Development and Evaluation of Novel and Highly Sensitive Single-Tube Nested Real-Time RT-PCR Assays for SARS-CoV-2 Detection
Abstract
:1. Introduction
2. Results
2.1. In Silico Analysis of Primers and Probes Used in the In-House Developed STN Real-Time RT-PCR Assays for SARS-CoV-2 Detection
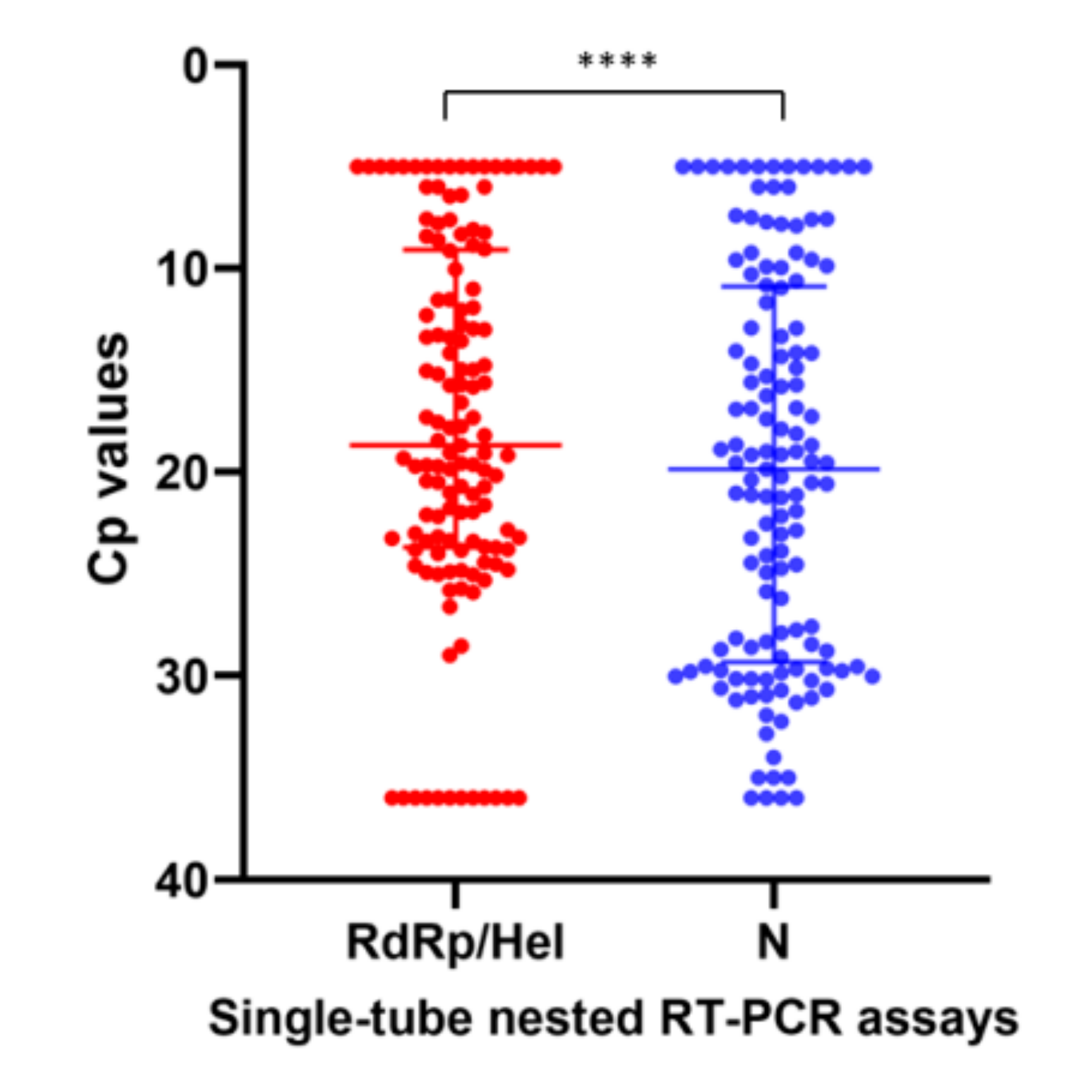
2.2. Analytical Performance of the STN RT-PCR Assays for SARS-CoV-2 Detection
2.3. Diagnostic Performance of the STN RT-PCR Assays for SARS-CoV-2 Detection
3. Discussion
4. Materials and Methods
4.1. Viruses, Clinical Specimens and Proficiency Testing Samples for Evaluation
4.2. Nucleic Acid Extraction
4.3. Primers and Probes
4.4. Real-Time RT-PCR Assays for SARS-CoV-2 RNA Detection
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BLASTn | Nucleotide Basic Local Alignment Search Tool |
| COVID-19 | Coronavirus disease 2019 |
| HCoV | Human coronavirus |
| Hel | Helicase |
| LOD | Limit of detection |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| N | Nucleocapsid |
| NCBI | National Center for Biotechnology Information, USA |
| NPA | Nasopharyngeal aspirate |
| NPS | Nasopharyngeal swab |
| PT | Proficiency testing |
| QCMD | Quality Control for Molecular Diagnostics |
| RdRp | RNA-dependent RNA polymerase |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| STN | Single-tube nested |
| TCID50 | Tissue culture infectious dose |
| Tm | Melting temperature |
| TNA | Total nucleic acid |
| WHO | World Health Organization |
References
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Lewnard, J.A.; Liu, V.X.; Jackson, M.L.; Schmidt, M.A.; Jewell, B.L.; Flores, J.P.; Jentz, C.; Northrup, G.R.; Mahmud, A.; Reingold, A.L.; et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: Prospective cohort study. BMJ 2020, 369, m1923. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Wong, S.C.; Chuang, V.W.; So, S.Y.; Chen, J.H.; Sridhar, S.; To, K.K.; Chan, J.F.; Hung, I.F.; Ho, P.L.; et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Wang, Q. Physical distancing, face masks, and eye protection for prevention of COVID-19. Lancet 2020. [Google Scholar] [CrossRef]
- Cheng, V.C.; Wong, S.C.; Chen, J.H.; Yip, C.C.; Chuang, V.W.; Tsang, O.T.; Sridhar, S.; Chan, J.F.; Ho, P.L.; Yuen, K.Y. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020, 41, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R.; et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.J.; Heinstein, C.; MacDonald, J.; Pettipas, J.; Hatchette, T.F.; Patriquin, G. A combined oropharyngeal/nares swab is a suitable alternative to nasopharyngeal swabs for the detection of SARS-CoV-2. J. Clin. Virol. 2020, 128, 104442. [Google Scholar] [CrossRef]
