Chronic Administration of Fipronil Heterogeneously Alters the Neurochemistry of Monoaminergic Systems in the Rat Brain
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Behavior
2.2. Quantitative Analysis of Monoamine Tissue Contents
2.2.1. Quantitative Analysis of DA System
2.2.2. Quantitative Analysis of 5-HT System
2.2.3. Quantitative Analysis of NA Tissue Contents
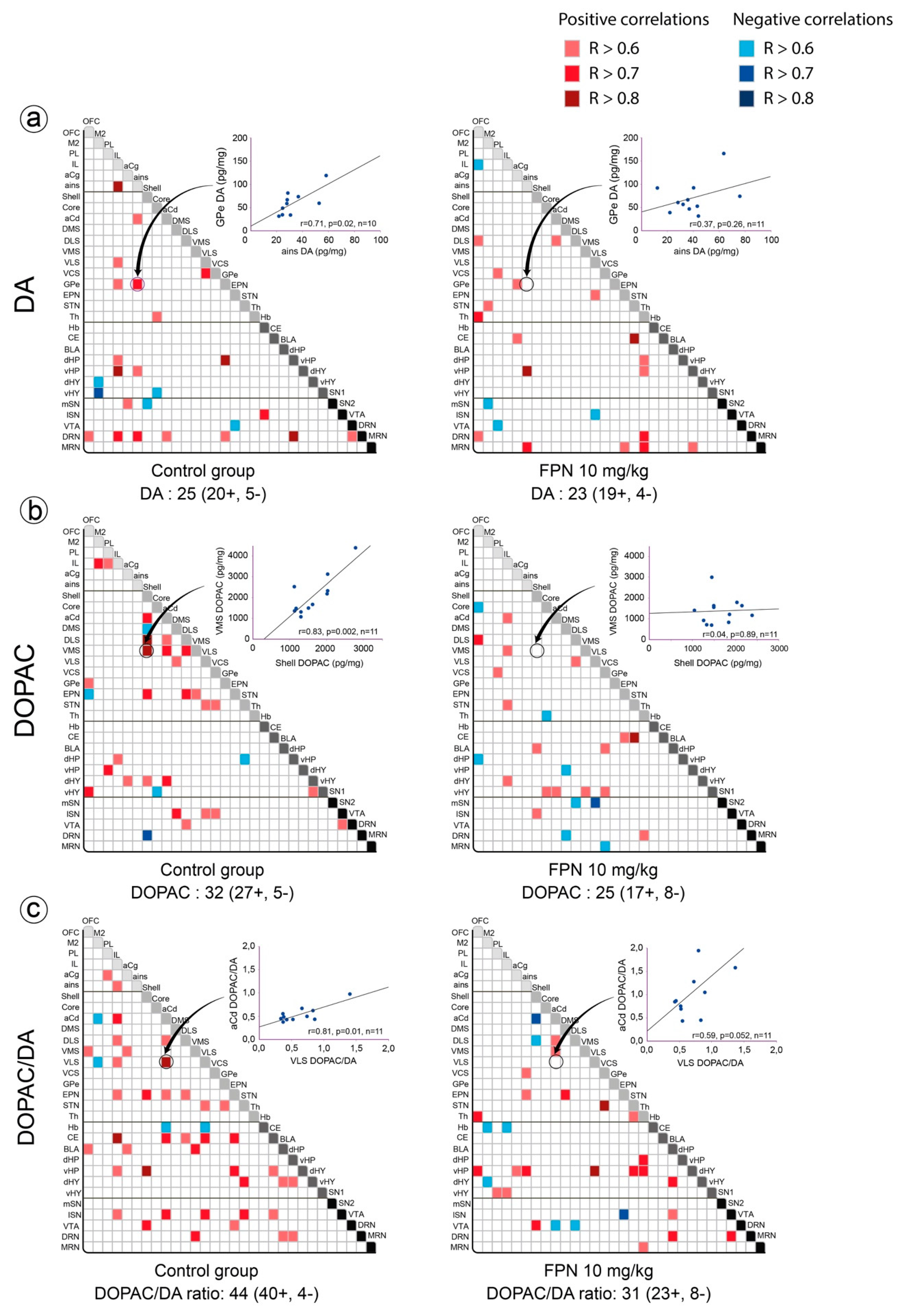
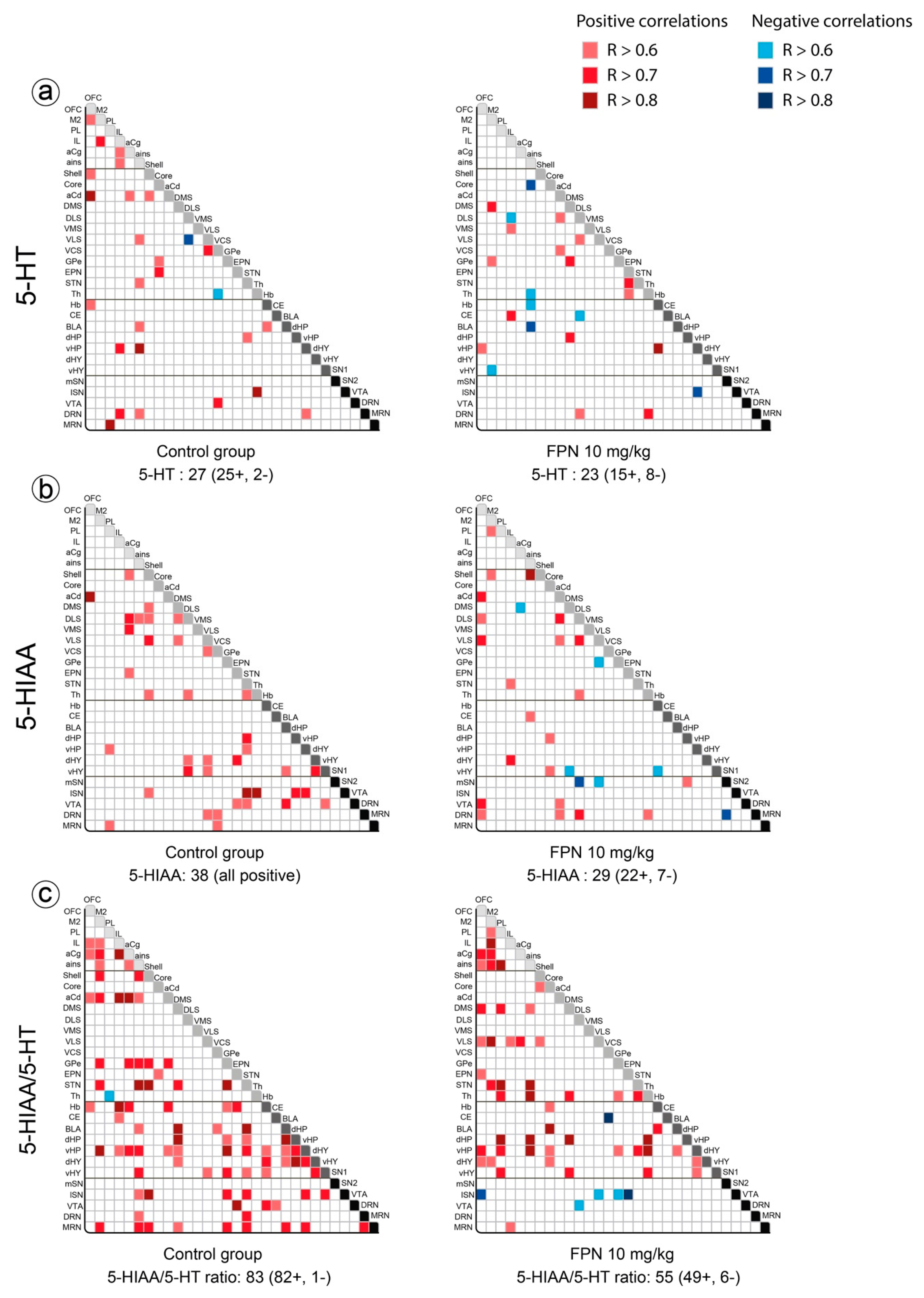
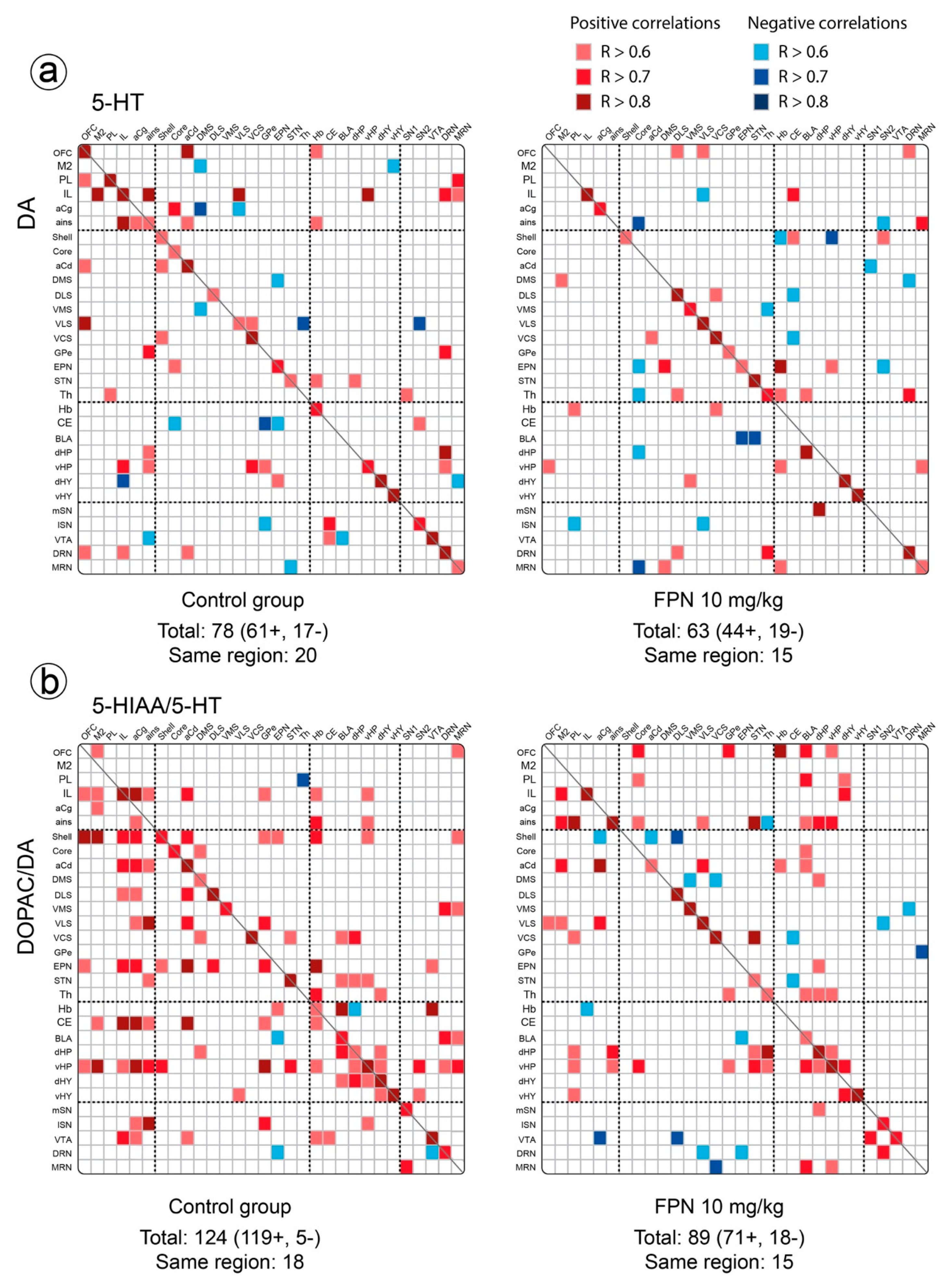
2.3. Qualitative and Correlative Analysis of Monoamine Tissue Contents
2.3.1. Within Monoaminergic Systems
Correlative Analysis of DA System
Correlative Analysis of the 5-HT System
Correlative Analysis of NA Tissue Contents
Correlative Analysis between Neurotransmitter and Their Metabolites Tissue Contents in a Single Brain Region
2.3.2. Between Monoaminergic Systems
Correlative Analysis between the DA and 5-HT Neurochemical Indexes
Correlative Analysis between NA and DA or 5-HT Tissue Contents
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Drug and Reagents
4.3. Treatment
4.4. Tissue Collection of Brain Regions
4.5. Tissue Processing and Neurochemical Analysis
4.6. Chromatographic Analysis
4.7. Statistical Data Analysis
5. Conclusions
6. Research Highlights
- The effect of the chronic administration of fipronil on the monoamine tissue content is analyzed across 30 brain regions of rats.
