P2 Receptors Influence hMSCs Differentiation towards Endothelial Cell and Smooth Muscle Cell Lineages
Abstract
:1. Introduction
2. Results
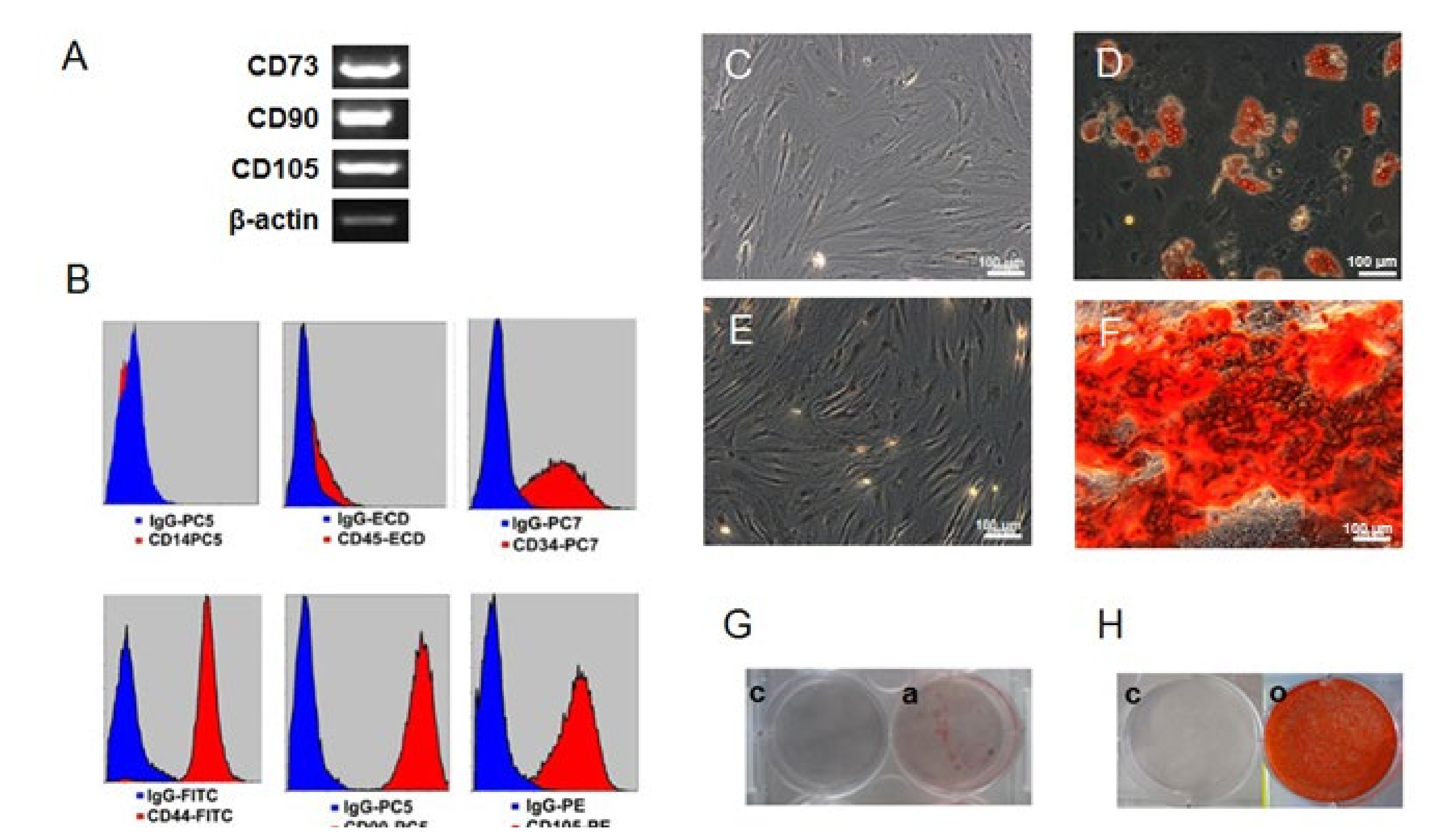
2.1. Confirmation of the Human Mesenchymal Stem Cells (hMSC) Character
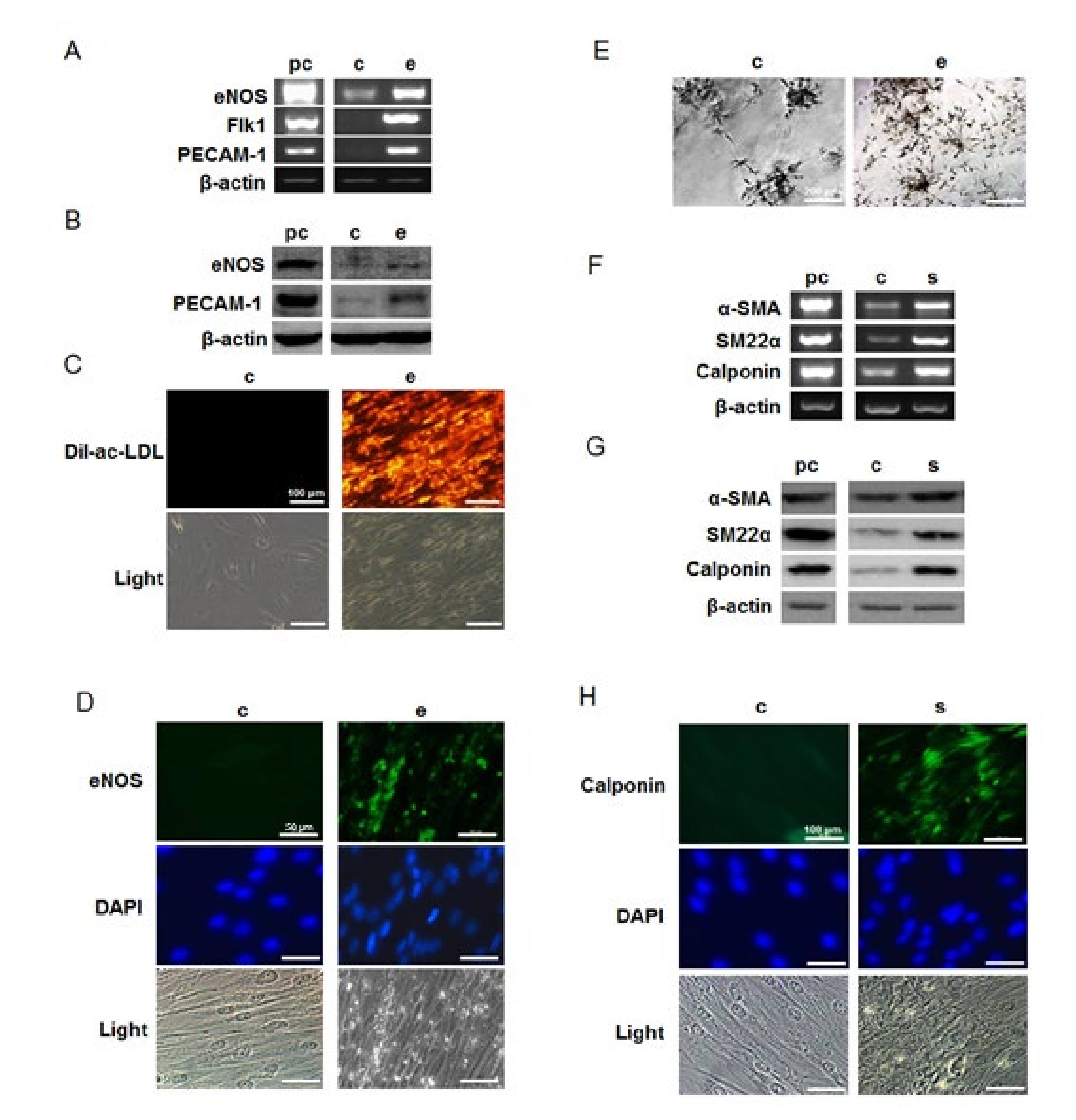
2.2. Differentiation of hMSCs towards Endothelial and Smooth Muscle Cells
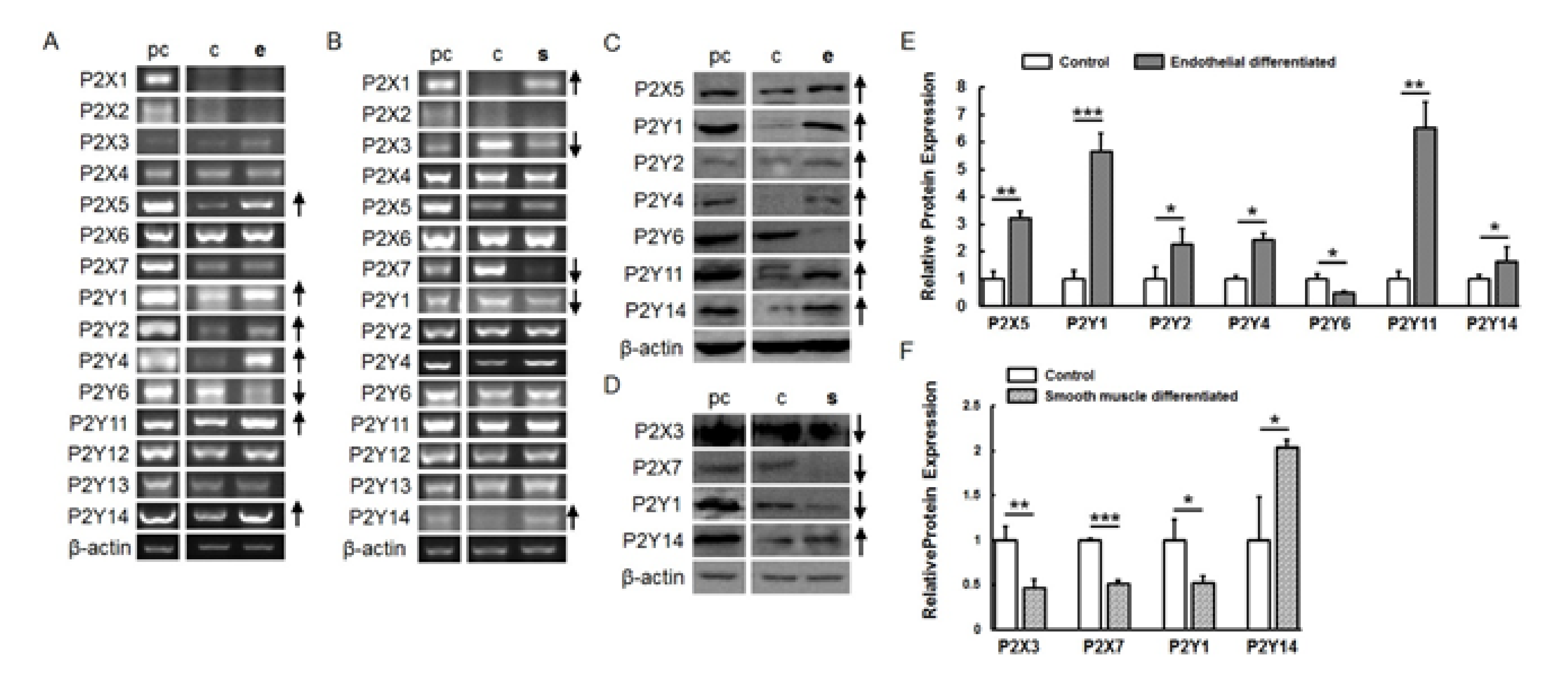
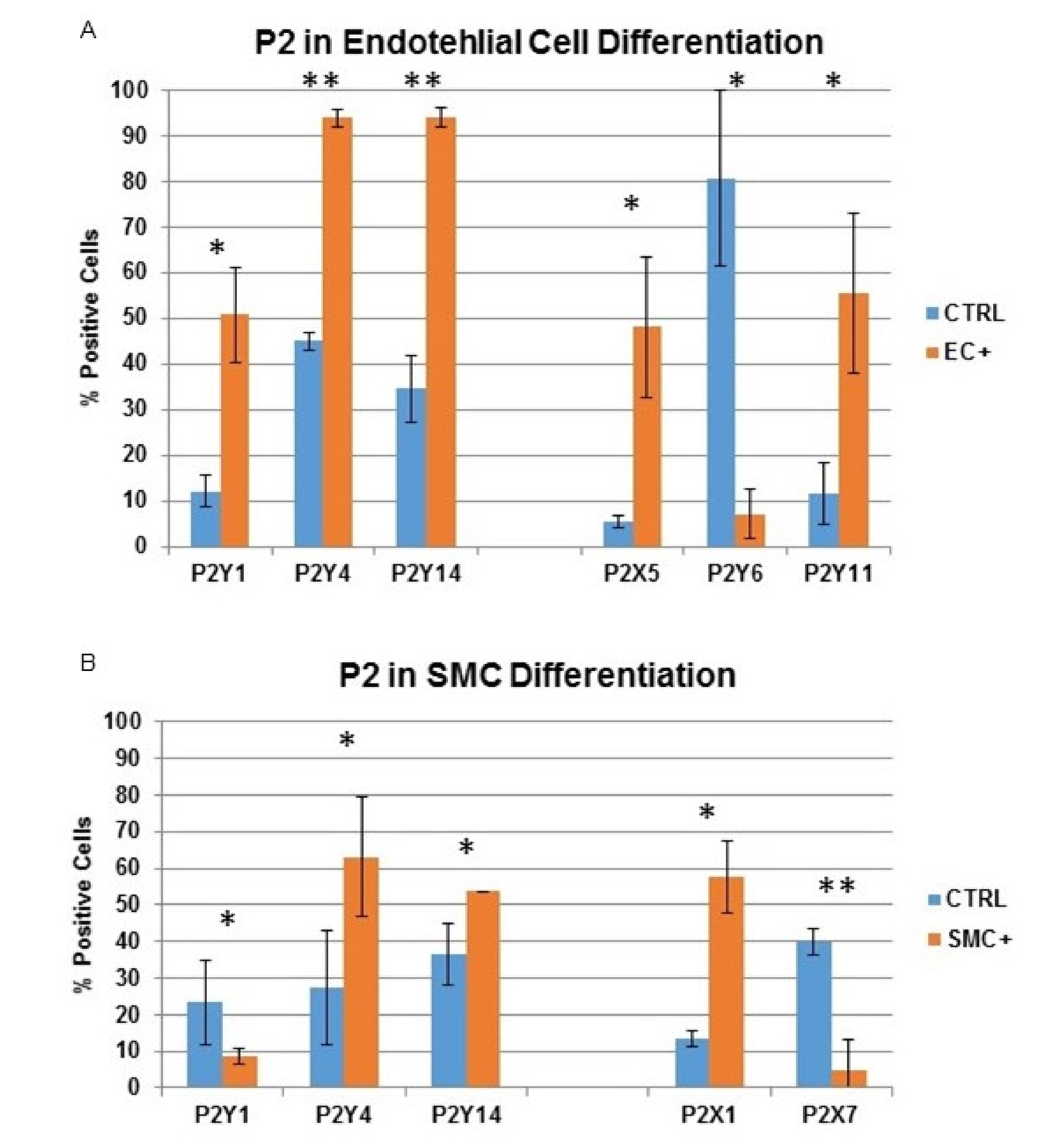
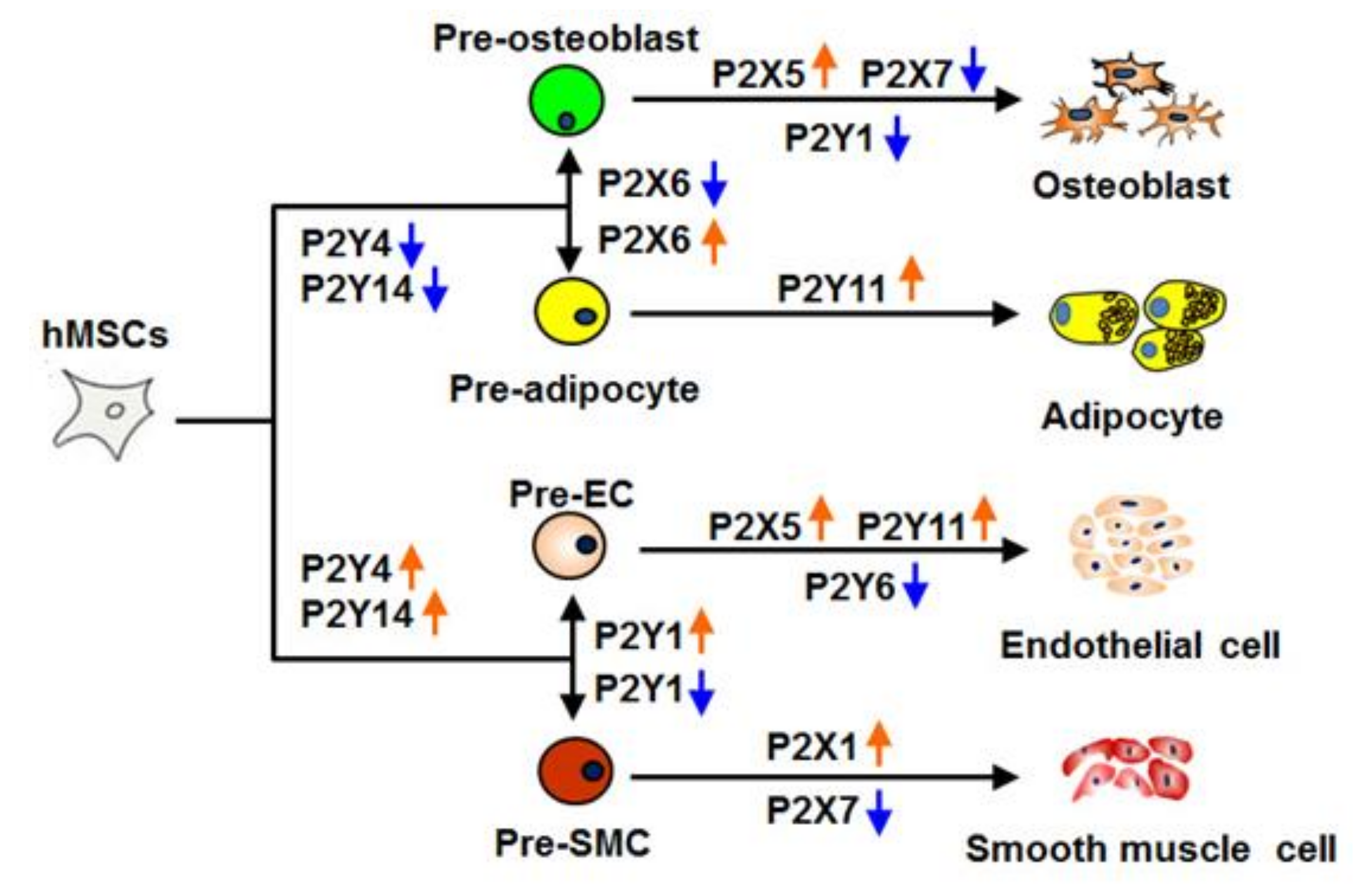
2.3. P2 Receptor Expression during Endothelial and Smooth Muscle Cell Differentiation
2.4. The Influence of Natural and Artificial P2 Agonists and Antagonists on Endothelial and Smooth Muscle Cell Differentiation
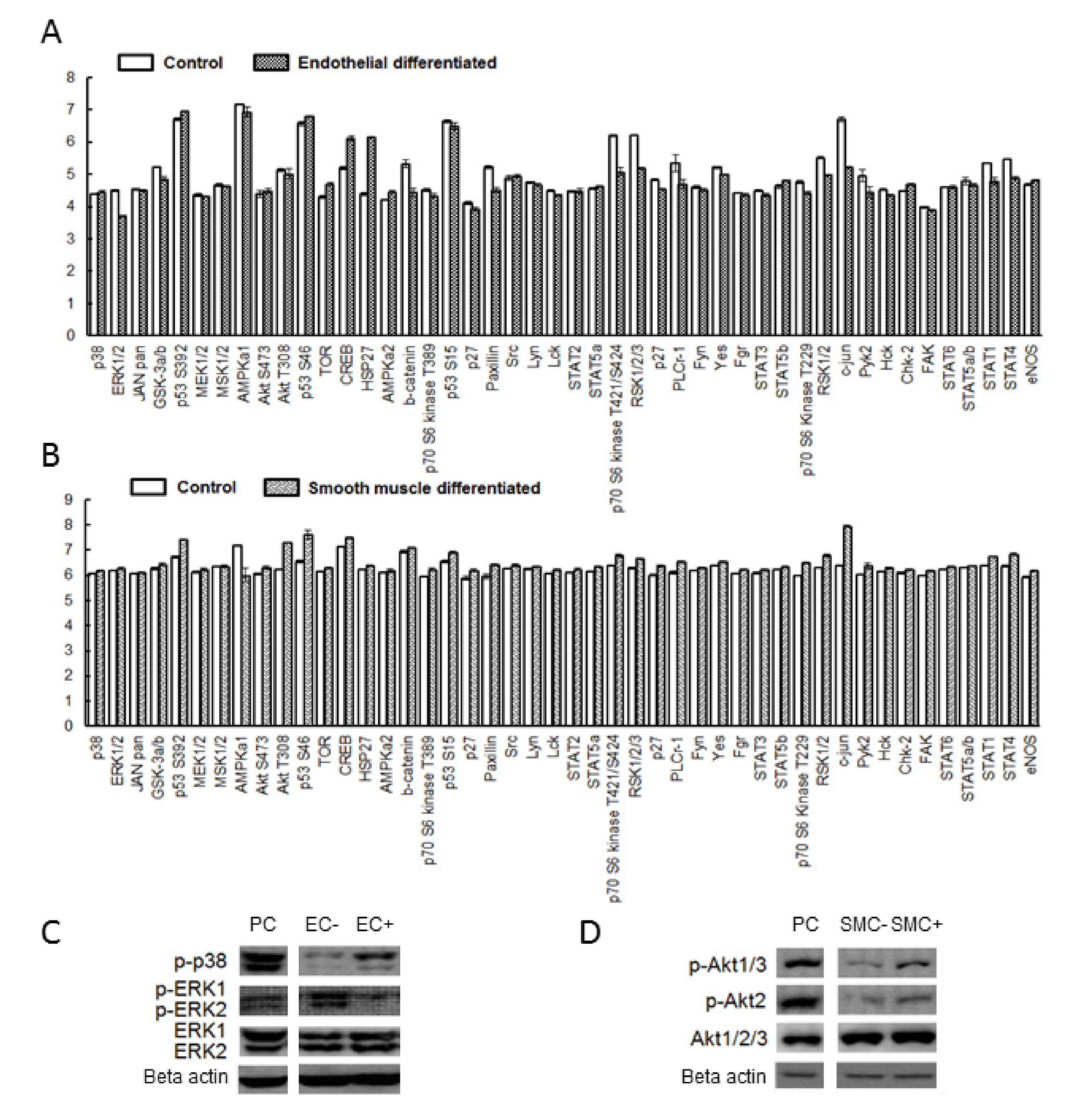
2.5. The Possible P2 Underlying Signaling Pathway Involved in Endothelial Cell and Smooth Muscle Cell Differentiation
3. Discussion
4. Materials and Methods
4.1. Cell Isolation, Culture and Characterization
4.2. Endothelial and Smooth Muscle Cell Differentiation
4.3. Semiquantitative RT-PCR
4.4. Western Blot
4.5. Immunochemical Staining and Quantification
4.6. Flow Cytometry Analysis
4.7. Determination of DiI-Ac-LDL Uptake
4.8. Matrigel Tube Formation Assay
4.9. Natural and Artificial Agonist and Antagonist Stimulation
4.10. Nucleotide Cleavage
4.11. Proteome Profiler Antibody Assay
4.12. Statistical Analysis
4.13. Ethics Sstatement
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A-74003 | Ozagrel hydrochloride ((2E)-3-[4-(1H-Imidazol-1-ylmethyl)phenyl]-2-propenoic acid hydrochloride) |
| ADP | Adenosine diphosphate |
| AKT | Protein kinase B |