- Wang, X.; Tan, L.; Wang, X.; Liu, W.; Lu, Y.; Cheng, L.; Sun, Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int. J. Infect. Dis. 2020, 94, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.Y.; Yam, W.C.; Wong, L.P.; Seto, W.H. Comparison of two automated DNA amplification systems with a manual one-tube nested PCR assay for diagnosis of pulmonary tuberculosis. J. Clin. Microbiol. 1997, 35, 1385–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Cai, K.; Zhang, R.; He, X.; Shen, X.; Liu, J.; Xu, J.; Qiu, F.; Lei, W.; Wang, J.; et al. A novel one-step single-tube nested quantitative Real-Time PCR assay for highly sensitive detection of SARS-CoV-2. Anal. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Yip, C.C.; To, K.K.; Tang, T.H.; Wong, S.C.; Leung, K.H.; Fung, A.Y.; Ng, A.C.; Zou, Z.; Tsoi, H.W.; et al. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J. Clin. Microbiol. 2020, 58, e00310-20. [Google Scholar] [CrossRef] [Green Version]
- Shirato, K.; Nao, N.; Katano, H.; Takayama, I.; Saito, S.; Kato, F.; Katoh, H.; Sakata, M.; Nakatsu, Y.; Mori, Y.; et al. Development of Genetic Diagnostic Methods for Novel Coronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- To, K.K.; Tsang, O.T.; Yip, C.C.; Chan, K.H.; Wu, T.C.; Chan, J.M.; Leung, W.S.; Chik, T.S.; Choi, C.Y.; Kandamby, D.H.; et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Lohse, S.; Pfuhl, T.; Berkó-Göttel, B.; Rissland, J.; Geißler, T.; Gärtner, B.; Becker, S.L.; Schneitler, S.; Smola, S. Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Chan, W.M.; Ip, J.D.; Chu, A.W.; Yip, C.C.; Lo, L.S.; Chan, K.H.; Ng, A.C.; Poon, R.W.; To, W.K.; Tsang, O.T.; et al. Identification of nsp1 gene as the target of SARS-CoV-2 real-time RT-PCR using nanopore whole genome sequencing. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Yip, C.C.; Ho, C.C.; Chan, J.F.; To, K.K.; Chan, H.S.; Wong, S.C.; Leung, K.H.; Fung, A.Y.; Ng, A.C.; Zou, Z.; et al. Development of a Novel, Genome Subtraction-Derived, SARS-CoV-2-Specific COVID-19-nsp2 Real-Time RT-PCR Assay and Its Evaluation Using Clinical Specimens. Int. J. Mol. Sci. 2020, 21, 2574. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.C.; Sridhar, S.; Cheng, A.K.; Leung, K.H.; Choi, G.K.; Chen, J.H.; Poon, R.W.; Chan, K.H.; Wu, A.K.; Chan, H.S.; et al. Evaluation of the commercially available LightMix® Modular E-gene kit using clinical and proficiency testing specimens for SARS-CoV-2 detection. J. Clin. Virol. 2020, 129, 104476. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.W.; Pan, Y.; Cheng, S.M.S.; Hui, K.P.Y.; Krishnan, P.; Liu, Y.; Ng, D.Y.M.; Wan, C.K.C.; Yang, P.; Wang, Q.; et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, I.F.; Cheng, V.C.; Li, X.; Tam, A.R.; Hung, D.L.; Chiu, K.H.; Yip, C.C.; Cai, J.P.; Ho, D.T.; Wong, S.C.; et al. SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: A case series. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Baric, R.S.; Fu, K.; Chen, W.; Yount, B. High recombination and mutation rates in mouse hepatitis virus suggest that coronaviruses may be potentially important emerging viruses. Adv. Exp. Med. Biol. 1995, 380, 571–576. [Google Scholar]
