Adenovirus Reveals New Pathway for Cholesterol Egress from the Endolysosomal System
Abstract
:1. Introduction
2. Non-Vesicular Lipid Transport at MCSs
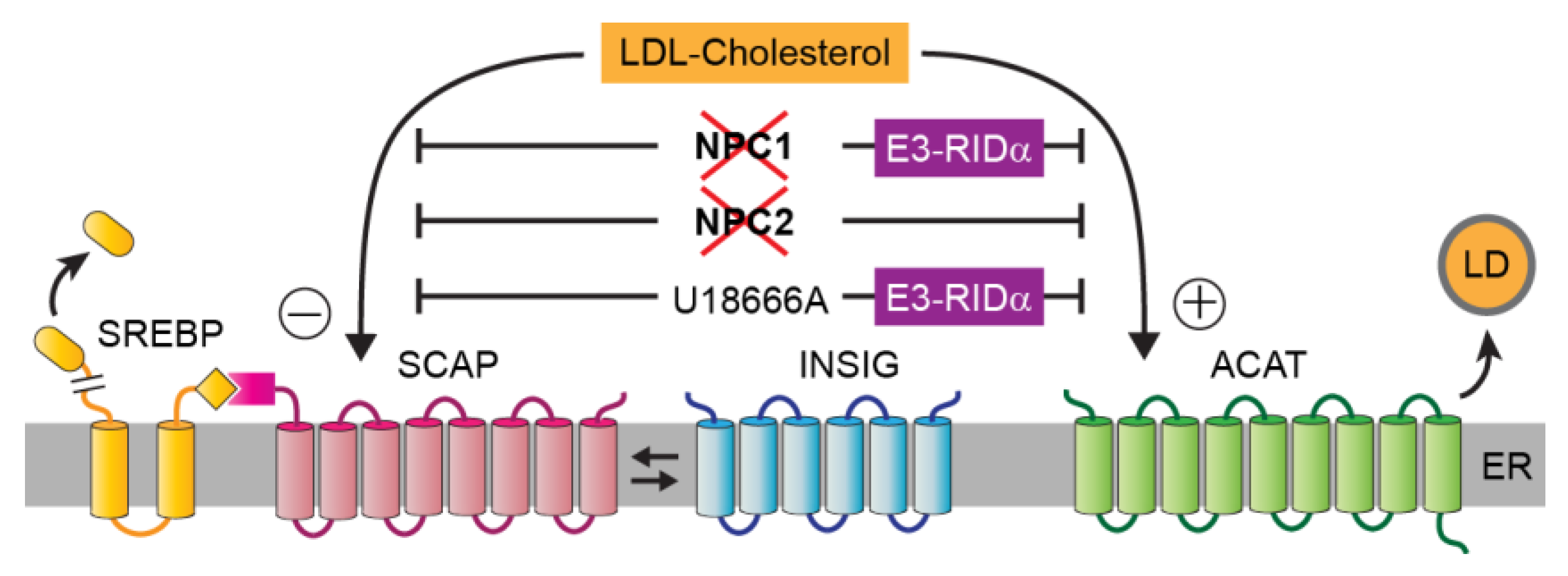
3. Cholesterol Homeostasis
4. Mechanisms of LE/Lys Movement and Positioning
5. Host Intracellular Trafficking Pathways Regulate the Early Stages of Adenovirus Infection
6. Adenovirus E3 Protein Hijacks ORP1L to Restore Cholesterol Homeostasis
7. Are There Physiological ORP1L-Dependent Cholesterol Transport Pathways?
8. Conclusions and Future Directions
Funding
Conflicts of Interest
Abbreviations
| ACAT | Acyl-CoA: cholesterol acyltransferase |
| ASMase | Acid sphingomyelinase |
| CAR | Coxsackievirus B adenovirus receptor |
| E3 | Early region three |
| EGFR | EGF receptor |
| ER | Endoplasmic reticulum |
| FFAT | Two phenylalanines (FF) in an acidic tract |
| LBPA | Lysobisphosphatidic acid |
| LDL | Low density lipoprotein |
| LE/Lys | Late endosome/lysosome |
| LIC1 | Light intermediate chain 1 |
| LSO | Lysosomal storage organelles |
| LTP | Lipid transfer protein |
| MCS | Membrane contact sites |
| MVB | Multivesicular body |
| MTOC | Mitotic organizing center |
| NPC | Niemann–Pick type C disease |
| ORD | OSBP-related domain |
| ORP | Oxysterol-binding protein (OSBP)-related protein |
| PH | Pleckstrin homology |
| PKA | Protein kinase A |
| SBD | Sterol binding domain |
| SREBP | Sterol regulatory element-binding protein |
| START | Steroidogenic acute regulatory protein-related lipid transfer |
| VRC | Viral replication complexes |
References
- Iaea, D.; Maxfield, F.R. Cholesterol trafficking and distribution. Essays Biochem. 2015, 57, 43–55. [Google Scholar] [PubMed]
- Ikonen, E. Mechanisms of cellular cholesterol compartmentalization: Recent insights. Curr. Opin. Cell Biol. 2018, 53, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Basseri, S.; Austin, R.C. Endoplasmic reticulum stress and lipid metabolism: Mechanisms and therapeutic potential. Biochem. Res. Int. 2012, 2012, 841362. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Prinz, W.A. Bridging the gap: Membrane contact sites in signaling, metabolism, and organelle dynamics. J. Cell Biol. 2015, 205, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Raiborg, C.; Wenzel, E.M.; Stenmark, H. ER-endosome contact sites: Molecular compositions and functions. EMBO J. 2015, 34, 1848–1858. [Google Scholar] [CrossRef] [Green Version]
- Knoblach, B.; Sun, X.; Coquelle, N.; Fagarasanu, A.; Poirier, R.L.; Rachubinski, R.A. An ER-peroxisome tether exerts peroxisome population control in yeast. EMBO J. 2013, 32, 2439–2453. [Google Scholar] [CrossRef] [Green Version]
- Lackner, L.L.; Ping, H.; Graef, M.; Murley, A.; Nunnari, J. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc. Natl. Acad. Sci. USA 2013, 110, E458–E467. [Google Scholar] [CrossRef] [Green Version]
