A Subset of Purposeless Oral Movements Triggered by Dopaminergic Agonists Is Modulated by 5-HT2C Receptors in Rats: Implication of the Subthalamic Nucleus
Abstract
:1. Introduction
2. Results
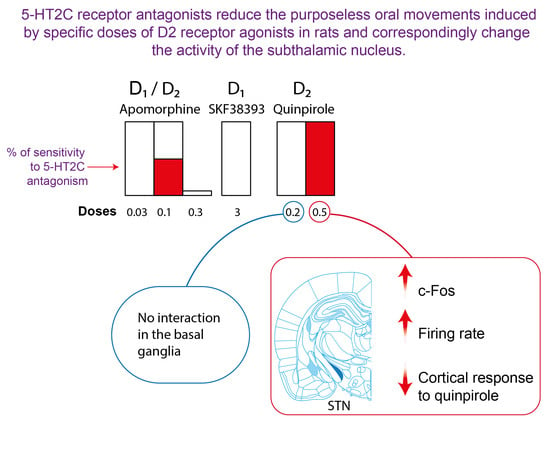
2.1. Effect of 5-HT2C Antagonists on Purposeless Oral Movements Induced by DA Agonists
2.2. Effect of 5-HT2C Antagonists on the Number of c-Fos-Immunolabeled Cells Induced by Quinpirole in the Basal Ganglia
2.3. Effect of Quinpirole/5-HT2C Antagonist on ACC Stimulation-Evoked Responses of SNr Neurons
2.4. Effect of SB 243213 and Quinpirole on Basal STN Neuron Activity
3. Discussion
3.1. 5-HT2C Receptors and Purposeless Oral Movements Induced by DA Agonists
3.2. Quinpirole and 5-HT2C Receptors Interact on STN Neuronal Activity: Focus on the Cortico-STN Hyperdirect Pathway
3.3. Correlation between the Orofacial Control Exerted by 5-HT2C Receptors and the Activity of the STN
4. Materials and Methods
4.1. Animals
4.2. Behavioral Testing
4.3. Immunohistochemistry
4.4. Surgeries
4.5. Electrical Stimulation of the Anterior Cingulate Cortex
4.6. Extracellular Single Unit Recordings of SNr and STN Neurons
4.7. Drugs
4.8. Data Analysis
4.8.1. Fos Immunohistochemistry
4.8.2. Electrophysiological Experiments
4.9. Pharmacological Treatment and Experimental Design
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | serotonin |
| 5-HT2C receptors | Serotonin2C receptors |
| DA | Dopamine |
| SNr | substantia nigra pars reticulata |
| STN | subthalamic nucleus |
| NAc | nucleus accumbens |
| DM | dorsomedial |
| DL | dorsolateral |
| VM | ventromedial |
| VL | ventrolateral |
| EPN | entopeduncular nucleus |
| GP | globus pallidus |
| ACC | anterior Cingular Cortex |
| VP | ventral pallidum |
| OCD | obsessive compulsive disorder |
| Rmag | response magnitude |
| PSTH | Peristimulus time histogram |
| PLSD | protected least significant difference |
References
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995, 18, 63–64. [Google Scholar] [CrossRef]
- Delong, M.; Wichmann, T. Update on models of basal ganglia function and dysfunction. Park. Relat. Disord. 2009, 15, S237–S240. [Google Scholar] [CrossRef] [Green Version]
- Mink, J.W. Neurobiology of basal ganglia and Tourette syndrome: Basal ganglia circuits and thalamocortical outputs. Adv. Neurol. 2006, 99, 89–98. [Google Scholar] [PubMed]
- Waddington, J.L. Spontaneous orofacial movements induced in rodents by very long-term neuroleptic drug administration: Phenomenology, pathophysiology and putative relationship to tardive dyskinesia. Psychopharmacology 1990, 101, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Bastide, M.F.; Meissner, W.G.; Picconi, B.; Fasano, S.; Fernagut, P.-O.; Feyder, M.; Francardo, V.; Alcacer, C.; Ding, Y.; Brambilla, R.; et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol. 2015, 132, 96–168. [Google Scholar] [CrossRef]
- Mink, J.W. The Basal Ganglia: Focused Selection and Inhibition of Competing Motor Programs. Prog. Neurobiol. 1996, 50, 381–425. [Google Scholar] [CrossRef]
- Nambu, A. Seven problems on the basal ganglia. Curr. Opin. Neurobiol. 2008, 18, 595–604. [Google Scholar] [CrossRef]
- Brus, R.; Plech, A.; Kostrzewa, R.M. Enhanced quinpirole response in rats lesioned neonatally with 5,7-dihydroxytryptamine. Pharmacol. Biochem. Behav. 1995, 50, 649–653. [Google Scholar] [CrossRef]
