P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases
Abstract
1. Extracellular Nucleotides and Purinergic Receptors
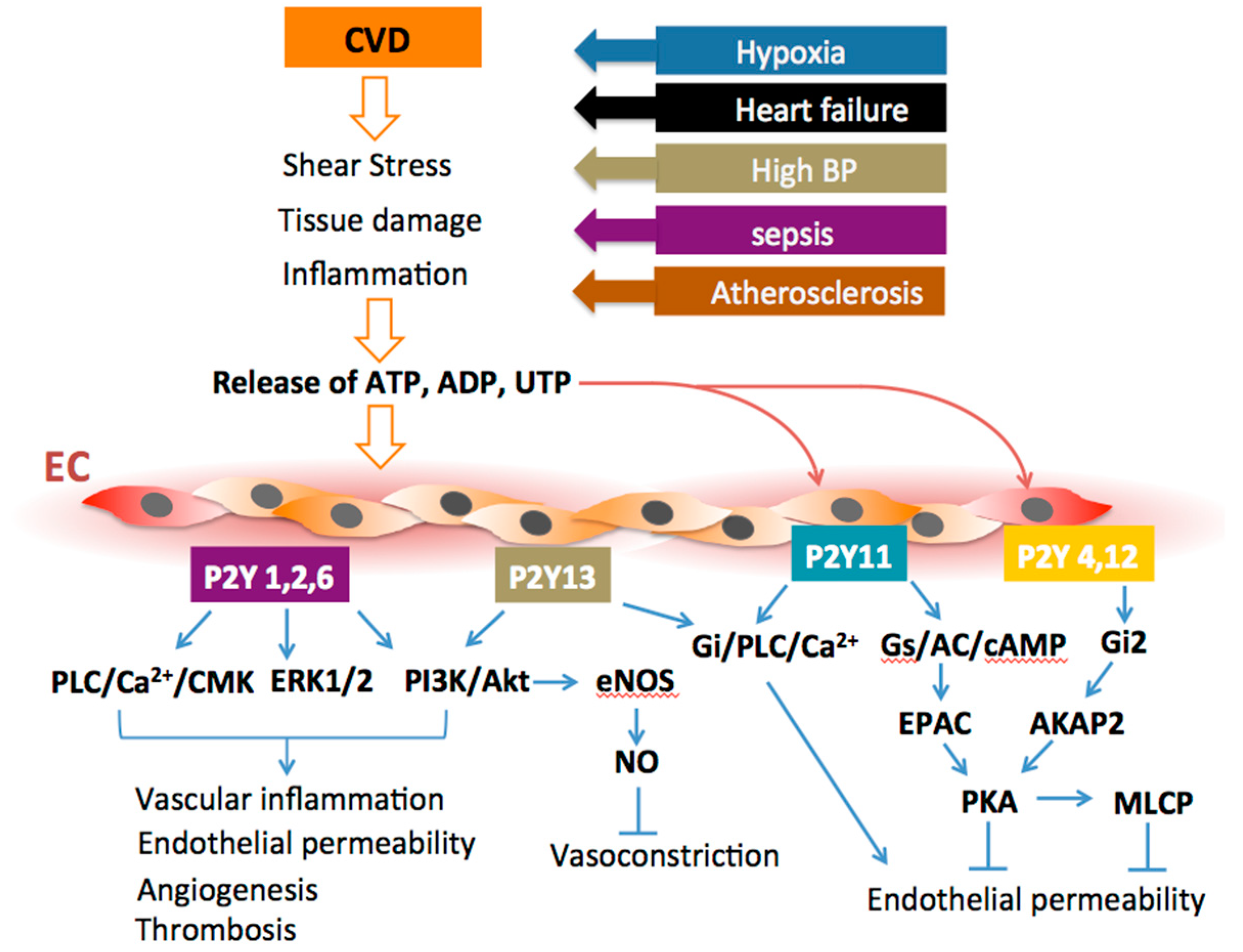
2. P2Y Receptor Signaling and Endothelial Dysfunction
2.1. P2Y Receptors and Vascular Tone Regulation
2.1.1. Hypertension
2.1.2. Vascular Tone and Aging
2.1.3. Vascular Tone and Diabetes
2.2. P2Y Receptors and Regulation of Oxidative Stress and Vascular Inflammation
2.2.1. Oxidative Stress
2.2.2. Vascular Inflammation
2.2.3. Atherosclerosis
2.2.4. Metabolic Syndrome
2.3. P2Y Receptors and Regulation of Vascular Barrier Function
2.3.1. P2Y Receptors and Vascular Permeability
2.3.2. P2Y Receptors and Vascular Barrier Protection
2.4. P2Y Receptors and Regulation of Vessel Growth
2.4.1. Vasa Vasorum Neovascularization
2.4.2. Angiogenic Purinergic Signaling in VVEC
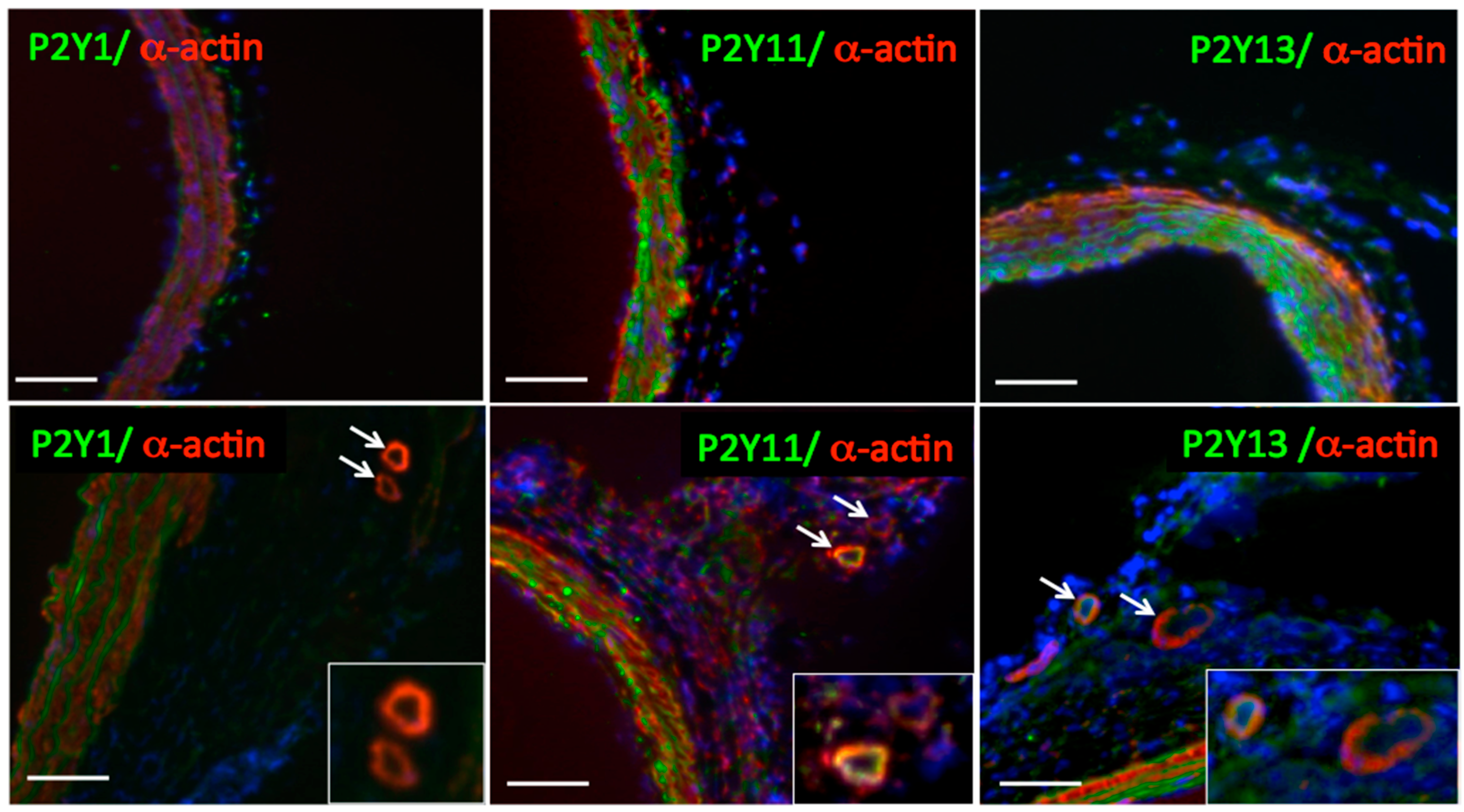
2.4.3. P2Y Receptor Subtypes and VV Neovascularization
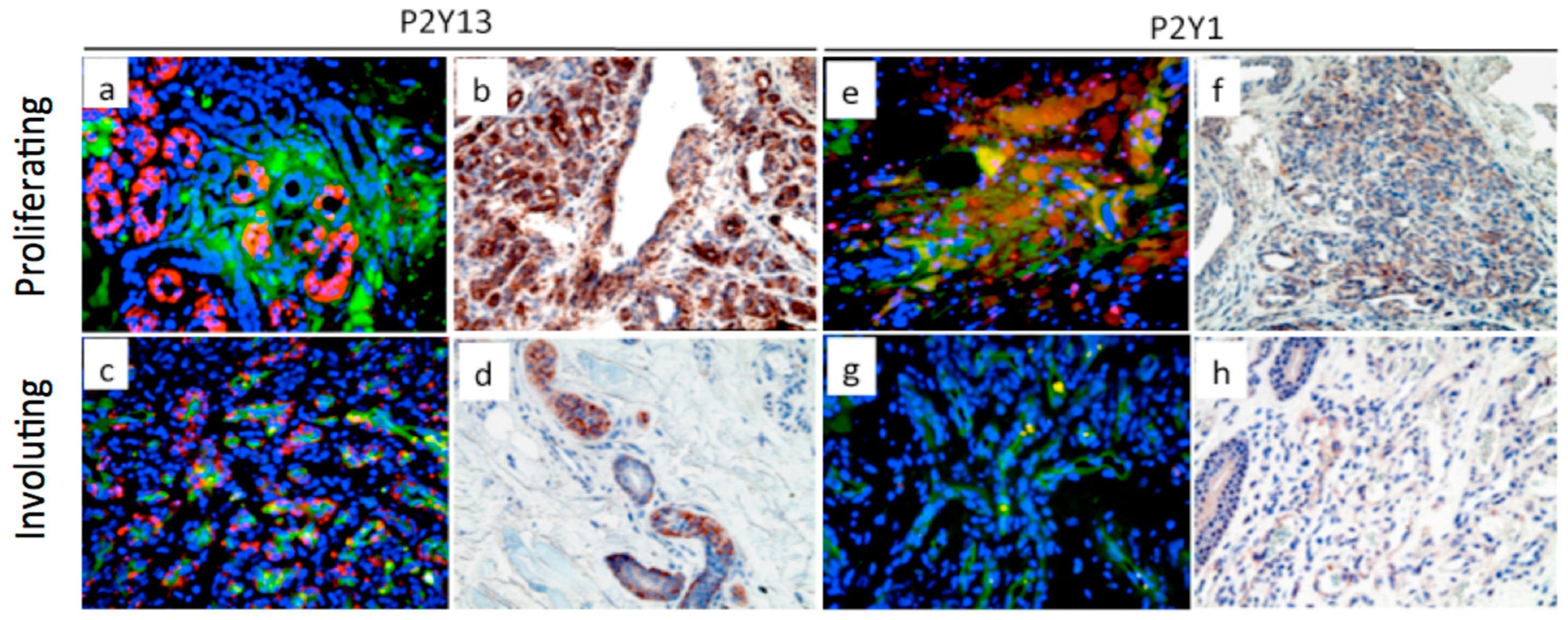
2.5. P2Y Receptors and Infantile Hemangioma Development
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Adenylate cyclase |
| ADP | Adenosine triphosphate |
| ADPβS | Adenosine-5′-0-(2-thiodiphosphate) |
| AKAP2 | PKA anchoring protein 2 |
| Akt | Serine/threonine protein kinase |
| ALI | acute lung injury |
| ApoE | Apolipoprotein A |
| ARDS | Acute respiratory distress syndrome |
| ATPγS | Adenosine 5′-(3-thiotriphosphate) |
| AT1R | Angiotensin II type I receptor |
| CAM | Cell adhesion molecule |
| CaMKII | Calmodulin kinase |
| cAMP | Cyclic adenosine monophosphate |
| CD39/ENTPDase | Ectonucleoside triphosphate diphosphohydrolase |
| CVDs | Cardiovascular diseases |
| Cx 37, 40 | Connexin 37, 40 |
| DAMP | damage-associated molecular pattern |
| EC | Endothelial cells |
| ECM | Extracellular Matrix |
| ED | Endothelial dysfunction |
| EDH | Endothelium-dependent hyperpolarization |
| eNOS | Endothelial nitric oxide synthase |
| EPAC | Exchange Protein Activated by cAMP |