- Fipronil non-homogeneously reduces dopamine and serotonin levels, the main defects being found in the striatum.
- Fipronil did not alter the noradrenaline tissue content, except in the nucleus accumbens.
- Fipronil completely modified the pattern of correlations, more prominently the dopamine and the serotonin turnovers, within and between brain regions.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aCd | anterior caudate |
| aCg | anterior cingulate cortex |
| ains | anterior insular cortex |
| BLA | basolateral nucleus of amygdala |
| CE | central nucleus of amygdala |
| CNS | central nervous system |
| core | core of the nucleus accumbens |
| DA | dopamine |
| dHP | dorsal part of hippocampus |
| dHY | dorsal part of hypothalamus |
| DLS | dorsolateral striatum |
| DMS | dorsomedial striatum |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| DRN | dorsal raphe nucleus |
| EPN | entopeduncular nucleus |
| FPN | fipronil |
| GABA | γ-aminobutyric acid |
| GABAA receptor | γ-aminobutyric acid A receptor |
| GPe | the globus pallidus pars externa |
| Hb | habenula |
| HPLC | high pressure liquid chromatography |
| HVA | homovanillic acid |
| IL | infralimbic cortex |
| M2 | motor cortex M2 |
| MAO-B | monoamine oxidase-B |
| MDMA | 3,4-methylenedioxy-methamphetamine |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRN | median raphe nucleus |
| NA | noradrenaline |
| NAc | nucleus accumbens |
| OFC | orbitofrontal cortex |
| PD | Parkinson’s disease |
| PL | prelimbic cortex |
| rpm | revolutions per minute |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine; serotonin |
| shell | shell of the nucleus accumbens |
| SN | substantia nigra |
| lSN | lateral part of substantia nigra |
| mSN | medial part of substantia nigra |
| STN | subthalamic nucleus |
| Th | thalamus |
| TH | tyrosine hydroxylase |
| VCS | ventro-caudal striatum |
| vHP | ventral part of hippocampus |
| vHY | ventral part of hypothalamus |
| VLS | ventrolateral striatum |
| VMS | ventromedial striatum |
| VTA | ventral tegmental area |
| 6-OHDA | 6-hydroxydopamine |
References
- Nandipati, S.; Litvan, I. Environmental exposures and parkinson’s disease. Int. J. Environ. Res. Public Health 2016, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jennings, A. Worldwide regulations of standard values of pesticides for human health risk control: A review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.R.; Shalat, S.L.; Buckley, B.; Winnik, B.; O’Suilleabhain, P.; Diaz-Arrastia, R.; Reisch, J.; German, D.C. Elevated serum pesticide levels and risk of parkinson disease. Arch. Neurol. 2009, 66, 870–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.R.; Roy, A.; Shalat, S.L.; von Stein, R.T.; Hossain, M.M.; Buckley, B.; Gearing, M.; Levey, A.I.; German, D.C. Elevated serum pesticide levels and risk for alzheimer disease. JAMA Neurol. 2014, 71, 284–290. [Google Scholar] [CrossRef]
- Tingle, C.C.; Rother, J.A.; Dewhurst, C.F.; Lauer, S.; King, W.J. Fipronil: Environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 2003, 176, 1–66. [Google Scholar]
- Raymond-Delpech, V.; Matsuda, K.; Sattelle, B.M.; Rauh, J.J.; Sattelle, D.B. Ion channels: Molecular targets of neuroactive insecticides. Invertebr. Neurosci. 2005, 5, 119–133. [Google Scholar] [CrossRef]
- Das, P.C.; Cao, Y.; Cherrington, N.; Hodgson, E.; Rose, R.L. Fipronil induces cyp isoforms and cytotoxicity in human hepatocytes. Chem. Biol. Interact. 2006, 164, 200–214. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Galal, M.K.; Ibrahim, M.A.; Allah, A.A.A.; Afify, M.M.; Refaat, R. The terminalia laxiflora modulates the neurotoxicity induced by fipronil in male albino rats. Biosci. Rep. 2019, 39, BSR20181363. [Google Scholar] [CrossRef] [Green Version]
- Godinho, A.F.; de Oliveira Souza, A.C.; Carvalho, C.C.; Horta, D.F.; De Fraia, D.; Anselmo, F.; Chaguri, J.L.; Faria, C.A. Memory impairment due to fipronil pesticide exposure occurs at the gabaa receptor level, in rats. Physiol. Behav. 2016, 165, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Cam, M.; Durieu, E.; Bodin, M.; Manousopoulou, A.; Koslowski, S.; Vasylieva, N.; Barnych, B.; Hammock, B.D.; Bohl, B.; Koch, P.; et al. Induction of amyloid-beta42 production by fipronil and other pyrazole insecticides. J. Alzheimer Dis. JAD 2018, 62, 1663–1681. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Daim, M.M.; Shaheen, H.M.; Abushouk, A.I.; Toraih, E.A.; Fawzy, M.S.; Alansari, W.S.; Aleya, L.; Bungau, S. Thymoquinone and diallyl sulfide protect against fipronil-induced oxidative injury in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 23909–23916. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abdeen, A. Protective effects of rosuvastatin and vitamin e against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem. Toxicol. 2018, 114, 69–77. [Google Scholar] [CrossRef]