- Gong, L.; Kostrzewa, R.M.; Fuller, R.W.; Perry, K.W. Supersensitization of the oral response to SKF 38393 in neonatal 6-OHDA-lesioned rats is mediated through a serotonin system. J. Pharmacol. Exp. Ther. 1992, 261, 1000–1007. [Google Scholar]
- Gong, L.; Kostrzewa, R.M.; Perry, K.W.; Fuller, R.W. Dose-related effects of a neonatal 6-OHDA lesion on SKF 38393- and m-chlorophenylpiperazine-induced oral activity responses of rats. Dev. Brain Res. 1993, 76, 233–238. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Jenner, P.; Marsden, C.D. Pharmacological characterisation of spontaneous or drug-associated purposeless chewing movements in rats. Psychopharmacology 1985, 85, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Waddington, J.L.; O’Sullivan, G.J.; Tomiyama, K. Regulation of Orofacial Movement: Dopamine Receptor Mechanisms And Mutant Models. Int. Rev. Neurobiol. 2011, 97, 39–60. [Google Scholar] [CrossRef]
- Gong, L.; Kostrzewa, R.M. Supersensitized oral responses to a serotonin agonist in neonatal 6-OHDA-treated rats. Pharmacol. Biochem. Behav. 1992, 41, 621–623. [Google Scholar] [CrossRef]
- Lagière, M.; Navailles, S.; Bosc, M.; Guthrie, M.; De Deurwaerdère, P. Serotonin2C Receptors and the Motor Control of Oral Activity. Curr. Neuropharmacol. 2013, 11, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Lagiere, M.; Navailles, S.; Mignon, L.; Roumegous, A.; Chesselet, M.-F.; De Deurwaerdère, P. The enhanced oral response to the 5-HT2 agonist Ro 60-0175 in parkinsonian rats involves the entopeduncular nucleus: Electrophysiological correlates. Exp. Brain Res. 2013, 230, 513–524. [Google Scholar] [CrossRef]
- Navailles, S.; Lagière, M.; Roumegous, A.; Polito, M.; Boujema, M.B.; Cador, M.; Dunlop, J.; Chesselet, M.-F.; Millan, M.J.; De Deurwaerdère, P. Serotonin2C ligands exhibiting full negative and positive intrinsic activity elicit purposeless oral movements in rats: Distinct effects of agonists and inverse agonists in a rat model of Parkinson’s disease. Int. J. Neuropsychopharmacol. 2013, 16, 593–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, B.R.; Jenner, P.; Marsden, C. Induction of purposeless chewing behaviour in rats by 5-HT agonist drugs. Eur. J. Pharmacol. 1989, 162, 101–107. [Google Scholar] [CrossRef]
- Rosengarten, H.; Schweitzer, J.W.; Friedhoff, A.J. The effect of novel antipsychotics in rat oral dyskinesia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 1999, 23, 1389–1404. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Lagiere, M.; Bosc, M.; Navailles, S. Multiple controls exerted by 5-HT2C receptors upon basal ganglia function: From physiology to pathophysiology. Exp. Brain Res. 2013, 230, 477–511. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Ramos, M.; Bharatiya, R.; Puginier, E.; Chagraoui, A.; Manem, J.; Cuboni, E.; Pierucci, M.; Deidda, G.; Casarrubea, M.; et al. Lorcaserin bidirectionally regulates dopaminergic function site-dependently and disrupts dopamine brain area correlations in rats. Neuropharmacology 2020, 166, 107915. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Di Matteo, V.; Di Mascio, M.; Esposito, E. Preferential modulation of mesolimbic vs. nigrostriatal dopaminergic function by serotonin2C/2B receptor agonists: A combined in vivo electrophysiological and microdialysis study. Synapse 2000, 35, 53–61. [Google Scholar] [CrossRef]