| ERK1/2 | Extracellular signal regulated kinases 1/2 |
| E-selectin | CD62 antigen-like family member E |
| GEF | Guanine nucleotide exchange factor |
| GPCR | G-protein coupled receptor |
| GPR120 | G-protein coupled receptor 120 |
| Gs, Gs | G protein subtypes |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IGF-I | Insulin-like growth factor 1 |
| IL-1, -6 | Interleukin-1, -6 |
| JNK | c-Jun N-terminal kinase |
| KCa3.1 | The calcium-activated potassium channel KCa3.1 |
| KLF | The Krüppel-like family of transcription factors |
| LDL | Low-density lipoproteins |
| LMVEC | Lung microvascular endothelial cells |
| LPS | Lipopolysaccharide |
| MeSADP | 2-(Methylthio)adenosine 5’-diphosphate |
| MeSATP | 2-(Methylthio)adenosine 5′-triphosphate |
| MLC | Myosin light chains |
| MLCP | MLC phosphatase |
| MMPs | Matrix metallopeptidases |
| mTOR | The mammalian target of rapamycin |
| β-NAD | β-Nicotinamide adenine dinucleotide |
| NFkB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOX2 | NADPH oxidase catalytic component |
| PAF | Platelet-activating factor |
| PB | Barometric pressure |
| PIEZO | Mechanically activated cation channels |
| PGI2 | Prostacyclin |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PKG | Protein kinase G |
| PLC | Phospholipase C |
| PLY | Pneumolysin |
| PI3K | Phosphatidyl inositol 3 kinase |
| PAH | Pulmonary arterial hypertension |
| p38 MAPK | p38 Mitogen-activated protein kinases |
| Rac 1 | A small GTPase protein |
| Rap 1 | A small GTPases protein |
| ROS | Reactive oxygen species |
| ROCK | Rho protein kinase |
| TER | Transendothelial electrical resistance |
| Tiam 1 | T-Lymphoma invasion and metastasis-inducing protein 1 |
| TNFαR | Tumor necrosis factor alpha receptor |
| TXA2 | Thromboxane A2 |
| TLR | Toll-like receptor |
| TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
| T1D, T2D | Type 1,2 diabetes |
| UTP | Uridine triphosphate |
| Vav | Guanine nucleotide exchange factor |
| VCAM-1 | Vascular cell adhesion protein 1 |