| AMPKα1 | 5′-AMP-activated protein kinase catalytic subunit alpha-1 |
| α-SMA | Alpha-smooth muscle actin |
| ATP | Adenosine triphosphate |
| BMP2 | Bone morphogenetic protein 2 |
| BSA | Bovine serum albumin |
| cAMP | Cyclic adenosine monophosphatase |
| CD | Cluster of differentiation |
| CREB | cAMP response element-binding protein |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DiL-Ac-LDL | Dil Acetylated Low Density Lipoprotein |
| DMEM | Dulbecco’s modiefied Eagle medium |
| eNOS | Nitric oxide synthase 3 |
| EPC | Endothelial progenitor cell |
| ERK1/2 | extracellular signal-regulated kinases 1/2 |
| ESC | Embryonic stem cell |
| FCS | Fetal calf serum |
| FLK-1 | Kinase insert domain receptor 1 |
| HSP27 | Heat shock protein 27 |
| HMEC | Human microvascular endothelial cell |
| IPSC | Induced pluripotent stem cell |
| JNK | c-Jun N-terminal kinases |
| LPL | Lipoprotein lipase |
| MAPK | Mitogen-activated protein kinase |
| MRS2365 | [[(1R,2R,3S,4R,5S)-4-[6-Amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo [3.1.0]hex-1-yl]methyl] diphosphoric acid mono ester trisodium salt |
| MRS2500 | (1R*,2S*)-4-[2-Iodo-6-(methylamino)-9H-purin-9-yl]-2-(phosphonooxy)bicyclo[3.1.0]hexane-1-Methanol dihydrogen phosphate ester tetraammonium salt |
| MSC | Mesenchymal stem cell |
| P2 | Purinergic receptor type 2 |
| PBS | Phosphate-buffered saline |
| PECAM-1 | Platelet endothelial cell adhesion molecule 1 |
| PI3K | Phosphatidyinositol 3-kinase |
| PPADS | Pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt |
| PPARy | Peroxisome proliferator-activated receptor gamma |
