFABP |
| Hewing et al. (2015) [24] | 167 recreational runners age 50.3 ± 11.4 years (89 females, 78 males) | marathon | sCyst-C |
| Andreazzoli et al. (2017) [41] | 18 professional male cyclists age 31.5 ± 4 years | mountain stage of one of the major European professional cycling competitions | pNGAL uNGAL |
| Mansour et al. (2017) [46] | 22 heathy amateur runners age 44 (range 22–63) years (13 females, 9 males) | marathon | uNGAL uKIM-1 uIL-6, uIL-8, uIL-18, uTNFα, uYKL-40, uMCP-1 |
| Bongers et al. (2017) [44] | 60 marchers age 29 ± 78 years (30 females, 30 males) | 30, 40 or 50 km for three consecutive days | uCyst-C, uNGAL, uNGAL/uCyst-C, uNGAL/Cr, uNGAL/uOsm uKIM-1, uKIM-1/uCyst-C, uKIM-1/uCr, uKIM-1/uOsm |
| Bongers et al. (2018) [26] | 35 active healthy males age 23 ± 3 years | 150-min cycle ergometer test at 80% of HRmax until 3% hypohydration (samples taken after 30 and 50 min) | sCyst-C, uCyst-C uNGAL, uNGAL/uCyst-C, uNGAL/uCr, uNGAL/uOsm uKIM-1, uKIM/uCyst-C, uKIM-1/uCr, uKIM-1/uOsm |
| Spada et al. (2018) [48] | 58 healthy volunteers age 24 (range 21–28) years (29 males, 29 females) | 4 min of HIIRT | uNGAL, uNGAL/uCr uIL-18, uIL-18/uCr uCalbindin, uCalbindin/uCr uTTF, uTTF/uCr |
| Wołyniec et al. (2018) [47] | 19 healthy amateur runners age 35.74 ± 6.99 years (9 females, 10 males) | treadmill run test | uNGAL, uNGAL/uCr uKIM-1, uKIM-1/uCr |
| McDermott et al. (2018) [39] | 40 healthy cyclists age 52 ± 9 years | endurance cycling event (5.7 ± 1.2 h) in heat (33.2 ± 5.0 °C, 38.4 ± 10.7% RH) | sNGAL |
| Chapman et al. (2019) [18] | 12 healthy adults age 24 ± 5 years (3 females, 9 males) | 4 h exercise in heat (35,1 °C, 61% RH) | pNGAL uNGAL, uNGAL/u.f. |
| Wolyniec et al. (2019) [45] | 16 Healthy amateur runners age 36.7 ± 8.2 years (2 females, 14 males) | 10- and 100-km runs | uCyst-C uNGAL, uNGAL/uCr uKIM-1, uKIM-1/uCr |
| Jouffroy et al. (2019) [51] | 47 healthy males age 43 ± 7 years | 80-km ultramarathon | uNGAL, uNGAL/uCr uKIM-1, uKIM-1/uCr |
| Poussel et al. (2020) [27] | 24 healthy runners age 36.5 (range 24–57) years (1 female, 23 males) | 120-km ultramarathon with 5700 m of positive elevation gain | sCyst-C uNGAL, uNGAL/uCr |
| Chapman et al. (2020) [43] | 13 healthy adults age 23 ± 2 years (3 females, 10 males) | 2 h exercise in a heat (temp 39 °C, 32 % RH) | uNGAL uIGFBP-7 uTIMP-2 |
| Kosaki et al. (2020) [53] | 116 adults without chronic kidney disease age 62 (range 24–83) years (31 females, 85 males) | incremental short maximal exercise using a cycling ergometer | uL-FABP/uCr |
| Semen et al. (2020) [50] | 54 healthy runners age 47 ± 15 years (21 females, 33 males) | half marathon after use of 400 mg single-dose ibuprofen: two groups: 1. supplemented with MOF-VVPP 2. Control | uNGAL, uCyst-C uIL-6, uIL-8, uIL-18, uTNFα |
| Semen et al. (2020) [49] | 1. 35 runners age 44 ± 2 years (17 females, 18 males) 2. 45 runners age 55 ± 2 years (24 females, 21 males) | 1. 10 km run 2. half marathon | uNGAL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołyniec, W.; Ratkowski, W.; Renke, J.; Renke, M. Changes in Novel AKI Biomarkers after Exercise. A Systematic Review. Int. J. Mol. Sci. 2020, 21, 5673. https://doi.org/10.3390/ijms21165673
Wołyniec W, Ratkowski W, Renke J, Renke M. Changes in Novel AKI Biomarkers after Exercise. A Systematic Review. International Journal of Molecular Sciences. 2020; 21(16):5673. https://doi.org/10.3390/ijms21165673
Chicago/Turabian StyleWołyniec, Wojciech, Wojciech Ratkowski, Joanna Renke, and Marcin Renke. 2020. "Changes in Novel AKI Biomarkers after Exercise. A Systematic Review" International Journal of Molecular Sciences 21, no. 16: 5673. https://doi.org/10.3390/ijms21165673
APA StyleWołyniec, W., Ratkowski, W., Renke, J., & Renke, M. (2020). Changes in Novel AKI Biomarkers after Exercise. A Systematic Review. International Journal of Molecular Sciences, 21(16), 5673. https://doi.org/10.3390/ijms21165673