| VEGFR2 | Vascular endothelial growth factor receptor-2 |
| VSMC | Vascular smooth muscle cell |
| VVEC | Vasa vasorum endothelial cells |
References
- Di Virgilio, F.; Solini, A. P2 receptors: New potential players in atherosclerosis. Br. J. Pharmacol. 2002, 135, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G. Purinergic signalling: Pathophysiological roles. Jpn. J. Pharmacol. 1998, 78, 113–145. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Burnstock, G. Purine and pyrimidine receptors. Cell Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef]
- Motte, S.; Pirotton, S.; Boeynaems, J.M. Evidence that a form of ATP uncomplexed with divalent cations is the ligand of P2y and nucleotide/P2u receptors on aortic endothelial cells. Br. J. Pharmacol. 1993, 109, 967–971. [Google Scholar] [CrossRef]
- Wang, L.; Karlsson, L.; Moses, S.; Hultgardh-Nilsson, A.; Andersson, M.; Borna, C.; Gudbjartsson, T.; Jern, S.; Erlinge, D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2002, 40, 841–853. [Google Scholar] [CrossRef]
- Burnstock, G.; Ulrich, H. Purinergic signaling in embryonic and stem cell development. Cell Mol. Life Sci. 2011, 68, 1369–1394. [Google Scholar] [CrossRef]
- Bours, M.J.; Swennen, E.L.; Di Virgilio, F.; Cronstein, B.N.; Dagnelie, P.C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006, 112, 358–404. [Google Scholar] [CrossRef]
- Burnstock, G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 2006, 58, 58–86. [Google Scholar] [CrossRef]
- Erlinge, D.; Burnstock, G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008, 4, 1–20. [Google Scholar] [CrossRef]
- Gerasimovskaya, E.V.; Ahmad, S.; White, C.W.; Jones, P.L.; Carpenter, T.C.; Stenmark, K.R. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J. Biol. Chem. 2002, 277, 44638–44650. [Google Scholar] [CrossRef]