- Beyeler, A.; Kadiri, N.; Navailles, S.; Ben Boujema, M.; Gonon, F.; Le Moine, C.; Gross, C.; De Deurwaerdère, P. Stimulation of serotonin2C receptors elicits abnormal oral movements by acting on pathways other than the sensorimotor one in the rat basal ganglia. Neuroscience 2010, 169, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Lagière, M.; Bosc, M.; Whitestone, S.; Manem, J.; Elboukhari, H.; Benazzouz, A.; Di Giovanni, G.; De Deurwaerdère, P. Does the Serotonin2C receptor segregate circuits of the basal ganglia responding to cingulate cortex stimulation? CNS Neurosci. Ther. 2017, 24, 741–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Deurwaerdère, P.; Chesselet, M.-F. Nigrostriatal Lesions Alter Oral Dyskinesia and c-Fos Expression Induced by the Serotonin Agonist 1-(m-Chlorophenyl) piperazine in Adult Rats. J. Neurosci. 2000, 20, 5170–5178. [Google Scholar] [CrossRef] [PubMed]
- Eberle-Wang, K.; Lucki, I.; Chesselet, M.-F. A role for the subthalamic nucleus in 5-HT2C-induced oral dyskinesia. Neuroscience 1996, 72, 117–128. [Google Scholar] [CrossRef]
- Parry, T.J.; Eberle-Wang, K.; Lucki, I.; Chesselet, M.-F. Dopaminergic Stimulation of Subthalamic Nucleus Elicits Oral Dyskinesia in Rats. Exp. Neurol. 1994, 128, 181–190. [Google Scholar] [CrossRef]
- Kelley, A.; Delfs, J. Excitatory amino acid receptors mediate the orofacial stereotypy elicited by dopaminergic stimulation of the ventrolateral striatum. Neuroscience 1994, 60, 85–95. [Google Scholar] [CrossRef]
- Plech, A.; Brus, R.; Kostrzewa, R.M.; Kalbfleisch, J.H. Enhanced oral activity responses to intrastriatal SKF 38393 andm-CPP are attenuated by intrastriatal mianserin in neonatal 6-OHDA-lesioned rats. Psychopharmacology 1995, 119, 466–473. [Google Scholar] [CrossRef]
- Kreiss, D.S.; De Deurwaerdère, P. Purposeless oral activity induced by meta -chlorophenylpiperazine (m-CPP): Undefined tic-like behaviors? J. Neurosci. Methods 2017, 292, 30–36. [Google Scholar] [CrossRef]
- Kontis, D.; Boulougouris, V.; Papakosta, V.M.; Kalogerakou, S.; Papadopoulos, S.; Poulopoulou, C.; Papadimitriou, G.N.; Tsaltas, E. Dopaminergic and serotonergic modulation of persistent behaviour in the reinforced spatial alternation model of obsessive–compulsive disorder. Psychopharmacology 2008, 200, 597–610. [Google Scholar] [CrossRef]
- Degos, B.; Deniau, J.-M.; Le Cam, J.; Mailly, P.; Maurice, N. Evidence for a direct subthalamo-cortical loop circuit in the rat. Eur. J. Neurosci. 2008, 27, 2599–2610. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, B.P.; Deniau, J.M.; Glowinski, J.; Thierry, A.M. Basal ganglia and processing of cortical information: Functional interactions between trans-striatal and trans-subthalamic circuits in the substantia nigra pars reticulata. Neuroscience 2003, 117, 931–938. [Google Scholar] [CrossRef]