| RO-3 | 5-[[4,5-Dimethoxy-2-(methylethyl)phenyl]methyl]-2,4-pyrimidinediamine |
| RSK1/2 | Ribosomal s6 kinase |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
| SM22α | Transgelin |
| STAT1 | Signal transducer and activator of transcription 1 |
| TOR | Target of rapamycin |
| UDP | Uridine diphosphate |
| UTP | Uridine-5′-triphosphate |
References
- Tobiasch, E. Adult Human Mesenchymal Stem Cells as Source for Future Tissue Engineering; Forschungsspitzen und Spitzenforschung: Heidelberg, Germany, 2009; pp. 329–338. [Google Scholar]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, O.K.; Kuo, T.K.; Chen, W.-M.; Lee, K.-D.; Hsieh, S.-L.; Chen, T.-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 2015, 348, 2151. [Google Scholar] [CrossRef] [Green Version]
- Contreras, O.; Rossi, F.M.; Brandan, E. Adherent muscle connective tissue fibroblasts are phenotypically and biochemically equivalent to stromal fibro/adipogenic progenitors. Matrix Biol. Plus 2019, 2, 100006. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.-I.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Saugspier, M.; Felthaus, O.; Viale-Bouroncle, S.; Driemel, O.; Reichert, T.E.; Schmalz, G.; Morsczeck, C. The differentiation and gene expression profile of human dental follicle cells. Stem Cells Dev. 2010, 19, 707–717. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Chandrakanthan, V.; Xaymardan, M.; Asli, N.S.; Li, J.; Ahmed, I.; Heffernan, C.; Menon, M.K.; Scarlett, C.J.; Rashidianfar, A.; et al. Adult cardiac-resident msc-like stem cells with a proepicardial origin. Cell Stem Cell 