- Woodward, H.N.; Anwar, A.; Riddle, S.; Taraseviciene-Stewart, L.; Fragoso, M.; Stenmark, K.R.; Gerasimovskaya, E.V. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L954–L964. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.; Lukashev, D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat. Rev. Immunol. 2005, 5, 712–721. [Google Scholar] [CrossRef]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef]
- Corriden, R.; Insel, P.A. Basal release of ATP: An autocrine-paracrine mechanism for cell regulation. Sci. Signal. 2010, 3, re1. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kugelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schoneberg, T.; Perez-Sen, R.; et al. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- Zemskov, E.; Lucas, R.; Verin, A.D.; Umapathy, N.S. P2Y receptors as regulators of lung endothelial barrier integrity. J. Cardiovasc. Dis. Res. 2011, 2, 14–22. [Google Scholar] [CrossRef]
- Burnstock, G.; Ralevic, V. Purinergic signaling and blood vessels in health and disease. Pharmacol. Rev. 2014, 66, 102–192. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signaling and vascular cell proliferation and death. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 364–373. [Google Scholar] [CrossRef]
- Gerasimovskaya, E.V.; Tucker, D.A.; Stenmark, K.R. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J. Appl. Physiol. 2005, 98, 722–731. [Google Scholar] [CrossRef]
- Van der Weyden, L.; Conigrave, A.D.; Morris, M.B. Signal transduction and white cell maturation via extracellular ATP and the P2Y11 receptor. Immunol. Cell Biol. 2000, 78, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic receptors as future targets for treatment of functional GI disorders. Gut 2008, 57, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 2008, 7, 575–590. [Google Scholar] [CrossRef] [PubMed]
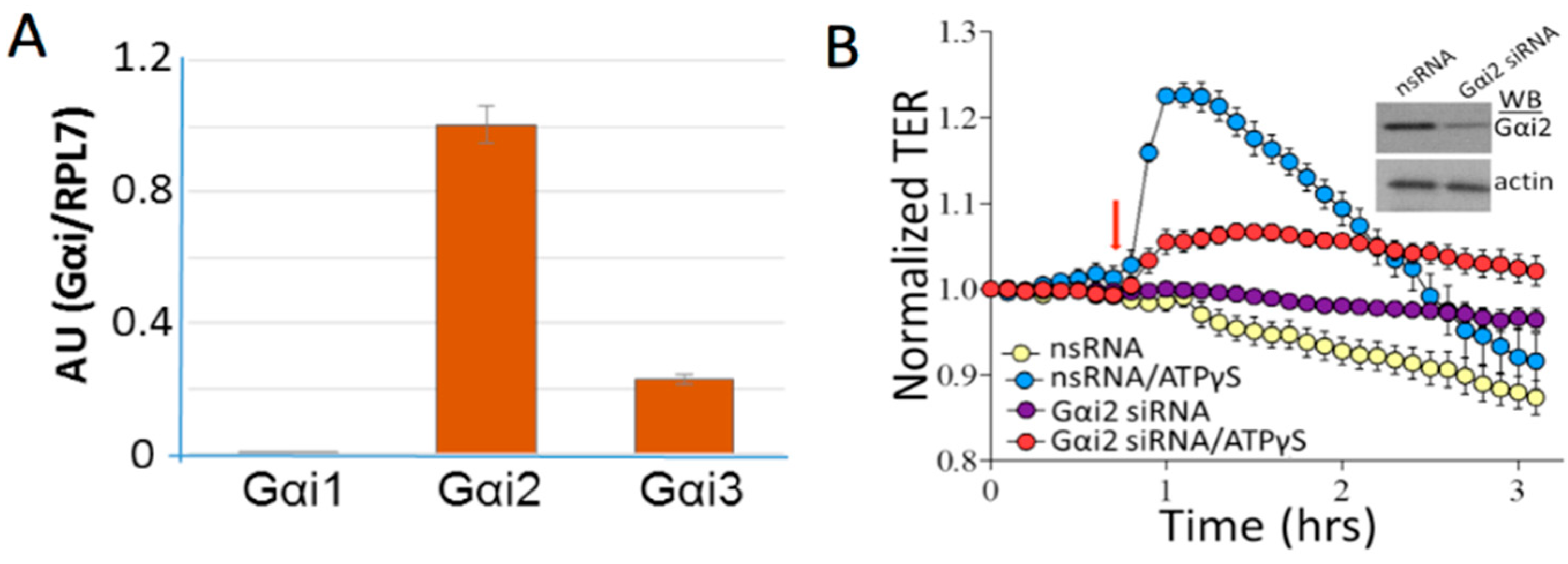
- Bátori, R.; Kumar, S.; Bordán, Z.; Cherian-Shaw, M.; Kovács-Kása, A.; MacDonald, J.A.; Fulton, D.J.R.; Erdődi, F.; Verin, A.D. Differential mechanisms of adenosine- and ATPγS-induced microvascular endothelial barrier strengthening. J. Cell. Physiol. 2019, 234, 5863–5879. [Google Scholar] [CrossRef] [PubMed]