- Maurice, N.; Deniau, J.-M.; Glowinski, J.; Thierry, A.-M. Relationships between the Prefrontal Cortex and the Basal Ganglia in the Rat: Physiology of the Cortico-Nigral Circuits. J. Neurosci. 1999, 19, 4674–4681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoessl, A.J.; Dourish, C.T.; Iversen, S.D. Apomorphine-induced yawning in rats is abolished by bilateral 6-hydroxydopamine lesions of the substantia nigra. Psychopharmacology 1987, 93, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.T.; Witkin, J.M.; Newman, A.H.; Svensson, K.A.; Grundt, P.; Cao, J.; Woods, J.H. Dopamine agonist-induced yawning in rats: A dopamine D3 receptor-mediated behavior. J. Pharmacol. Exp. Ther. 2005, 314, 310–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, F.; Corda, M.G.; Melis, M.R.; Piludu, M.A.; Löber, S.; Hübner, H.; Gmeiner, P.; Argiolas, A.; Giorgi, O. Dopamine agonist-induced penile erection and yawning: A comparative study in outbred Roman high- and low-avoidance rats. Pharmacol. Biochem. Behav. 2013, 109, 59–66. [Google Scholar] [CrossRef]
- Bonhaus, D.W.; Bach, C.; DeSouza, A.; Salazar, F.R.; Matsuoka, B.D.; Zuppan, P.; Chan, H.W.; Eglen, R.M. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2b) receptor gene products: Comparison with 5-HT2a and 5-HT2c receptors. Br. J. Pharmacol. 1995, 115, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Kreiss, D.S.; Coffman, C.F.; Fiacco, N.R.; Granger, J.C.; Helton, B.M.; Jackson, J.C.; Kim, L.V.; Mistry, R.S.; Mizer, T.M.; Palmer, L.V.; et al. Ritualistic Chewing Behavior induced by mCPP in the rat is an animal model of Obsessive Compulsive Disorder. Pharmacol. Biochem. Behav. 2013, 104, 119–124. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Marshall, J.F. D1 dopamine receptors influence Fos immunoreactivity in the globus pallidus and subthalamic nucleus of intact and nigrostriatal-lesioned rats. Brain Res. 1995, 703, 156–164. [Google Scholar] [CrossRef]
- Di Giovanni, G.; De Deurwaerdère, P. New therapeutic opportunities for 5-HT2C receptor ligands in neuropsychiatric disorders. Pharmacol. Ther. 2016, 157, 125–162. [Google Scholar] [CrossRef]
- Kennett, G.; Wood, M.D.; Bright, F.V.; Trail, B.; Riley, G.; Holland, V.; Avenell, K.Y.; Stean, O.T.; Upton, N.; Bromidge, S.M.; et al. SB 242084, a Selective and Brain Penetrant 5-HT 2C Receptor Antagonist. Neuropharmacology 1997, 36, 609–620. [Google Scholar] [CrossRef]
- Wood, M.; Reavill, C.; Trail, B.; Wilson, A.; Stean, T.; Kennett, G.; Lightowler, S.; Blackburn, T.; Thomas, D.; Gager, T.; et al. SB-243213; a selective 5-HT2C receptor inverse agonist with improved anxiolytic profile: Lack of tolerance and withdrawal anxiety. Neuropharmacology 2001, 41, 186–199. [Google Scholar] [CrossRef]
- Berg, K.A.; Navailles, S.; Sanchez, T.A.; Silva, Y.M.; Wood, M.D.; Spampinato, U.; Clarke, W.P. Differential effects of 5-methyl-1-2-(2-methyl-3-pyridyl)oxyl-5-pyridylcarbamoyl-6-trifluoro methylindone (sb 243213) on 5-hydroxytryptamine(2c) receptor-mediated responses. J. Pharmacol. Exp. Ther. 2006, 319, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Deurwaerdère, P.; Navailles, S.; Berg, A.K.; Clarke, W.P.; Spampinato, U. Constitutive Activity of the Serotonin2C Receptor Inhibits In Vivo Dopamine Release in the Rat Striatum and Nucleus Accumbens. J. Neurosci. 2004, 24, 3235–3241. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Le Moine, C.; Chesselet, M.-F. Selective blockade of serotonin2C receptor enhances Fos expression specifically in the striatum and the subthalamic nucleus within the basal ganglia. Neurosci. Lett. 2010, 469, 251–255. [Google Scholar] [CrossRef]