- Bockler, S.; Westermann, B. Mitochondrial ER contacts are crucial for mitophagy in yeast. Dev. Cell 2014, 28, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER tubules mark sites of mitochondrial division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.R.; DiBenedetto, J.R.; West, M.; Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell 2013, 24, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Kojima, H.; Nagano, T.; Okabe, T.; Wakita, T.; Shimizu, H. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 2011, 85, 2364–2372. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, X.; Tangchaiburana, S.; Ndeh, R.; Markham, J.E.; Tsegaye, Y.; Dunn, T.M.; Wang, G.-L.; Bellizzi, M.; Parsons, J.F.; et al. An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 2008, 20, 3163–3179. [Google Scholar] [CrossRef] [Green Version]
- Roulin, P.S.; Murer, L.P.; Greber, U.F. A single point mutation in the rhinovirus 2B protein reduces the requirement for phosphatidylinositol 4-kinase class III beta in viral replication. J. Virol. 2018, 92, e01462-18. [Google Scholar]
- Dorobantu, C.M.; Albulescu, L.; Harak, C.; Feng, Q.; van Kampen, M.; Strating, J.R.P.M.; Gorbalenya, A.E.; Lohmann, V.; van der Schaar, H.M.; van Kuppeveld, F.J.M. Modulation of the host lipid landscape to promote RNA virus replication: The picornavirus encephalomyocarditis virus converges on the pathway used by hepatitis C virus. PLoS Pathog. 2014, 11, e1005185. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa-Sasaki, K.; Sasaki, J.; Taniguchi, K. A complex comprising phosphatidylinositol 4-Kinase III, ACBD3, and Aichi virus proteins enhances phosphatidylinositol 4-phosphate synthesis and is critical for formation of the viral replication complex. J. Virol. 2014, 88, 6586–6598. [Google Scholar] [CrossRef] [Green Version]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef] [Green Version]
- Toulmay, A.; Prinz, W.A. Lipid transfer and signaling at organelle contact sites: The tip of the iceberg. Curr. Opin. Cell Biol. 2011, 23, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Sprong, H.; van der Sluijs, P.; van Meer, G. How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol. 2001, 2, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Somerharju, P. Is spontaneous translocation of polar lipids between cellular organelles negligible? Lipid Insights 2016, 8, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, L.H.; Gatta, A.T.; Levine, T.P. Lipid transfer proteins: The lipid commute via shuttles, bridges and tubes. Nat. Rev. Mol. Cell Biol. 2019, 20, 85–101. [Google Scholar] [CrossRef]
- Selitrennik, M.; Lev, S. The role of phosphatidylinositol-transfer proteins at membrane contact sites. Biochem. Soc. Trans. 2016, 44, 419–424. [Google Scholar] [CrossRef]
- von Filseck, J.M.; Mesmin, B.; Bigay, J.l.; Antonny, B.; Drin, G. Building lipid PIPelines throughout the cell by ORP/Osh proteins. Biochem. Soc. Trans. 2014, 42, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Im, Y.J.; Hurley, J.H.; Prinz, W.A. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J. Cell Biol. 2006, 173, 107–119. [Google Scholar] [CrossRef]
- Morley, S.; Cecchini, M.; Zhang, W.; Virgulti, A.; Noy, N.; Atkinson, J.; Manor, D. Mechanisms of ligand transfer by the hepatic tocopherol transfer protein. J. Biol. Chem. 2008, 283, 17797–17804. [Google Scholar] [CrossRef] [Green Version]
- Antonny, B.; Bigay, J.; Mesmin, B. The oxysterol-binding protein cycle: Burning off PI (4) P to transport cholesterol. Ann. Rev. Biochem. 2018, 87, 809–837. [Google Scholar] [CrossRef]