- Lau, S.K.; Li, K.S.; Huang, Y.; Shek, C.T.; Tse, H.; Wang, M.; Choi, G.K.; Xu, H.; Lam, C.S.; Guo, R.; et al. Ecoepidemiology and comp lete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J. Virol. 2010, 84, 2808–2819. [Google Scholar] [CrossRef] [Green Version]
- Woo, P.C.; Lau, S.K.; Yip, C.C.; Huang, Y.; Tsoi, H.W.; Chan, K.H.; Yuen, K.Y. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J. Virol. 2006, 80, 7136–7145. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Chan, J.F.; Yuen, T.T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.; Tsang, J.O.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV: Implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef]
- Chan, J.F.; Zhang, A.J.; Yuan, S.; Poon, V.K.; Chan, C.C.; Lee, A.C.; Chan, W.M.; Fan, Z.; Tsoi, H.W.; Wen, L.; et al. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Chan, J.F.; Wang, Y.; Yuen, T.T.; Chai, Y.; Hou, Y.; Shuai, H.; Yang, D.; Hu, B.; Huang, X.; et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.; Choi, G.K.; Tsang, A.K.; Tee, K.M.; Lam, H.Y.; Yip, C.C.; To, K.K.; Cheng, V.C.; Yeung, M.L.; Lau, S.K.; et al. Development and Evaluation of Novel Real-Time Reverse Transcription-PCR Assays with Locked Nucleic Acid Probes Targeting Leader Sequences of Human-Pathogenic Coronaviruses. J. Clin. Microbiol. 2015, 53, 2722–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.H.; Yip, C.C.; Poon, R.W.; Chan, K.H.; Cheng, V.C.; Hung, I.F.; Chan, J.F.; Yuen, K.Y.; To, K.K. Evaluating the use of posterior oropharyngeal saliva in a point-of-care assay for the detection of SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.C.; Chan, W.M.; Ip, J.D.; Seng, C.W.; Leung, K.H.; Poon, R.W.; Ng, A.C.; Wu, W.L.; Zhao, H.; Chan, K.H.; et al. Nanopore Sequencing Reveals Novel Targets for Detection and Surveillance of Human and Avian Influenza A Viruses. J. Clin. Microbiol. 2020, 58, e02127-19. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]

| Molecular Assays | Reference Standard * | Kappa Value (95% CI) † | McNemar’s Test | ||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| STN COVID-19-RdRp/Hel assay | |||||
| Positive | 99 | 0 | 1.00 (1.00–1.00) | p = 1.000 | |
| Negative | 0 | 114 | |||
| STN COVID-19-N assay | |||||
| Positive | 99 | 0 | 1.00 (1.00–1.00) | p = 1.000 | |
| Negative | 0 | 114 | |||
| Non-nested COVID-19-RdRp/Hel assay [14] | |||||
| Positive | 99 | 0 | 1.00 (1.00–1.00) | p = 1.000 | |
| Negative | 0 | 114 | |||
| Non-nested N assay [15] | |||||
| Positive | 94 | 0 | 0.95 (0.91–0.99) | p = 0.063 | |
| Negative | 5 | 114 | |||
| Cp Value | ||||
|---|---|---|---|---|
| Pool * | Single Test (Non-Nested COVID-19-RdRp/Hel Assay) | Pool Test (Non-Nested COVID-19-RdRp/Hel Assay | Pool Test (STN COVID-19-RdRp/Hel Assay) | Pool Test (STN COVID-19-N Assay) |