- Lyubchenko, T.; Woodward, H.; Veo, K.D.; Burns, N.; Nijmeh, H.; Liubchenko, G.A.; Stenmark, K.R.; Gerasimovskaya, E.V. P2Y1 and P2Y13 purinergic receptors mediate Ca2+ signaling and proliferative responses in pulmonary artery vasa vasorum endothelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C266–C275. [Google Scholar] [CrossRef]
- Kaczmarek, E.; Erb, L.; Koziak, K.; Jarzyna, R.; Wink, M.R.; Guckelberger, O.; Blusztajn, J.K.; Trinkaus-Randall, V.; Weisman, G.A.; Robson, S.C. Modulation of endothelial cell migration by extracellular nucleotides: Involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb. Haemost. 2005, 93, 735–742. [Google Scholar]
- Jacobson, J.R.; Dudek, S.M.; Singleton, P.A.; Kolosova, I.A.; Verin, A.D.; Garcia, J.G. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L289–L295. [Google Scholar] [CrossRef][Green Version]
- Lemoli, R.M.; Ferrari, D.; Fogli, M.; Rossi, L.; Pizzirani, C.; Forchap, S.; Chiozzi, P.; Vaselli, D.; Bertolini, F.; Foutz, T.; et al. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood 2004, 104, 1662–1670. [Google Scholar] [CrossRef]
- Rossi, L.; Manfredini, R.; Bertolini, F.; Ferrari, D.; Fogli, M.; Zini, R.; Salati, S.; Salvestrini, V.; Gulinelli, S.; Adinolfi, E.; et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood 2007, 109, 533–542. [Google Scholar] [CrossRef]
- Satterwhite, C.M.; Farrelly, A.M.; Bradley, M.E. Chemotactic, mitogenic, and angiogenic actions of UTP on vascular endothelial cells. Am. J. Physiol. 1999, 276, H1091–H1097. [Google Scholar] [CrossRef]
- Noll, T.; Holschermann, H.; Koprek, K.; Gunduz, D.; Haberbosch, W.; Tillmanns, H.; Piper, H.M. ATP reduces macromolecule permeability of endothelial monolayers despite increasing [Ca2+]i. Am. J. Physiol. 1999, 276, H1892–H1901. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, E.; Fauler, M.; Fois, G.; Frick, M. P2 Purinergic Signaling in the Distal Lung in Health and Disease. Int. J. Mol. Sci. 2020, 21, 4973. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Brouns, I.; Adriaensen, D.; Timmermans, J.P. Purinergic signaling in the airways. Pharmacol. Rev. 2012, 64, 834–868. [Google Scholar] [CrossRef]