- Wong, L.H.; Copic, A.; Levine, T.P. Advances on the transfer of lipids by lipid transfer proteins. Trends Biochem. Sci. 2017, 42, 516–530. [Google Scholar] [CrossRef] [Green Version]
- Lange, Y. Disposition of intracellular cholesterol in human fibroblasts. J. Lipid Res. 1991, 32, 329–339. [Google Scholar]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.S. A receptor-mediated pathway for cholesterol homeostasis. In Nobel Lectures, Physiology or Medicine 1981–1990; World Scientific Publishing Co.: Singapore, 1985. [Google Scholar]
- Chevallier, J.; Chamoun, Z.; Jiang, G.; Prestwich, G.; Sakai, N.; Matile, S.; Parton, R.G.; Gruenberg, J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J. Biol. Chem. 2008, 283, 27871–27880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vance, J.E.; Karten, B. Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J. Lipid Res. 2014, 55, 1609–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, S.R. NPC intracellular cholesterol transporter 1 (NPC1)-mediated cholesterol export from lysosomes. J. Biol. Chem. 2019, 294, 1706–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Benoff, B.; Liou, H.-L.; Lobel, P.; Stock, A.M. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick Type C2 disease. J. Biol. Chem. 2007, 282, 23525–23531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCauliff, L.A.; Langan, A.; Li, R.; Ilnytska, O.; Bose, D.; Waghalter, M.; Lai, K.; Kahn, P.C.; Storch, J. Intracellular cholesterol trafficking is dependent upon NPC2 interaction with lysobisphosphatidic acid. eLife 2019, 8, e50832. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Coutavas, E.; Shi, H.; Hao, Q.; Blobel, G. Structure of human Niemann Pick C1 protein. Proc. Natl. Acad. Sci. USA 2016, 113, 8212–8217. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shi, Y.; Song, J.; Qi, J.; Lu, G.; Yan, J.; Gao, G.F. Ebola viral glycoprotein bound to its endosomal receptor Niemann-Pick C1. Cell 2016, 164, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef] [Green Version]
- Infante, R.E.; Wang, M.L.; Radhakrishnan, A.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 15287–15292. [Google Scholar] [CrossRef] [Green Version]
- Charman, M.; Kennedy, B.E.; Osborne, N.; Karten, B. MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein. J. Lipid Res. 2010, 51, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Deffieu, M.S.; Lee, P.L.; Saha, P.; Pfeffer, S.R. Glycosylation inhibition reduces cholesterol accumulation in NPC1 protein-deficient cells. Proc. Natl. Acad. Sci. USA 2015, 112, 14876–14881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Kumar, J.; Ferguson, C.; Schulz, T.A.; Ong, Y.S.; Hong, W.; Prinz, W.A.; Parton, R.G.; Brown, A.J.; Yang, H. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J. Cell Biol. 2011, 192, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoglinger, D.; Burgoyne, T.; Sanchez-Heras, E.; Hartwig, P.; Colaco, A.; Newton, J.; Futter, C.E.; Spiegel, S.; Platt, F.M.; Eden, E.R. NPC1 regulates ER contacts with endocytic organelles to mediate cholesterol egress. Nat. Commun. 2019, 10, 4276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.; Torta, F.; Masai, K.; Lucast, L.; Czapla, H.; Tanner, L.B.; Narayanaswamy, P.; Wenk, M.R.; Nakatsu, F.; De Camilli, P. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 2015, 349, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Sturley, S.L.; Patterson, M.C.; Pentchev, P. Unraveling the sterol-trafficking defect in Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA 2009, 106, 2093–2094. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-Y.; Chang, C.C.Y.; Ohgami, N.; Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 2006, 22, 129–157. [Google Scholar] [CrossRef]