- Park, J.H.; Park, Y.S.; Lee, J.B.; Park, K.H.; Paik, M.K.; Jeong, M.; Koh, H.C. Meloxicam inhibits fipronil-induced apoptosis via modulation of the oxidative stress and inflammatory response in sh-sy5y cells. J. Appl. Toxicol. 2016, 36, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, Y.S.; Koh, H.C. Progressive loss of nigrostriatal dopaminergic neurons induced by inflammatory responses to fipronil. Toxicol. Lett. 2016, 258, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Bharatiya, R.; Bratzu, J.; Lobina, C.; Corda, G.; Cocco, C.; De Deurwaerdere, P.; Argiolas, A.; Melis, M.R.; Sanna, F. The pesticide fipronil injected into the substantia nigra of male rats decreases striatal dopamine content: A neurochemical, immunohistochemical and behavioral study. Behav. Brain Res. 2020, 384, 112562. [Google Scholar] [CrossRef] [PubMed]
- Engeln, M.; De Deurwaerdere, P.; Li, Q.; Bezard, E.; Fernagut, P.O. Widespread monoaminergic dysregulation of both motor and non-motor circuits in parkinsonism and dyskinesia. Cereb. Cortex 2015, 25, 2783–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pifl, C.; Schingnitz, G.; Hornykiewicz, O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience 1991, 44, 591–605. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Nicklas, W.J.; Vyas, I.; Duvoisin, R.C. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci. Lett. 1985, 62, 389–394. [Google Scholar] [CrossRef]
- Delaville, C.; Navailles, S.; Benazzouz, A. Effects of noradrenaline and serotonin depletions on the neuronal activity of globus pallidus and substantia nigra pars reticulata in experimental parkinsonism. Neuroscience 2012, 202, 424–433. [Google Scholar] [CrossRef]
- Tinakoua, A.; Bouabid, S.; Faggiani, E.; De Deurwaerdere, P.; Lakhdar-Ghazal, N.; Benazzouz, A. The impact of combined administration of paraquat and maneb on motor and non-motor functions in the rat. Neuroscience 2015, 311, 118–129. [Google Scholar] [CrossRef]
- Delaville, C.; Chetrit, J.; Abdallah, K.; Morin, S.; Cardoit, L.; De Deurwaerdere, P.; Benazzouz, A. Emerging dysfunctions consequent to combined monoaminergic depletions in parkinsonism. Neurobiol. Dis. 2012, 45, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Navailles, S.; De Deurwaerdere, P. Contribution of serotonergic transmission to the motor and cognitive effects of high-frequency stimulation of the subthalamic nucleus or levodopa in parkinson’s disease. Mol. Neurobiol. 2012, 45, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdere, P.; Di Giovanni, G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Prog. Neurobiol. 2017, 151, 175–236. [Google Scholar] [CrossRef] [PubMed]
- Delaville, C.; Deurwaerdere, P.D.; Benazzouz, A. Noradrenaline and parkinson’s disease. Front. Syst. Neurosci. 2011, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soubrie, P.; Reisine, T.D.; Glowinski, J. Functional aspects of serotonin transmission in the basal ganglia: A review and an in vivo approach using the push-pull cannula technique. Neuroscience 1984, 13, 605–625. [Google Scholar] [CrossRef]
- Spoont, M.R. Modulatory role of serotonin in neural information processing: Implications for human psychopathology. Psychol. Bull. 1992, 112, 330–350. [Google Scholar] [CrossRef]
- De Deurwaerdere, P.; Gaetani, S.; Vaughan, R.A. Old neurochemical markers, new functional directions? An editorial for ‘distinct gradients of various neurotransmitter markers in caudate nucleus and putamen of the human brain’ on page 650. J. Neurochem. 2020, 152, 623–626. [Google Scholar] [CrossRef]
- Dellu-Hagedorn, F.; Fitoussi, A.; De Deurwaerdere, P. Correlative analysis of dopaminergic and serotonergic metabolism across the brain to study monoaminergic function and interaction. J. Neurosci. Methods 2017, 280, 54–63. [Google Scholar] [CrossRef]
- Fitoussi, A.; Dellu-Hagedorn, F.; De Deurwaerdere, P. Monoamines tissue content analysis reveals restricted and site-specific correlations in brain regions involved in cognition. Neuroscience 2013, 255, 233–245. [Google Scholar] [CrossRef]
- Bjorklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Lindvall, O. Catecholaminergic brain stem regulatory systems. In Handbook of Physiology. The Nervous System. Iv. Intrinsic Regulatory Systems of the Brain; Mountcastle, V.B., Bloom, F.E., Geiger, S.R., Eds.; American Physiological Society: Rockville, MD, USA, 1986; pp. 677–700. [Google Scholar]