- Matsuyama, H.; Amaya, F.; Hashimoto, S.; Ueno, H.; Beppu, S.; Mizuta, M.; Shime, N.; Ishizaka, A.; Hashimoto, S. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: An experimental study. Respir. Res. 2008, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, I.A.; Mirzapoiazova, T.; Moreno-Vinasco, L.; Sammani, S.; Garcia, J.G.; Verin, A.D. Protective effect of purinergic agonist ATPgammaS against acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L319–L324. [Google Scholar] [CrossRef]
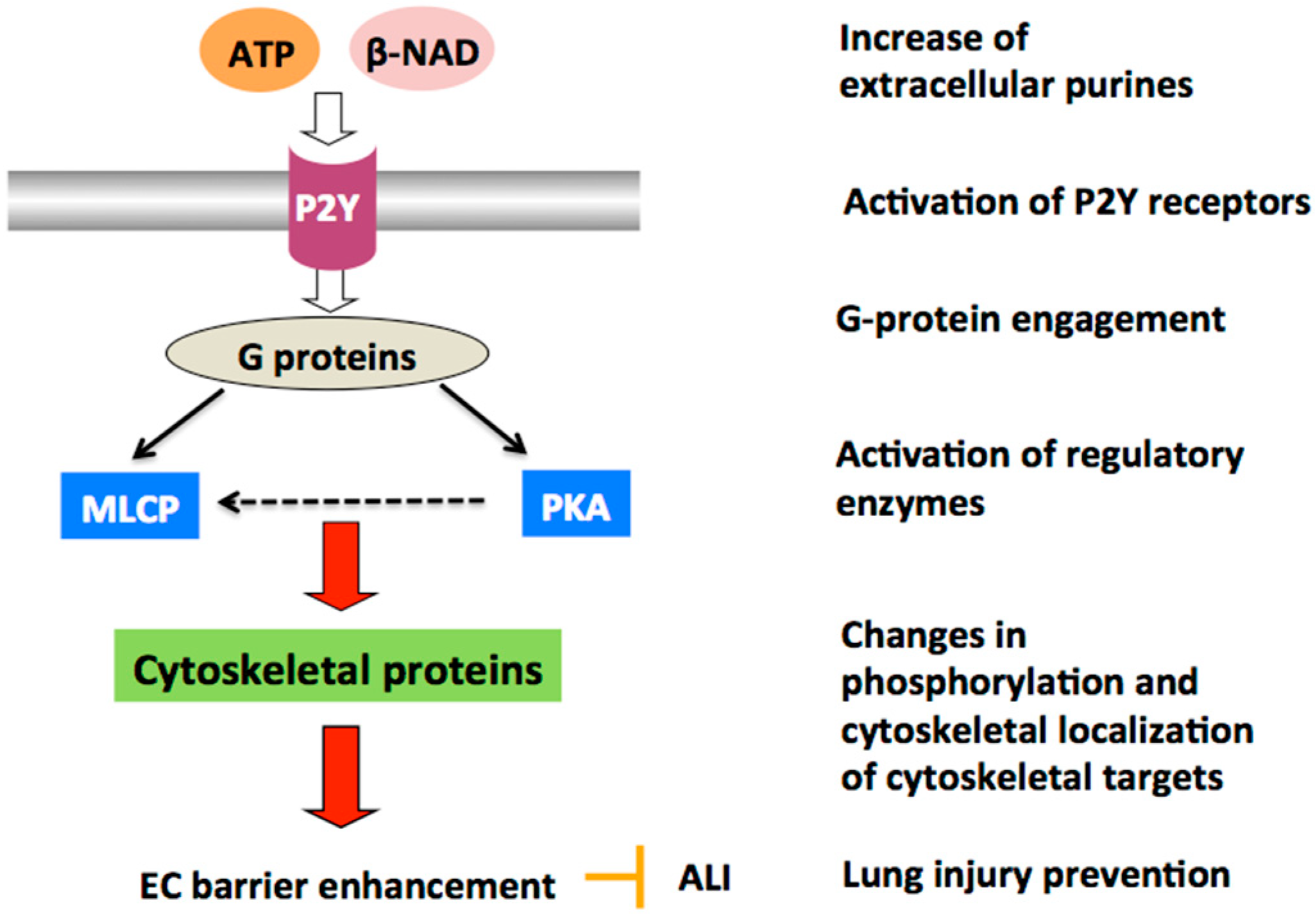
- Umapathy, N.S.; Zemskov, E.A.; Gonzales, J.; Gorshkov, B.A.; Sridhar, S.; Chakraborty, T.; Lucas, R.; Verin, A.D. Extracellular beta-nicotinamide adenine dinucleotide (beta-NAD) promotes the endothelial cell barrier integrity via PKA- and EPAC1/Rac1-dependent actin cytoskeleton rearrangement. J. Cell. Physiol. 2010, 223, 215–223. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Gonzales, J.; Fulzele, S.; Kim, K.M.; Lucas, R.; Verin, A.D. beta-Nicotinamide adenine dinucleotide attenuates lipopolysaccharide-induced inflammatory effects in a murine model of acute lung injury. Exp. Lung Res. 2012, 38, 223–232. [Google Scholar] [CrossRef][Green Version]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 2007, 100, 174–190. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef]
- Li, W.H.; Qiu, Y.; Zhang, H.Q.; Tian, X.X.; Fang, W.G. P2Y2 Receptor and EGFR Cooperate to Promote Prostate Cancer Cell Invasion via ERK1/2 Pathway. PLoS ONE 2015, 10, e0133165. [Google Scholar] [CrossRef]