| 1 | 32.21 | 32.62 | 21.03 | 24.68 |
| 2 | 33.31 | − | 23.25 | 30.25 |
| 3 | 34.80 | − | − | − |
| 4 | 35.76 | − | − | − |
| Primer/Probe | Sequence (5′–3′) | Gene Target | Reference |
|---|---|---|---|
| In-house single-tube nested real-time RT-PCR | |||
| Outer forward | AGGTATTGGGAACCTGAGTTTTATGAGGCTATGTACACAC | RdRp/Hel | This study |
| Outer reverse | ACCTGGAGCATTGCAAACATACGGATTAACAGACAAGAC | ||
| Inner forward | CGCATACAGTCTTRCAGGCT | ||
| Inner reverse | GTGTGATGTTGAWATGACATGGTC | ||
| Probe | FAM- TTAAGATGTGGTGCTTGCATACGTAGAC -lABkFQ | ||
| Outer forward | AATTGCACAATTTGCCCCCAGCGCTTCA | N | This study |
| Outer reverse | TGCGTCAATATGCTTATTCAGCAAAATGACTTGATCTTTGA | ||
| Inner forward | GCGTTCTTCGGAATGTCG | ||
| Inner reverse | TTGGATCTTTGTCATCCAATTTG | ||
| Probe | FAM- AACGTGGTTGACCTACACAGST -lABkFQ | ||
| In-house non-nested real-time RT-PCR | |||
| COVID-19-RdRp/Hel-F | CGCATACAGTCTTRCAGGCT | RdRp/Hel | [14] |
| COVID-19-RdRp/Hel-R | GTGTGATGTTGAWATGACATGGTC | ||
| COVID-19-RdRp/Hel-P | FAM- TTAAGATGTGGTGCTTGCATACGTAGAC -lABkFQ | ||
| NIID_2019-n COV_N_F2 | AAATTTTGGGGACCAGGAAC | N | [15] |
| NIID_2019-n COV_N_R2 | TGGCAGCTGTGTAGGTCAAC | ||
| NIID_2019-n COV_N_P2 | FAM- ATGTCGCGCATTGGCATGGA -BHQ | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yip, C.C.-Y.; Sridhar, S.; Leung, K.-H.; Ng, A.C.-K.; Chan, K.-H.; Chan, J.F.-W.; Tsang, O.T.-Y.; Hung, I.F.-N.; Cheng, V.C.-C.; Yuen, K.-Y.; et al. Development and Evaluation of Novel and Highly Sensitive Single-Tube Nested Real-Time RT-PCR Assays for SARS-CoV-2 Detection. Int. J. Mol. Sci. 2020, 21, 5674. https://doi.org/10.3390/ijms21165674
Yip CC-Y, Sridhar S, Leung K-H, Ng AC-K, Chan K-H, Chan JF-W, Tsang OT-Y, Hung IF-N, Cheng VC-C, Yuen K-Y, et al. Development and Evaluation of Novel and Highly Sensitive Single-Tube Nested Real-Time RT-PCR Assays for SARS-CoV-2 Detection. International Journal of Molecular Sciences. 2020; 21(16):5674. https://doi.org/10.3390/ijms21165674
Chicago/Turabian StyleYip, Cyril Chik-Yan, Siddharth Sridhar, Kit-Hang Leung, Anthony Chin-Ki Ng, Kwok-Hung Chan, Jasper Fuk-Woo Chan, Owen Tak-Yin Tsang, Ivan Fan-Ngai Hung, Vincent Chi-Chung Cheng, Kwok-Yung Yuen, and et al. 2020. "Development and Evaluation of Novel and Highly Sensitive Single-Tube Nested Real-Time RT-PCR Assays for SARS-CoV-2 Detection" International Journal of Molecular Sciences 21, no. 16: 5674. https://doi.org/10.3390/ijms21165674
APA StyleYip, C. C.-Y., Sridhar, S., Leung, K.-H., Ng, A. C.-K., Chan, K.-H., Chan, J. F.-W., Tsang, O. T.-Y., Hung, I. F.-N., Cheng, V. C.-C., Yuen, K.-Y., & To, K. K.-W. (2020). Development and Evaluation of Novel and Highly Sensitive Single-Tube Nested Real-Time RT-PCR Assays for SARS-CoV-2 Detection. International Journal of Molecular Sciences, 21(16), 5674. https://doi.org/10.3390/ijms21165674