- Kadiri, N.; Lagière, M.; Le Moine, C.; Millan, M.J.; De Deurwaerdère, P.; Navailles, S. Diverse effects of 5-HT2C receptor blocking agents on c-Fos expression in the rat basal ganglia. Eur. J. Pharmacol. 2012, 689, 8–16. [Google Scholar] [CrossRef]
- Navailles, S.; Lagiere, M.; Le Moine, C.; De Deurwaerdère, P. Role of 5-HT2C receptors in the enhancement of c-Fos expression induced by a 5-HT2B/2C inverse agonist and 5-HT2 agonists in the rat basal ganglia. Exp. Brain Res. 2013, 230, 525–535. [Google Scholar] [CrossRef]
- Ryan, L.J.; Sanders, D.J. Subthalamic nucleus and globus pallidus lesions alter activity in nigrothalamic neurons in rats. Brain Res. Bull. 1994, 34, 19–26. [Google Scholar] [CrossRef]
- Heckman, P.; Schweimer, J.; Sharp, T.; Prickaerts, J.; Blokland, A. Phosphodiesterase 4 inhibition affects both the direct and indirect pathway: An electrophysiological study examining the tri-phasic response in the substantia nigra pars reticulata. Brain Struct. Funct. 2017, 223, 739–748. [Google Scholar] [CrossRef]
- Nambu, A.; Tokuno, H.; Takada, M. Functional significance of the cortico–subthalamo–pallidal hyperdirect pathway. Neurosci. Res. 2002, 43, 111–117. [Google Scholar] [CrossRef]
- Degos, B.; Deniau, J.-M.; Thierry, A.-M.; Glowinski, J.; Pezard, L.; Maurice, N. Neuroleptic-Induced Catalepsy: Electrophysiological Mechanisms of Functional Recovery Induced by High-Frequency Stimulation of the Subthalamic Nucleus. J. Neurosci. 2005, 25, 7687–7696. [Google Scholar] [CrossRef] [PubMed]
- Hassani, O.-K.; Féger, J. Effects of intrasubthalamic injection of dopamine receptor agonists on subthalamic neurons in normal and 6-hydroxydopamine-lesioned rats: An electrophysiological and c-Fos study. Neuroscience 1999, 92, 533–543. [Google Scholar] [CrossRef]
- Aristieta, A.; Morera-Herreras, T.; Ruíz-Ortega, J.A.; Miguelez, C.; Vidaurrazaga, I.; Arrue, A.; Zumárraga, M.; Ugedo, L. Modulation of the subthalamic nucleus activity by serotonergic agents and fluoxetine administration. Psychopharmacology 2013, 231, 1913–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, G.; Rosales, M.G.; Hernández, S.; Sierra, A.; Aceves, J. 5-Hydroxytryptamine increases spontaneous activity of subthalamic neurons in the rat. Neurosci. Lett. 1995, 192, 17–20. [Google Scholar] [CrossRef]
- Stanford, I.; Kantaria, M.; Chahal, H.; Loucif, K.; Wilson, C. 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: Action at 5-HT2C, 5-HT4 and 5-HT1A receptors. Neuropharmacology. 2005, 49, 1228–1234. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, L.; Kitai, S.T. Modulation of Spontaneous Firing in Rat Subthalamic Neurons by 5-HT Receptor Subtypes. J. Neurophysiol. 2005, 93, 1145–1157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.J.; Liu, X.; Liu, J.; Wang, S.; Ali, U.; Wu, Z.H.; Wang, T. Subthalamic neurons show increased firing to 5-HT2C receptor activation in 6-hydroxydopamine-lesioned rats. Brain Res. 2009, 1256, 180–189. [Google Scholar] [CrossRef]
- Chagraoui, A.; Whitestone, S.; Baassiri, L.; Manem, J.; Di Giovanni, G.; De Deurwaerdère, P. Neurochemical impact of the 5-HT2C receptor agonist WAY-163909 on monoamine tissue content in the rat brain. Neurochem. Int. 2019, 124, 245–255. [Google Scholar] [CrossRef]