- Hale, M.W.; Lowry, C.A. Functional topography of midbrain and pontine serotonergic systems: Implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl.) 2011, 213, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, H.W. Serotonin-immunoreactive neurons and their projections in the cns. In Handbook of Chemical Neuroanatomy—Classical Transmitters and Transmitters Receptors in the Cns Part ii; Björklund, A., Hökfelt, T., Kuhar, M.J., Eds.; Elsevier Science LTD: Amsterdam, The Netherlands, 1984; pp. 68–125. [Google Scholar]
- Aston-Jones, G. Locus coeruleus, a5 and a7 noradrenergic cell groups. In The Rat Nervous System, 3rd ed.; Academic Press: New York, NY, USA, 2004; pp. 259–284. [Google Scholar]
- Chagraoui, A.; Boulain, M.; Juvin, L.; Anouar, Y.; Barriere, G.; Deurwaerdere, P. L-dopa in parkinson’s disease: Looking at the “false” neurotransmitters and their meaning. Int. J. Mol. Sci. 2019, 21, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puginier, E.; Bharatiya, R.; Chagraoui, A.; Manem, J.; Cho, Y.H.; Garret, M.; De Deurwaerdere, P. Early neurochemical modifications of monoaminergic systems in the r6/1 mouse model of huntington’s disease. Neurochem. Int. 2019, 128, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Kartheek, R.M.; David, M. Assessment of fipronil toxicity on wistar rats: A hepatotoxic perspective. Toxicol. Rep. 2018, 5, 448–456. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Herkenham, M.; Thibault, J. The neostriatal mosaic: Ii. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J. Neurosci. 1987, 7, 3915–3934. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef]
- Lucas, G.; De Deurwaerdere, P.; Porras, G.; Spampinato, U. Endogenous serotonin enhances the release of dopamine in the striatum only when nigro-striatal dopaminergic transmission is activated. Neuropharmacology 2000, 39, 1984–1995. [Google Scholar] [CrossRef]
- Spampinato, U.; Esposito, E.; Samanin, R. Serotonin agonists reduce dopamine synthesis in the striatum only when the impulse flow of nigro-striatal neurons is intact. J. Neurochem. 1985, 45, 980–982. [Google Scholar] [CrossRef]
- Ichikawa, J.; Meltzer, H.Y. Doi, a 5-ht2a/2c receptor agonist, potentiates amphetamine-induced dopamine release in rat striatum. Brain Res. 1995, 698, 204–208. [Google Scholar] [CrossRef]
- Lucas, G.; De Deurwaerdere, P.; Caccia, S.; Umberto, S. The effect of serotonergic agents on haloperidol-induced striatal dopamine release in vivo: Opposite role of 5-ht (2a) and 5-ht (2c) receptor subtypes and significance of the haloperidol dose used. Neuropharmacology 2000, 39, 1053–1063. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Fadayel, G.M.; Sullivan, C.K.; Taylor, V.L. 5-ht2 receptors exert a state-dependent regulation of dopaminergic function: Studies with mdl 100,907 and the amphetamine analogue, 3,4-methylenedioxymethamphetamine. Eur. J. Pharmacol. 1992, 223, 65–74. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Sullivan, C.K.; Fadayel, G.M. Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J. Neurochem. 1994, 62, 1382–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.J.; Taylor, V.L.; Abbate, G.M.; Nieduzak, T.R. 5-ht2 antagonists stereoselectively prevent the neurotoxicity of 3,4-methylenedioxymethamphetamine by blocking the acute stimulation of dopamine synthesis: Reversal by l-dopa. J. Pharmacol. Exp. Ther. 1991, 256, 230–235. [Google Scholar] [PubMed]
- De Deurwaerdere, P.; Moison, D.; Navailles, S.; Porras, G.; Spampinato, U. Regionally and functionally distinct serotonin3 receptors control in vivo dopamine outflow in the rat nucleus accumbens. J. Neurochem. 2005, 94, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Imperato, A.; Angelucci, L. 5-ht3 receptors control dopamine release in the nucleus accumbens of freely moving rats. Neurosci. Lett. 1989, 101, 214–217. [Google Scholar] [CrossRef]
- Lucas, G.; Di Matteo, V.; De Deurwaerdere, P.; Porras, G.; Martin-Ruiz, R.; Artigas, F.; Esposito, E.; Spampinato, U. Neurochemical and electrophysiological evidence that 5-ht4 receptors exert a state-dependent facilitatory control in vivo on nigrostriatal, but not mesoaccumbal, dopaminergic function. Eur. J. Neurosci. 2001, 13, 889–898. [Google Scholar] [CrossRef]
- Porras, G.; Di Matteo, V.; De Deurwaerdere, P.; Esposito, E.; Spampinato, U. Central serotonin4 receptors selectively regulate the impulse-dependent exocytosis of dopamine in the rat striatum: In vivo studies with morphine, amphetamine and cocaine. Neuropharmacology 2002, 43, 1099–1109. [Google Scholar] [CrossRef]
- Pozzi, L.; Trabace, L.; Invernizzi, R.; Samanin, R. Intranigral gr-113808, a selective 5-ht4 receptor antagonist, attenuates morphine-stimulated dopamine release in the rat striatum. Brain Res. 1995, 692, 265–268. [Google Scholar] [CrossRef]
- Carboni, E.; Acquas, E.; Frau, R.; Di Chiara, G. Differential inhibitory effects of a 5-ht3 antagonist on drug-induced stimulation of dopamine release. Eur. J. Pharmacol. 1989, 164, 515–519. [Google Scholar] [CrossRef]