- Aho, J.; Helenius, M.; Vattulainen-Collanus, S.; Alastalo, T.P.; Koskenvuo, J. Extracellular ATP protects endothelial cells against DNA damage. Purinergic Signal. 2016, 12, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Iring, A.; Strilic, B.; Albarran Juarez, J.; Kaur, H.; Troidl, K.; Tonack, S.; Burbiel, J.C.; Muller, C.E.; Fleming, I.; et al. P2Y(2) and Gq/G(1)(1) control blood pressure by mediating endothelial mechanotransduction. J. Clin. Investig. 2015, 125, 3077–3086. [Google Scholar] [CrossRef]
- Marziano, C.; Hong, K.; Cope, E.L.; Kotlikoff, M.I.; Isakson, B.E.; Sonkusare, S.K. Nitric Oxide-Dependent Feedback Loop Regulates Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Cooperativity and Endothelial Function in Small Pulmonary Arteries. J. Am. Heart Assoc. 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, M.; Arvai, A.S.; Tainer, J.A.; Getzoff, E.D. Structural basis for endothelial nitric oxide synthase binding to calmodulin. EMBO J. 2003, 22, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Rieg, J.A.; Burt, J.M.; Ruth, P.; Rieg, T. P2Y(2) receptor activation decreases blood pressure via intermediate conductance potassium channels and connexin 37. Acta Physiol. 2015, 213, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lankhuizen, I.M.; van Beusekom, H.M.; Cheng, C.; Duncker, D.J.; Merkus, D. Uridine Adenosine Tetraphosphate-Induced Coronary Relaxation Is Blunted in Swine with Pressure Overload: A Role for Vasoconstrictor Prostanoids. Front. Pharmacol. 2018, 9, 255. [Google Scholar] [CrossRef]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef]
- Fujii, N.; Nishiyasu, T.; Sigal, R.J.; Boulay, P.; McGarr, G.W.; Kenny, G.P. Aging attenuates adenosine triphosphate-induced, but not muscarinic and nicotinic, cutaneous vasodilation in men. Microcirculation 2018, 25, e12462. [Google Scholar] [CrossRef]
- McCluskey, C.; Mooney, L.; Paul, A.; Currie, S. Compromised cardiovascular function in aged rats corresponds with increased expression and activity of calcium/calmodulin dependent protein kinase IIdelta in aortic endothelium. Vasc. Pharmacol. 2019, 118–119, 106560. [Google Scholar] [CrossRef]