2011, 9, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tobiasch, E. The role of purinergic receptors in stem cells in their derived consecutive tissues. In Adult Stem Cell Standardization; di Nardo, P., Ed.; River Publishers: Roma, Italy, 2011; pp. 73–98. [Google Scholar]
- Pittenger, M.F. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Yang, J.Q.; Heydarkhan, S.; Xu, Y.; Zuk, P.A.; MacLellan, W.R.; Beygui, R.E. Human adipose stem cells: A potential cell source for cardiovascular tissue engineering. Cells Tissues Organs 2008, 187, 263–274. [Google Scholar] [CrossRef]
- Wosnitza, M.; Hemmrich, K.; Groger, A.; Gräber, S.; Pallua, N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation 2007, 75, 12–23. [Google Scholar] [CrossRef]
- Burnstock, G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 2006, 58, 58–86. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.-M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Han, H.J. ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-Kinase/Akt, and mitogen-activated protein kinase signaling pathways. Stem Cells 2006, 24, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Manfredini, R.; Bertolini, F.; Ferrari, D.; Fogli, M.; Zini, R.; Salati, S.; Salvestrini, V.; Gulinelli, S.; Adinolfi, E.; et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood 2006, 109, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Braun, N.; Shukla, V.; Füllgrabe, M.; Schomerus, C.; Korf, H.; Gachet, C.; Ikehara, Y.; Sévigny, J.; Robson, S.; et al. Extracellular nucleotide signaling in adult neural stem cells: Synergism with growth factor-mediated cellular proliferation. Develoment 2006, 133, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Zippel, N.; Limbach, C.A.; Ratajski, N.; Urban, C.; Luparello, C.; Pansky, A.; Kassack, M.U.; Tobiasch, E. Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Dev. 2012, 21, 884–900. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Balasubramanian, R.; Deflorian, F.; Gao, Z.-G. G protein-coupled adenosine (P1) and P2Y receptors: Ligand design and receptor interactions. Purinergic Signal. 2012, 8, 419–436. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zhang, P.; Moudgill, N.; Hager, E.; Tarola, N.; DiMatteo, C.; McIlhenny, S.; Tulenko, T.; DiMuzio, P.J. Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev. 2011, 20, 977–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabhaneni, K.C.; Tkachuk, S.; Kiyan, Y.; Shushakova, N.; Haller, H.; Dumler, I.; Eden, G. Urokinase receptor mediates mobilization, migration, and differentiation of mesenchymal stem cells. Cardiovasc. Res. 2010, 90, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2002, 5, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2009, 28, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Khan, D.; Delling, J.; Tobiasch, E. Mechanisms underlying the osteo and adipo-differentiation of human mesenchymal stem cells. Sci. World J. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, S.T.F.; Asgari, A.; Lokmic, Z.T.; Nee, S.R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [Green Version]
- Zippel, N.; Schulze, M.; Tobiasch, E. Biomaterials and mesenchymal stem cells for regenerative medicine. Recent Pat. Biotechnol. 2010, 4, 1–22. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, M.; Fang, L.; Lv, Q.; He, Q.; Deng, M.; Liu, X.; Chen, X.; Chen, M.; Xie, X.; et al. Extracellular nucleotide inhibits cell proliferation and negatively regulates Toll-like receptor 4 signalling in human progenitor endothelial cells. Cell Biol. Int. 2012, 36, 625–633. [Google Scholar] [CrossRef]
- Laurie, N.A.; Donovan, S.L.; Shih, C.-S.; Zhang, J.; Mills, N.; Fuller, C.; Teunisse, A.; Lam, S.; Ramos, Y.F.M.; Mohan, A.; et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006, 444, 61–66. [Google Scholar] [CrossRef]
- Kawano, S.; Otsu, K.; Kuruma, A.; Shoji, S.; Yanagida, E.; Muto, Y.; Yoshikawa, F.; Hirayama, Y.; Mikoshiba, K.; Furuichi, T. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 2006, 39, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Orriss, I.; Knight, G.E.; Utting, J.C.; Taylor, S.E.; Burnstock, G.; Arnett, T.R. Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell. Physiol. 2009, 220, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Sen, C.K.; Wells, A. Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev. 2008, 17, 897–908. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Jiang, Y.; Chu, L.; Hao, H.; Liua, Z.; Verfaillie, C.M.; Zweier, J.; Gupta, K.; Liu, Z. MAPK/ERK signalling mediates VEGF-induced bone marrow stem cell differentiation into endothelial cell. J. Cell. Mol. Med. 2008, 12, 2395–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Karlsson, L.; Moses, S.; Hultgårdh-Nilsson, A.; Andersson, M.; Borna, C.; Gudbjartsson, T.; Jern, S.; Erlinge, D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2002, 40, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Erb, L.; Liao, Z.; Seye, C.I.; Weisman, G.A. P2 receptors: Intracellular signaling. Pflügers Arch. Eur. J. Physiol. 2006, 452, 552–562. [Google Scholar] [CrossRef]
- Schulze, M.; Tobiasch, E. Artificial scaffolds and mesenchymal stem cells for hard tissues. Biomed. Inorg. Polym. 2011, 126, 153–194. [Google Scholar] [CrossRef]
- Meng, X.; Chen, M.; Su, W.; Tao, X.; Sun, M.; Zou, X.; Ying, R.; Wei, W.; Wang, B. The differentiation of mesenchymal stem cells to vascular cells regulated by the HMGB1/RAGE axis: Its application in cell therapy for transplant arteriosclerosis. Stem Cell Res. Ther. 2018, 9, 85. [Google Scholar] [CrossRef]
- Lu, W.; Xiu, X.; Zhao, Y.; Gui, M. Improved proliferation and differentiation of bone marrow mesenchymal stem cells into vascular endothelial cells with sphingosine 1-phosphate. Transplant. Proc. 2015, 47, 2035–2040. [Google Scholar] [CrossRef]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. 2017, 17, 1667–1675. [Google Scholar] [CrossRef]
- Joddar, B.; Kumar, S.A.; Kumar, A. A contact-based method for differentiation of human mesenchymal stem cells into an endothelial cell-phenotype. Cell Biophys. 2017, 76, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, K.; Uusitalo-Kylmälä, L.; Hentunen, T.A.; Heino, T.J. Angiogenic potential of human mesenchymal stromal cell and circulating mononuclear cell cocultures is reflected in the expression profiles of proangiogenic factors leading to endothelial cell and pericyte