- Cianciola, N.L.; Chung, S.; Manor, D.; Carlin, C.R. Adenovirus modulates Toll-like receptor 4 signaling by reprogramming ORP1L-VAP protein contacts for cholesterol transport from endosomes to the endoplasmic reticulum. J. Virol. 2017, 91, e01904–e01916. [Google Scholar] [CrossRef] [Green Version]
- Cianciola, N.L.; Greene, D.J.; Morton, R.E.; Carlin, C.R. Adenovirus RIDa uncovers a novel pathway requiring ORP1L for lipid droplet formation independent of NPC1. Mol. Biol. Cell 2013, 24, 3309–3325. [Google Scholar] [CrossRef]
- Lange, Y.; Strebel, F.; Steck, T.L. Role of the plasma membrane in cholesterol esterification in rat hepatoma cells. J. Biol. Chem. 1993, 268, 13838–13843. [Google Scholar]
- Ilnytska, O.; Santiana, M.; Hsu, N.-Y.; Du, W.-L.; Chen, Y.-H.; Viktorova, E.G.; Belov, G.; Brinker, A.; Storch, J.; Moore, C.; et al. Enteroviruses harness the cellular endocytic machinery to remodel the host cell cholesterol landscape for effective viral replication. Cell Host Microbe 2013, 14, 281–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristiana, I.; Yang, H.; Brown, A.J. Different kinetics of cholesterol delivery to components of the cholesterol homeostatic machinery: Implications for cholesterol trafficking to the endoplasmic reticulum. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Srivastava, K.; Daphna-Iken, D.; Traub, L.M.; Schaffer, J.E.; Ory, D.S. Cholesterol overload promotes morphogenesis of a Niemann-Pick C (NPC)-like compartment independent of inhibition of NPC1 or HE1/NPC2 function. J. Biol. Chem. 2001, 276, 46414–46421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dove, D.E.; Su, Y.R.; Zhang, W.; Jerome, W.G.; Swift, L.L.; Linton, M.F.; Fazio, S. ACAT1 deficiency disrupts cholesterol efflux and alters cellular morphology in macrophages. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Sobo, K.; Le Blanc, I.; Luyet, P.-P.; Fivaz, M.; Ferguson, C.; Parton, R.G.; Gruenberg, J.; van der Goot, F.G. Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking. PLoS ONE 2007, 2, e851. [Google Scholar] [CrossRef]
- Pacheco, C.; Lieberman, A. The pathogenesis of Niemann-Pick type C disease: A role for autophagy? Expert Rev. Mol. Med. 2008, 10, e26. [Google Scholar] [CrossRef] [Green Version]
- Ganley, I.G.; Pfeffer, S.R. Cholesterol accumulation sequesters Rab9 and disrupts late endosome function in NPC1-deficient cells. J. Biol. Chem. 2006, 281, 17890–17899. [Google Scholar] [CrossRef] [Green Version]
- Granger, E.; McNee, G.; Allan, V.; Woodman, P. The role of the cytoskeleton and molecular motors in endosomal dynamics. Sem. Cell Dev. Biol. 2014, 31, 20–29. [Google Scholar] [CrossRef]
- Guerra, F.; Bucci, C. Multiple Roles of the Small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef]