- Papakosta, V.-M.; Kalogerakou, S.; Kontis, D.; Anyfandi, E.; Theochari, E.; Boulougouris, V.; Papadopoulos, S.; Panagis, G.; Tsaltas, E. 5-HT2C receptor involvement in the control of persistence in the Reinforced Spatial Alternation animal model of obsessive–compulsive disorder. Behav. Brain Res. 2013, 243, 176–183. [Google Scholar] [CrossRef]
- Flaisher-Grinberg, S.; Klavir, O.; Joel, D. The role of 5-HT2A and 5-HT2C receptors in the signal attenuation rat model of obsessive–compulsive disorder. Int. J. Neuropsychopharmacol. 2008, 11, 811–825. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, P.J.; Soko, A.D.; Higgins, G.A. Impulsive action in the 5-choice serial reaction time test in 5-HT2C receptor null mutant mice. Psychopharmacology 2012, 226, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.J.; Tampakeras, M.; Sinyard, J.; Higgins, G.A. Opposing effects of 5-HT2A and 5-HT2C receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology 2007, 195, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.A.; Silenieks, L.B.; Lau, W.; De Lannoy, I.A.M.; Lee, D.K.H.; Izhakova, J.; Coen, K.; Le, A.D.; Fletcher, P.J. Evaluation of chemically diverse 5-HT2C receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology 2012, 226, 475–490. [Google Scholar] [CrossRef]
- Tucci, M.C.; Dvorkin-Gheva, A.; Johnson, E.; Wong, M.; Szechtman, H. 5-HT2A/C receptors do not mediate the attenuation of compulsive checking by mCPP in the quinpirole sensitization rat model of obsessive–compulsive disorder (OCD). Behav. Brain Res. 2015, 279, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.C.; Dvorkin-Gheva, A.; Sharma, R.; Taji, L.; Cheon, P.; Peel, J.; Kirk, A.; Szechtman, H. Separate mechanisms for development and performance of compulsive checking in the quinpirole sensitization rat model of obsessive-compulsive disorder (OCD). Psychopharmacology 2014, 231, 3707–3718. [Google Scholar] [CrossRef]
- Enespoli, E.; Rizzo, F.; Boeckers, T.; Schulze, U.; Hengerer, B. Altered dopaminergic regulation of the dorsal striatum is able to induce tic-like movements in juvenile rats. PLoS ONE 2018, 13, e0196515. [Google Scholar] [CrossRef]
- Straathof, M.; Blezer, E.L.; Van Heijningen, C.; Smeele, C.E.; Van Der Toorn, A.; Buitelaar, J.K.; Glennon, J.C.; Otte, W.M.; Dijkhuizen, R.M.; De Ruiter, S.; et al. Structural and functional MRI of altered brain development in a novel adolescent rat model of quinpirole-induced compulsive checking behavior. Eur. Neuropsychopharmacol. 2020, 33, 58–70. [Google Scholar] [CrossRef]
- Winter, C.; Mundt, A.P.; Jalali, R.; Joel, D.; Harnack, D.; Morgenstern, R.; Juckel, G.; Kupsch, A. High frequency stimulation and temporary inactivation of the subthalamic nucleus reduce quinpirole-induced compulsive checking behavior in rats. Exp. Neurol. 2008, 210, 217–228. [Google Scholar] [CrossRef]
- Szechtman, H.; Ahmari, S.E.; Beninger, R.J.; Eilam, D.; Harvey, B.H.; Edemann-Callesen, H.; Winter, C. Obsessive-compulsive disorder: Insights from animal models. Neurosci. Biobehav. Rev. 2017, 76, 254–279. [Google Scholar] [CrossRef] [Green Version]