differentiation. J. Tissue Eng. Regen. Med. 2017, 12, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Tancharoen, W.; Aungsuchawan, S.; Pothacharoen, P.; Bumroongkit, K.; Puaninta, C.; Pangjaidee, N.; Narakornsak, S.; Markmee, R.; Laowanitwattana, T.; Thaojamnong, C. Human platelet lysate as an alternative to fetal bovine serum for culture and endothelial differentiation of human amniotic fluid mesenchymal stem cells. Mol. Med. Rep. 2019, 19, 5123–5132. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Babczyk, P.; Pansky, A.; Kassack, M.U.; Tobiasch, E. P2 Receptors Influence hMSCs Differentiation towards Endothelial Cell and Smooth Muscle Cell Lineages. Int. J. Mol. Sci. 2020, 21, 6210. https://doi.org/10.3390/ijms21176210
Zhang Y, Babczyk P, Pansky A, Kassack MU, Tobiasch E. P2 Receptors Influence hMSCs Differentiation towards Endothelial Cell and Smooth Muscle Cell Lineages. International Journal of Molecular Sciences. 2020; 21(17):6210. https://doi.org/10.3390/ijms21176210
Chicago/Turabian StyleZhang, Yu, Patrick Babczyk, Andreas Pansky, Matthias Ulrich Kassack, and Edda Tobiasch. 2020. "P2 Receptors Influence hMSCs Differentiation towards Endothelial Cell and Smooth Muscle Cell Lineages" International Journal of Molecular Sciences 21, no. 17: 6210. https://doi.org/10.3390/ijms21176210
APA StyleZhang, Y., Babczyk, P., Pansky, A., Kassack, M. U., & Tobiasch, E. (2020). P2 Receptors Influence hMSCs Differentiation towards Endothelial Cell and Smooth Muscle Cell Lineages. International Journal of Molecular Sciences, 21(17), 6210. https://doi.org/10.3390/ijms21176210