- Rocha, N.; Kuijl, C.; van der Kant, R.; Janssen, L.; Houben, D.; Janssen, H.; Zwart, W.; Neefjes, J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150Glued and late endosome positioning. J. Cell Biol. 2009, 185, 1209–1225. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.; Rocha, N.; Zwart, W.; Jordens, I.; Janssen, L.; Kuijl, C.; Olkkonen, V.; Neefjes, J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor {beta}lll spectrin. J. Cell Biol. 2007, 12, 459–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrand, C.; Corti, M.; Goodson, H.; Cosson, P.; Cavalli, V.; Mayran, N.; Fauré, J.; Gruenberg, J. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002, 21, 1289–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, S.; Hogg, J.C. Adenovirus infections and lung disease. Curr. Opin. Pharm. 2007, 7, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Meier, O.; Greber, U.F. Adenovirus endocytosis. J. Gene Med. 2004, 6, S152–S163. [Google Scholar] [CrossRef]
- Luisoni, S.; Suomalainen, M.; Boucke, K.; Tanner, L.B.; Wenk, M.R.; Guan, X.L.; Grzybek, M.; Coskun, A.; Greber, U.F. Co-option of membrane wounding enables virus penetration into cells. Cell Host Microbe 2016, 18, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Burckhardt, C.J.; Suomalainen, M.; Schoenenberger, P.; Boucke, K.; Hemmi, S.; Greber, U.F. Drifting motions of the adenovirus receptor CAR and immobile integrins initiate virus uncoating and membrane lytic protein exposure. Cell Host Microbe 2011, 10, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.W.; Almeida, P.E.; Corrotte, M. Damage control: Cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014, 24, 734–742. [Google Scholar] [CrossRef] [Green Version]
- Hendrickx, R.; Stichling, N.; Koelen, J.; Kuryk, L.; Lipiec, A.; Greber, U. Innate immunity to adenovirus. Hum. Gene Ther. 2014, 25, 265–284. [Google Scholar] [CrossRef] [Green Version]
- Carlin, C.R. New insights to adenovirus-directed innate immunity in respiratory epithelial cells. Microorganisms 2019, 7, 216. [Google Scholar] [CrossRef] [Green Version]
- Suomalainen, M.; Nakano, M.Y.; Boucke, K.; Keller, S.; Greber, U.F. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 2001, 20, 1310–1319. [Google Scholar] [CrossRef]
- Scherer, J.; Yi, J.; Vallee, R.B. PKA-dependent dynein switching from lysosomes to adenovirus: A novel form of host-virus competition. J. Cell Biol. 2014, 205, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Choi, A.M.K. Autophagy in the lung. Proc. Am. Thorac. Soc. 2010, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Carlin, C.R. Host cell autophagy modulates early stages of adenovirus infections in airway epithelial cells. J. Virol. 2013, 87, 2307–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.M.J.; Mellouk, N.; Osborne, S.E.; Ammendolia, D.A.; Dyer, D.N.; Li, R.; Brunen, D.; van Rijn, J.M.; Huang, J.; Czuczman, M.A.; et al. An ATG16L1-dependent pathway promotes plasma membrane repair and limits Listeria monocytogenes cell-to-cell spread. Nat. Microbiol. 2018, 3, 1472–1485. [Google Scholar] [CrossRef] [PubMed]
- Imelli, N.; Meier, O.; Boucke, K.; Hemmi, S.; Greber, U.F. Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2. J. Virol. 2004, 78, 3089–3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Li, G.-M.; Li, Y.-G.; Yamate, M.; Li, S.-M.; Ikuta, K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007, 9, 96–102. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef]