- Welter, M.-L.; Burbaud, P.; Fernandez-Vidal, S.; Bardinet, E.; Coste, J.; Piallat, B.; Borg, M.; Besnard, S.; Sauleau, P.; Devaux, B.; et al. Basal ganglia dysfunction in OCD: Subthalamic neuronal activity correlates with symptoms severity and predicts high-frequency stimulation efficacy. Transl. Psychiatry 2011, 1, e5. [Google Scholar] [CrossRef]
- Haynes, W.I.; Clair, A.-H.; Fernandez-Vidal, S.; Gholipour, B.; Morgiève, M.; Mallet, L. Altered anatomical connections of associative and limbic cortico-basal-ganglia circuits in obsessive-compulsive disorder. Eur. Psychiatry 2018, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Eberle-Wang, K.; Chesselet, M.-F. Increased m-CPP-induced oral dyskinesia after lesion of serotonergic neurons. Pharmacol. Biochem. Behav. 2001, 68, 347–353. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Delaville, C.; Chetrit, J.; Abdallah, K.; Morin, S.; Cardoit, L.; De Deurwaerdère, P.; Benazzouz, A. Emerging dysfunctions consequent to combined monoaminergic depletions in parkinsonism. Neurobiol. Dis. 2012, 45, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Gao, D.; Bouali-Benazzouz, R.; Benabid, A.-L.; Benazzouz, A. Effect of microiontophoretic application of dopamine on subthalamic nucleus neuronal activity in normal rats and in rats with unilateral lesion of the nigrostriatal pathway. Eur. J. Neurosci. 2001, 14, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Tinakoua, A.; Bouabid, S.; Faggiani, E.; De Deurwaerdère, P.; Lakhdar-Ghazal, N.; Benazzouz, A. The impact of combined administration of paraquat and maneb on motor and non-motor functions in the rat. Neuroscience 2015, 311, 118–129. [Google Scholar] [CrossRef]
- Bunney, B.S.; Walters, J.R.; Roth, R.H.; Aghajanian, G.K. Dopaminergic neurons: Effect of antipsychotic drugs and amphetamine on single cell activity. J. Pharmacol. Exp. Ther. 1973, 185, 560–571. [Google Scholar]
- Collins, P.; Broekkamp, C.L.E.; Jenner, P.; Marsden, C.D. Drugs acting at D-1 and D-2 dopamine receptors induce identical purposeless chewing in rats which can be differentiated by cholinergic manipulation. Psychopharmacology 1991, 103, 503–512. [Google Scholar] [CrossRef]
- Dekeyne, A.; La Cour, C.M.; Gobert, A.; Brocco, M.; Lejeune, F.; Serres, F.; Sharp, T.; Daszuta, A.; Soumier, A.; Papp, M.; et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology 2008, 199, 549–568. [Google Scholar] [CrossRef]
- Millan, M.J.; Maiofiss, L.; Cussac, D.; Audinot, V.; Boutin, J.-A.; Newman-Tancredi, A. Differential Actions of Antiparkinson Agents at Multiple Classes of Monoaminergic Receptor. I. A Multivariate Analysis of the Binding Profiles of 14 Drugs at 21 Native and Cloned Human Receptor Subtypes. J. Pharmacol. Exp. Ther. 2002, 303, 791–804. [Google Scholar] [CrossRef] [Green Version]
- Georges, F.; Aston-Jones, G. Activation of Ventral Tegmental Area Cells by the Bed Nucleus of the Stria Terminalis: A Novel Excitatory Amino Acid Input to Midbrain Dopamine Neurons. J. Neurosci. 2002, 22, 5173–5187. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Di Giovanni, G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: Therapeutic implications. Prog. Neurobiol. 2017, 151, 175–236. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.A.; Fletcher, P.J. Therapeutic Potential of 5-HT2CReceptor Agonists for Addictive Disorders. ACS Chem. Neurosci. 