- Ren, Y.; Feng, J. Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. J. Neurochem. 2007, 103, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, J.E.; Meyer, A.; Seidler, F.J.; Slotkin, T.A. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ. Health Perspect. 2005, 113, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Canadas, F.; Cardona, D.; Sunol, C.; Campa, L.; Sanchez-Amate, M.C.; Flores, P.; Sanchez-Santed, F. Long-term monoamine changes in the striatum and nucleus accumbens after acute chlorpyrifos exposure. Toxicol. Lett. 2008, 176, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Seidler, F.J. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: Chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol. Appl. Pharmacol. 2008, 233, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Stansley, B.J.; Yamamoto, B.K. Behavioral impairments and serotonin reductions in rats after chronic l-dopa. Psychopharmacology (Berl.) 2015, 232, 3203–3213. [Google Scholar] [CrossRef]
- Cooper, J.R.; Bloom, F.E.; Roth, R.H. The Biochemical Basis of Neuropharmacology, 8th ed.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- De Deurwaerdere, P.; Binda, C.; Corne, R.; Leone, C.; Valeri, A.; Valoti, M.; Ramsay, R.R.; Fall, Y.; Marco-Contelles, J. Comparative analysis of the neurochemical profile and mao inhibition properties of n-(furan-2-ylmethyl)-n-methylprop-2-yn-1-amine. ACS Chem. Neurosci. 2017, 8, 1026–1035. [Google Scholar] [CrossRef] [Green Version]
- Finberg, J.P. Update on the pharmacology of selective inhibitors of mao-a and mao-b: Focus on modulation of cns monoamine neurotransmitter release. Pharmacol. Ther. 2014, 143, 133–152. [Google Scholar] [CrossRef]
- Cases, O.; Seif, I.; Grimsby, J.; Gaspar, P.; Chen, K.; Pournin, S.; Müller, U.; Aguet, M.; Babinet, C.; Shih, J.C.; et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking maoa. Science 1995, 268, 1763–1766. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.C.; Lesch, K.P.; Murphy, D.L. Serotonin uptake into dopamine neurons via dopamine transporters: A compensatory alternative. Brain Res. 2002, 942, 109–119. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Lovenberg, W. In vitro demonstration of dopamine uptake by neostriatal serotonergic neurons of the rat. Neurosci. Lett. 1985, 59, 9–14. [Google Scholar] [CrossRef]
- Chagraoui, A.; Whitestone, S.; Baassiri, L.; Manem, J.; Di Giovanni, G.; De Deurwaerdere, P. Neurochemical impact of the 5-ht2c receptor agonist way-163909 on monoamine tissue content in the rat brain. Neurochem. Int. 2019, 124, 245–255. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdere, P.; Ramos, M.; Bharatiya, R.; Puginier, E.; Chagraoui, A.; Manem, J.; Cuboni, E.; Pierucci, M.; Deidda, G.; Casarrubea, M.; et al. Lorcaserin bidirectionally regulates dopaminergic function site-dependently and disrupts dopamine brain area correlations in rats. Neuropharmacology 2020, 166, 107915. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, G.; De Deurwaerdere, P. New therapeutic opportunities for 5-ht2c receptor ligands in neuropsychiatric disorders. Pharmacol. Ther. 2016, 157, 125–162. [Google Scholar] [CrossRef] [PubMed]
- Whitestone, S.; Deurwaerdere, P.; Baassiri, L.; Manem, J.; Anouar, Y.; Di Giovanni, G.; Bharatiya, R.; Chagraoui, A. Effect of the 5-ht2c receptor agonist way-163909 on serotonin and dopamine metabolism across the rat brain: A quantitative and qualitative neurochemical study. Int. J. Mol. Sci. 2019, 20, 2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giovanni, G.; Bharatiya, R.; Puginier, E.; Ramos, M.; De Deurwaerdère, S.; Chagraoui, A.; De Deurwaerdère, P. Lorcaserin alters serotonin and noradrenaline tissue content and their interaction with dopamine in the rat brain. Front. Pharmacol. 2020, 11, 962. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [Green Version]
- Dellu-Hagedorn, F.; Rivalan, M.; Fitoussi, A.; De Deurwaerdere, P. Inter-individual differences in the impulsive/compulsive dimension: Deciphering related dopaminergic and serotonergic metabolisms at rest. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2018, 373, 20170154. [Google Scholar] [CrossRef]
- Hortnagl, H.; Pifl, C.; Hortnagl, E.; Reiner, A.; Sperk, G. Distinct gradients of various neurotransmitter markers in caudate nucleus and putamen of the human brain. J. Neurochem. 2020, 152, 650–662. [Google Scholar] [CrossRef]
- Vasylieva, N.; Barnych, B.; Wan, D.; El-Sheikh, E.A.; Nguyen, H.M.; Wulff, H.; McMahen, R.; Strynar, M.; Gee, S.J.; Hammock, B.D. Hydroxy-fipronil is a new urinary biomarker of exposure to fipronil. Environ. Int. 2017, 103, 91–98. [Google Scholar] [CrossRef]