- Meher, G.; Bhattacharjya, S.; Chakraborty, H. Membrane cholesterol modulates oligomeric status and peptide-membrane interaction of severe acute respiratory syndrome coronavirus fusion peptide. J. Phys. Chem. B 2019, 123, 10654–10662. [Google Scholar] [CrossRef]
- Katsiki, N.; Banach, M.; Mikhailidis, D.P. Lipid-lowering therapy and renin-angiotensin-aldosterone system inhibitors in the era of the COVID-19 pandemic. Arch. Med. Sci. 2020, 16, 485–489. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Hoffman, P.; Carlin, C. Adenovirus E3 protein causes constitutively internalized EGF receptors to accumulate in a prelysosomal compartment, resulting in enhanced degradation. Mol. Cell Biol. 1994, 14, 3695–3706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Carlin, C.R. Adenovirus early region 3 RIDa protein limits NFkB signaling through stress-activated EGF receptors. PLoS Pathog. 2019, 15, e1008017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.H.; Cianciola, N.L.; Mills, J.L.; Sonnichsen, F.D.; Carlin, C. Adenovirus RIDa regulates endosome maturation by mimicking GTP-Rab7. J. Cell Biol. 2007, 179, 965–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianciola, N.L.; Carlin, C.R. Adenovirus RID-a activates an autonomous cholesterol regulatory mechanism that rescues defects linked to Niemann-Pick disease type C. J. Cell Biol. 2009, 187, 537–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, Y.J.; Raychaudhuri, S.; Prinz, W.A.; Hurley, J.H. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature 2005, 437, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Slotte, J.P.; Bierman, E.L. Movement of plasma-membrane sterols to the endoplasmic reticulum in cultured cells. Biochem. J. 1987, 248, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shisler, J.; Yang, C.; Walter, B.; Ware, C.; Gooding, L. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J. Virol. 1997, 71, 8299–8306. [Google Scholar] [CrossRef] [Green Version]
- Tollefson, A.E.; Hermiston, T.W.; Lichtenstein, D.L.; Colle, C.F.; Tripp, R.A.; Dimitrov, T.; Toth, K.; Wells, C.E.; Doherty, P.C.; Wold, W.S.M. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature 1997, 392, 726–730. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugimoto, Y.; Ohsaki, Y.; Ueno, M.; Kato, S.; Kitamura, Y.; Hosokawa, H.; Davies, J.; Ioannou, Y.; Vanier, M.; et al. Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: A potential basis for glial cell activation in the NPC brain. J. Neurosci. 2007, 27, 1879–1891. [Google Scholar]
- Jiang, H.; White, E.; Gomez-Manzano, C.; Fueyo, J. Adenovirus’s last trick. Autophagy 2008, 4, 118–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boura, E.; Nencka, R. Phosphatidylinositol 4-kinases: Function, structure, and inhibition. Exp. Cell Res. 2015, 337, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; van der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobuna, H.; Inoue, T.; Shibata, M.; Gengyo-Ando, K.; Yamamoto, A.; Mitani, S.; Arai, H. Multivesicular body formation requires OSBP-related proteins and cholesterol. PLoS Genet. 2010, 6, e1001055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eden, E.R.; Sanchez-Heras, E.; Tsapara, A.; Sobota, A.; Levine, T.P.; Futter, C.E. Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev. Cell 2016, 37, 473–483. [Google Scholar] [CrossRef] [Green Version]