2015, 6, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- Howell, L.L.; Cunningham, K.A. Serotonin 5-HT2 Receptor Interactions with Dopamine Function: Implications for Therapeutics in Cocaine Use Disorder. Pharmacol. Rev. 2015, 67, 176–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millan, M.J. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol. Ther. 2006, 110, 135–370. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.; Dekeyne, A.; Gobert, A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology 1998, 37, 953–955. [Google Scholar] [CrossRef]
- Millan, M.J.; Kamal, M.; Jockers, R.; Chanrion, B.; Labasque, M.; La Cour, C.M.; Marin, P.; Bockaert, J. The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5-HT2C receptors. Int. J. Neuropsychopharmacol. 2010, 14, 768–783. [Google Scholar] [CrossRef] [Green Version]
- Creed, M.C.; Oraha, A.; Nobrega, J.N. Effects of 5-HT2A and 5-HT2C receptor antagonists on acute and chronic dyskinetic effects induced by haloperidol in rats. Behav. Brain Res. 2011, 219, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Dahl, M.-L.; Spina, E.; Scordo, M.G. Further evidence for the association between 5-HT2C receptor gene polymorphisms and extrapyramidal side effects in male schizophrenic patients. Eur. J. Clin. Pharmacol. 2008, 64, 477–482. [Google Scholar] [CrossRef]
- Pact, V.; Giduz, T. Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology 1999, 53, 1154. [Google Scholar] [CrossRef]
- Richtand, N.M.; McNamara, R. Serotonin and Dopamine Interactions in Psychosis Prevention. Prog. Brain Res. 2008, 172, 141–153. [Google Scholar] [CrossRef]





| Early Excitation E1 | Inhibition I | Late Excitation E2 | |
|---|---|---|---|
| initial Rmag | 16.6 ± 1.1 | −15.3 ± 1.4 | 33.0 ± 2.7 |
| Duration (ms) | 8.1 ± 0.5 | 12.1 ± 0.8 | 12.7 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagière, M.; Bosc, M.; Whitestone, S.; Benazzouz, A.; Chagraoui, A.; Millan, M.J.; De Deurwaerdère, P. A Subset of Purposeless Oral Movements Triggered by Dopaminergic Agonists Is Modulated by 5-HT2C Receptors in Rats: Implication of the Subthalamic Nucleus. Int. J. Mol. Sci. 2020, 21, 8509. https://doi.org/10.3390/ijms21228509
Lagière M, Bosc M, Whitestone S, Benazzouz A, Chagraoui A, Millan MJ, De Deurwaerdère P. A Subset of Purposeless Oral Movements Triggered by Dopaminergic Agonists Is Modulated by 5-HT2C Receptors in Rats: Implication of the Subthalamic Nucleus. International Journal of Molecular Sciences. 2020; 21(22):8509. https://doi.org/10.3390/ijms21228509
Chicago/Turabian StyleLagière, Mélanie, Marion Bosc, Sara Whitestone, Abdelhamid Benazzouz, Abdeslam Chagraoui, Mark J. Millan, and Philippe De Deurwaerdère. 2020. "A Subset of Purposeless Oral Movements Triggered by Dopaminergic Agonists Is Modulated by 5-HT2C Receptors in Rats: Implication of the Subthalamic Nucleus" International Journal of Molecular Sciences 21, no. 22: 8509. https://doi.org/10.3390/ijms21228509
APA StyleLagière, M., Bosc, M., Whitestone, S., Benazzouz, A., Chagraoui, A., Millan, M. J., & De Deurwaerdère, P. (2020). A Subset of Purposeless Oral Movements Triggered by Dopaminergic Agonists Is Modulated by 5-HT2C Receptors in Rats: Implication of the Subthalamic Nucleus. International Journal of Molecular Sciences, 21(22), 8509. https://doi.org/10.3390/ijms21228509