- Müller, C.P.; Pum, M.E.; Amato, D.; Schüttler, J.; Huston, J.P.; Silva, M.A. The in vivo neurochemistry of the brain during general anesthesia. J. Neurochem. 2011, 119, 419–446. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lassiter, T.L.; MacKillop, E.A.; Ryde, I.T.; Seidler, F.J.; Slotkin, T.A. Is fipronil safer than chlorpyrifos? Comparative developmental neurotoxicity modeled in pc12 cells. Brain Res. Bull. 2009, 78, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| (a) | ||||||||
| DA | DOPAC | HVA | DOPAC/DA | |||||
| Brain Region | Control | FPN | Control | FPN | Control | FPN | Control | FPN |
| OFC | 16.62 ± 3.99 | 12.93 ± 2.17 | 38.96 ± 4.65 | 37.54 ± 3.43 | 19.16 ± 2.89 | 15.84 ± 2.15 | 2.59 ± 0.37 | 3.09 ± 0.29 |
| M2 | 28.20 ± 4.60 | 13.19 ± 0.98 * | 57.28 ± 9.03 | 32.24 ± 3.01 * | 16.13 ± 2.33 | 11.58 ± 2.22 | 2.39 ± 0.15 | 2.18 ± 0.22 |
| PL | 20.37 ± 2.71 | 35.56 ± 3 *** | 66.65 ± 6.49 | 85.35 ± 7.00 | 28.42 ± 3.45 | 28.54 ± 2.73 | 3.66 ± 0.25 | 2.73 ± 0.25 * |
| IL | 34.42 ± 8.14 | 29.02 ± 3.64 | 83.49 ± 6.36 | 109.7 ± 16.8 | 31.93 ± 5.25 | 36.85 ± 5.56 | 3.30 ± 0.33 | 3.65 ± 0.39 |
| aCg | 16.74 ± 2.15 | 16.62 ± 2.29 | 63.14 ± 8.34 | 75.58 ± 15.9 | 49.62 ± 5.96 | 60.72 ± 13.0 | 3.64 ± 0.26 | 4.24 ± 0.52 |
| ains | 38.46 ± 6.14 | 33.44 ± 4.75 | 77.47 ± 8.51 | 92.29 ± 13.59 | 81.96 ± 7.18 | 92.48 ± 10.3 | 2.05 ± 0.23 | 3.11 ± 0.55 |
| Shell | 1096 ± 92.6 | 845.9 ± 86 | 1643 ± 157 | 1581 ± 103 | 262.6 ± 17.9 | 286.6 ± 24.9 | 1.75 ± 0.26 | 1.85 ± 0.15 |
| Core | 1291 ± 80.3 | 941.0 ± 117 * | 1601 ± 121 | 1194 ± 159 | 325.5 ± 26.4 | 260.5 ± 28.1 | 1.33 ± 0.08 | 1.45 ± 0.13 |
| aCd | 5039 ± 435 | 2432 ± 317 *** | 2829 ± 128 | 1832 ± 186 *** | 696.9 ± 44.33 | 435.9 ± 40.16 *** | 0.58 ± 0.07 | 0.74 ± 0.09 |
| DMS | 2816 ± 220 | 1643 ± 129 *** | 1523 ± 134 | 874.4 ± 71.6 *** | 291.1 ± 20.4 | 178.9± 13.1 *** | 0.53 ± 0.05 | 0.52 ± 0.03 |
| DLS | 2820 ± 206 | 1230 ± 160 *** | 1159 ± 96.4 | 729.8± 63.5 *** | 370.1 ± 22.5 | 261.2 ± 18.2 ** | 0.42 ± 0.04 | 0.63 ± 0.08 * |
| VMS | 2835 ± 164 | 1124 ± 147 *** | 1921 ± 199 | 1242 ± 120 ** | 432.9 ± 43.6 | 277.2 ± 24.5 * | 0.79 ± 0.09 | 1.19 ± 0.17 |
| VLS | 2057 ± 274 | 1207 ± 162 * | 1135 ± 127 | 1082 ± 113 | 357.3 ± 25.6 | 412.1 ± 37.5 | 0.54 ± 0.05 | 0.94 ± 0.15 * |
| VCS | 1799 ± 139 | 1276 ± 170 * | 689.6 ± 90.3 | 651.7 ± 65.9 | 199.7 ± 20.3 | 174.9 ± 15.0 | 0.38 ± 0.04 | 0.52 ± 0.05 * |
| GPe | 66.24 ± 9.38 | 69.35 ± 11.1 | 101.9 ± 11.2 | 101.4 ± 10.8 | 64.18 ± 6.08 | 71.45 ± 9.26 | 1.51 ± 0.13 | 1.45 ± 0.14 |
| EPN | 88.01 ± 9.03 | 68.08 ± 8.54 | 58.89 ± 7.73 | 47.93 ± 8.55 | 16.44 ± 2.05 | 16.78 ± 3.69 | 0.62 ± 0.05 | 0.59 ± 0.05 |
| STN | 100.4 ± 7.34 | 92.42 ± 13.18 | 63.37 ± 4.43 | 65.97 ± 6.73 | 12.18 ± 1.08 | 12.48 ± 1.78 | 0.59 ± 0.05 | 0.59 ± 0.04 |
| Th | 8.39 ± 1.28 | 6.22 ± 1.01 | 13.20 ± 1.12 | 12.73 ± 1.30 | 4.43 ± 0.72 | 4.85 ± 0.75 | 1.75 ± 0.25 | 2.20 ± 0.19 |
| Hb | 16.19 ± 2.63 | 12.47 ± 1.53 | 23.45 ± 4.06 | 19.14 ± 3.86 | 7.76 ± 1.07 | 7.13 ± 1.08 | 1.41 ± 0.29 | 1.61 ± 0.16 |
| CE | 878.5 ± 90.6 | 645.7 ± 116 | 324.5 ± 22.2 | 244.9 ± 32.7 | 48.91 ± 3.81 | 51.22 ± 9.57 | 0.37 ± 0.04 | 0.40 ± 0.03 |
| BLA | 601.8 ± 99.3 | 575.1 ± 148 | 308.3 ± 35.6 | 435.3 ± 67.1 | 84.80 ± 8.90 | 97.84 ± 14.0 | 0.55 ± 0.06 | 0.81 ± 0.14 |
| dHP | 2.33 ± 0.50 | 2.06 ± 0.42 | 5.90 ± 1.01 | 9.34 ± 0.94 * | 4.82 ± 0.79 | 4.94 ± 0.64 | 2.18 ± 0.33 | 4.88 ± 1.25 |
| vHP | 3.82 ± 1.49 | 2.87 ± 0.75 | 6.93 ± 0.45 | 8.56 ± 0.55 * | 3.44 ± 0.33 | 4.96 ± 0.39 ** | 2.91 ± 0.65 | 3.34 ± 0.59 |
| dHY | 72.06 ± 9.62 | 81.76 ± 9.32 | 42.97 ± 6.17 | 44.79 ± 3.99 | 4.90 ± 0.95 | 5.01 ± 0.42 | 0.59 ± 0.05 | 0.55 ± 0.04 |
| vHY | 45.43 ± 7.24 | 33.79 ± 3.92 | 44.94 ± 5.48 | 43.97 ± 8.65 | 5.51 ± 0.49 | 6.85 ± 0.71 | 0.84 ± 0.09 | 1.23 ± 0.28 |
| mSN | 318.5 ± 52.2 | 157.6 ± 27.5 * | 157.8 ± 16.1 | 93.94 ± 12.5 * | 55.25 ± 5.41 | 28.68 ± 4.65 ** | 0.52 ± 0.04 | 0.62 ± 0.06 |
| lSN | 227.2 ± 33.5 | 139.3 ± 16.8 * | 86.04 ± 13.3 | 64.97 ± 8 | 27.00 ± 4.07 | 22.18 ± 2.87 | 0.36 ± 0.02 | 0.48 ± 0.04 * |
| VTA | 319.3 ± 58.8 | 291.8 ± 50.6 | 534.5 ± 97.5 | 689.3 ± 127 | 142.8 ± 13.1 | 124.9 ± 13.2 | 1.63 ± 0.09 | 2.28 ± 0.19 ** |
| DRN | 53.78 ± 8.23 | 51.09 ± 7.30 | 34.64 ± 2.93 | 30.31 ± 3.40 | 9.38 ± 1.29 | 10.35 ± 1.27 | 0.69 ± 0.07 | 0.61 ± 0.06 |
| MRN | 43.85 ± 4.64 | 34.31 ± 3.95 | 32.41 ± 2.63 | 27.29 ± 2.02 | 25.41 ± 2.53 | 22.53 ± 2.49 | 0.61 ± 0.05 | 0.79 ± 0.07 * |
| (b) | ||||||||
| NA | 5-HT | 5-HIAA | 5-HIAA/5-HT | |||||
| Brain Region | Control | FPN | Control | FPN | Control | FPN | Control | FPN |
| OFC | nd | nd | 89.67 ± 15.79 | 73.27 ± 7.68 | 594.3 ± 65.1 | 560.4 ± 68.1 | 6.99 ± 0.86 | 7.62 ± 0.74 |
| M2 | nd | nd | 170.5 ± 24.3 | 140.8 ± 19.2 | 675.5 ± 82.6 | 628.3 ± 46.8 | 4.14 ± 0.70 | 5.73 ± 0.75 |
| PL | 43.15 ± 6.33 | 36.07 ± 3.83 | 55.77 ± 7.74 | 49.76 ± 5.67 | 362.8 ±38.4 | 404.9 ± 32.2 | 6.60 ± 0.51 | 8.67 ± 1.04 |