- van der Goot, F.G.; Gruenberg, J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006, 16, 514–521. [Google Scholar] [CrossRef]
- Zhao, K.; Ridgway, N.D. Oxysterol-binding protein-related protein 1L regulates cholesterol egress from the endo-lysosomal system. Cell Rep. 2017, 19, 1807–1818. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Du, X.; Wang, H.; Wang, J.; Lu, C.; Chen, X.; Zhu, Z.; Luo, Z.; Yu, L.; Brown, A.J.; et al. Allosteric enhancement of ORP1-mediated cholesterol transport by PI(4,5)P2/PI(3,4)P2. Nat. Commun. 2019, 10, 829. [Google Scholar] [CrossRef] [Green Version]
- Berk, A.J. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 2005, 24, 7673–7685. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, V.; Gennaro, M.L. Foam cells: One size doesn’t fit all. Trends Immunol. 2019, 40, 1163–1179. [Google Scholar] [CrossRef]
- Singer, K.; Cheng, W.-C.; Kreutz, M.; Ho, P.-C.; Siska, P.J. Immunometabolism in cancer at a glance. Dis. Models Mech. 2018, 11, dmm034272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.-H.; Fu, Y.-C.; Zhang, D.-W.; Yin, K.; Tang, C.-K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Fujiwara, Y.; Chang, C.C.Y.; Chang, T.-Y.; Takeya, M.; Sakashita, N. Association of ACAT1-positive vesicles with late endosomes/lysosomes in cholesterol-rich human macrophages. J. Atheroscler. Thromb. 2010, 17, 740–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakashita, N.; Chang, C.C.Y.; Lei, X.; Fujiwara, Y.; Takeya, M.; Chang, T.-Y. Cholesterol loading in macrophages stimulates formation of ER-derived vesicles with elevated ACAT1 activity. J. Lipid Res. 2010, 51, 1263–1272. [Google Scholar] [CrossRef]
- Lu, F.; Liang, Q.; Abi-Mosleh, L.; Das, A.; De Brabander, J.K.; Goldstein, J.L.; Brown, M.S. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. eLife 2015, 4, e12177. [Google Scholar] [CrossRef]
- Johansson, M.; Lehto, M.; Tanhuanpaa, K.; Cover, T.L.; Olkkonen, V.M. The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol. Biol. Cell 2005, 16, 5480–5492. [Google Scholar] [CrossRef]
- Cruz, A.L.S.; Barreto, E.d.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Krycer, J.R.; Sharpe, L.J.; Luu, W.; Brown, A.J. The Akt-SREBP nexus: Cell signaling meets lipid metabolism. Trends Endocrin. Metab. 2010, 21, 268–276. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlin, C.; Manor, D. Adenovirus Reveals New Pathway for Cholesterol Egress from the Endolysosomal System. Int. J. Mol. Sci. 2020, 21, 5808. https://doi.org/10.3390/ijms21165808
Carlin C, Manor D. Adenovirus Reveals New Pathway for Cholesterol Egress from the Endolysosomal System. International Journal of Molecular Sciences. 2020; 21(16):5808. https://doi.org/10.3390/ijms21165808
Chicago/Turabian StyleCarlin, Cathleen, and Danny Manor. 2020. "Adenovirus Reveals New Pathway for Cholesterol Egress from the Endolysosomal System" International Journal of Molecular Sciences 21, no. 16: 5808. https://doi.org/10.3390/ijms21165808