| IL | 52.20 ± 10.4 | 53.94 ± 6.99 | 83.63 ± 16.8 | 67.84 ± 8.41 | 415.8 ± 50.6 | 516.9 ± 73.1 | 5.16 ± 0.67 | 7.50 ± 0.88 * |
| aCg | 66.20 ± 5.14 | 69.99 ± 8.57 | 45.75 ± 4.69 | 44.06 ± 6.67 | 348.3 ± 43.3 | 543.4 ± 80.0 * | 7.87 ± 1.09 | 10.34 ± 0.94 |
| ains | 98.14 ± 14.4 | 93.90 ± 7.73 | 174.4 ± 19.5 | 184.3 ± 16.7 | 645.9 ± 40.9 | 629.2 ± 68.4 | 3.79 ± 0.45 | 4.09 ± 0.53 |
| Shell | 120.4 ± 15.3 | 115.3 ± 15.4 | 136.1 ± 16.0 | 89.27 ± 10.4 * | 300.4 ± 20.78 | 310.1 ± 24.57 | 2.25 ± 0.29 | 3.62 ± 0.49 * |
| Core | 75.38 ± 4.91 | 50.29 ± 7.38 * | 114.3 ± 5.98 | 80.13 ± 6.81 ** | 276.6 ± 21.2 | 244.7 ± 36.4 | 2.58 ± 0.17 | 2.91 ± 0.22 |
| aCd | nd | nd | 97.66 ± 7.04 | 70.03 ± 9.71 * | 410.2 ± 18.5 | 347.4 ± 32.8 | 4.12 ± 0.21 | 5.21 ± 0.42 * |
| DMS | nd | nd | 59.45 ± 4.89 | 57.12 ± 8.68 | 199.2 ± 16.8 | 405.57± 46.4 *** | 3.18 ± 0.23 | 7.03 ± 0.45 *** |
| DLS | nd | nd | 68.68 ± 6.04 | 56.19 ± 9.29 | 264.7 ± 24.0 | 244.6 ± 28.7 | 3.57 ± 0.17 | 4.47 ± 0.33 * |
| VMS | nd | nd | 199.8 ± 17.4 | 115.9 ± 15.0 * | 459.1 ± 50.5 | 352.0 ± 30.4 | 2.83 ± 0.15 | 3.08 ± 0.32 |
| VLS | nd | nd | 67.55 ± 8.54 | 42.08 ± 4.76 * | 242.0 ± 19.1 | 227.6 ± 28.6 | 3.56 ± 0.28 | 5.58 ± 0.44 *** |
| VCS | 49.96 ± 3.77 | 37.16 ± 5.16 | 184.5 ± 15.9 | 113.3 ± 18.2 ** | 235.3 ± 20.7 | 182.7 ± 21.2 | 1.27 ± 0.11 | 1.64 ± 0.09 * |
| GPe | nd | nd | 181.7 ± 11.9 | 211.5 ± 29.7 | 666.7 ± 80.4 | 602.4 ± 64.3 | 3.40 ± 0.41 | 2.84 ± 0.37 |
| EPN | 95.32 ± 9.49 | 80.36 ± 13.05 | 219.1 ± 22.3 | 158.2 ± 22.7 | 627.9 ± 62.8 | 414.5 ± 43.4 * | 2.71 ± 0.12 | 2.67 ± 0.20 |
| STN | 284.9 ± 18.72 | 266.6 ± 30.3 | 359.2 ± 30.5 | 267.1 ± 38.1 | 846.7 ± 62.9 | 741.2 ± 63.4 | 2.27 ± 0.19 | 2.43 ± 0.12 |
| Th | 185.5 ± 8.99 | 172.2 ± 15.3 | 100.0 ± 16.8 | 84.82 ± 7.43 | 530.9 ± 51.9 | 507.4 ± 41.2 | 5.78 ± 0.76 | 5.69 ± 0.56 |
| Hb | 53.9 ± 7.66 | 46.58 ± 6.13 | 122.8 ± 22.3 | 103.2 ± 17.5 | 390.7 ± 66.1 | 412.7 ± 50.1 | 3.31 ± 0.85 | 3.42 ± 0.55 |
| CE | 184.9 ± 20.9 | 184.4 ± 19.1 | 250.1 ± 32.6 | 201.7 ± 24.5 | 442.3 ± 63.2 | 382.8 ± 40.5 | 1.74 ± 0.16 | 1.93 ± 0.17 |
| BLA | 126.0 ± 9.29 | 99.37 ± 11.1 | 214.5 ± 18.1 | 181.3 ± 28.9 | 428.2 ± 27.6 | 451.3 ± 37.3 | 1.97 ± 0.21 | 2.72 ± 0.33 |
| dHP | 218.9 ± 21.1 | 223.5 ± 16.1 | 133.3 ± 15.9 | 121.3 ± 14.2 | 428.1 ± 46.8 | 480.6 ± 35.3 | 3.21 ± 0.40 | 4.19 ± 0.68 |
| vHP | 191.8 ± 22.5 | 231.1 ± 18.3 | 165.3 ± 19.9 | 187.9 ± 21.8 | 684.9 ± 50.7 | 830.4 ± 92.8 | 3.91 ± 0.51 | 5.23 ± 0.59 |
| dHY | 702.6 ± 143 | 645.9 ± 137 | 253.6 ± 34.4 | 219.2 ± 22.3 | 522.9 ± 71.5 | 509.0 ± 48.9 | 2.03 ± 0.14 | 2.28 ± 0.11 |
| vHY | 636.9 ± 111 | 510.5 ± 55.9 | 171.4 ± 19.2 | 143.5 ± 16.1 | 392.2 ± 43.7 | 503.6 ± 71.7 | 2.27 ± 0.19 | 3.46 ± 0.56 |
| mSN | 119.4 ± 13.4 | 95.23 ± 19.4 | 845.4 ± 46.6 | 501.4 ± 62.3 *** | 941.5 ± 35.1 | 771.2 ± 72.4 * | 1.14 ± 0.05 | 1.52 ± 0.17 |
| lSN | 121.3 ± 13.3 | 109.3 ± 9.69 | 500.1 ± 54.6 | 544.6 ± 52.5 | 574.1 ± 74.9 | 731.7 ± 54.1 | 1.11 ± 0.05 | 1.36 ± 0.11 |
| VTA | 474.1 ± 38.9 | 370.9 ± 47.2 | 688.2 ± 60.8 | 494.2 ± 79.0 | 885.5 ± 88.9 | 728.3 ± 86.1 | 1.24 ± 0.09 | 1.50 ± 0.09 |
| DRN | 568.9 ± 83.8 | 501.9 ± 70.9 | 1150 ± 151 | 1228 ± 193 | 2119 ± 186 | 2279 ± 317 | 1.65 ± 0.07 | 1.87 ± 0.06 * |
| MRN | 352.4 ± 30.6 | 302.8 ± 38.4 | 1183 ± 101 | 1125 ± 121 | 2960 ± 262 | 3206 ± 370 | 2.47 ± 0.09 | 2.84 ± 0.07 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharatiya, R.; Chagraoui, A.; De Deurwaerdere, S.; Argiolas, A.; Melis, M.R.; Sanna, F.; De Deurwaerdere, P. Chronic Administration of Fipronil Heterogeneously Alters the Neurochemistry of Monoaminergic Systems in the Rat Brain. Int. J. Mol. Sci. 2020, 21, 5711. https://doi.org/10.3390/ijms21165711
Bharatiya R, Chagraoui A, De Deurwaerdere S, Argiolas A, Melis MR, Sanna F, De Deurwaerdere P. Chronic Administration of Fipronil Heterogeneously Alters the Neurochemistry of Monoaminergic Systems in the Rat Brain. International Journal of Molecular Sciences. 2020; 21(16):5711. https://doi.org/10.3390/ijms21165711
Chicago/Turabian StyleBharatiya, Rahul, Abdeslam Chagraoui, Salomé De Deurwaerdere, Antonio Argiolas, Maria Rosaria Melis, Fabrizio Sanna, and Philippe De Deurwaerdere. 2020. "Chronic Administration of Fipronil Heterogeneously Alters the Neurochemistry of Monoaminergic Systems in the Rat Brain" International Journal of Molecular Sciences 21, no. 16: 5711. https://doi.org/10.3390/ijms21165711
APA StyleBharatiya, R., Chagraoui, A., De Deurwaerdere, S., Argiolas, A., Melis, M. R., Sanna, F., & De Deurwaerdere, P. (2020). Chronic Administration of Fipronil Heterogeneously Alters the Neurochemistry of Monoaminergic Systems in the Rat Brain. International Journal of Molecular Sciences, 21(16), 5711. https://doi.org/10.3390/ijms21165711