- Smith, A.R.; Visioli, F.; Frei, B.; Hagen, T.M. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: Evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell 2006, 5, 391–400. [Google Scholar] [CrossRef]
- Nishimura, A.; Sunggip, C.; Tozaki-Saitoh, H.; Shimauchi, T.; Numaga-Tomita, T.; Hirano, K.; Ide, T.; Boeynaems, J.M.; Kurose, H.; Tsuda, M.; et al. Purinergic P2Y6 receptors heterodimerize with angiotensin AT1 receptors to promote angiotensin II-induced hypertension. Sci. Signal. 2016, 9, ra7. [Google Scholar] [CrossRef]
- Fujii, N.; Meade, R.D.; McNeely, B.D.; Nishiyasu, T.; Sigal, R.J.; Kenny, G.P. Type 2 diabetes specifically attenuates purinergic skin vasodilatation without affecting muscarinic and nicotinic skin vasodilatation and sweating. Exp. Physiol. 2018, 103, 212–221. [Google Scholar] [CrossRef]
- Handa, P.; Tateya, S.; Rizzo, N.O.; Cheng, A.M.; Morgan-Stevenson, V.; Han, C.Y.; Clowes, A.W.; Daum, G.; O’Brien, K.D.; Schwartz, M.W.; et al. Reduced vascular nitric oxide-cGMP signaling contributes to adipose tissue inflammation during high-fat feeding. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2827–2835. [Google Scholar] [CrossRef]
- Ishida, K.; Matsumoto, T.; Taguchi, K.; Kamata, K.; Kobayashi, T. Mechanisms underlying reduced P2Y(1) -receptor-mediated relaxation in superior mesenteric arteries from long-term streptozotocin-induced diabetic rats. Acta Physiol. 2013, 207, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Dang, X.; Sprague, R.S.; Mustafa, S.J.; Zhou, Z. Alteration of purinergic signaling in diabetes: Focus on vascular function. J. Mol. Cell Cardiol. 2020, 140, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.O.; Maloney, E.; Pham, M.; Luttrell, I.; Wessells, H.; Tateya, S.; Daum, G.; Handa, P.; Schwartz, M.W.; Kim, F. Reduced NO-cGMP signaling contributes to vascular inflammation and insulin resistance induced by high-fat feeding. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 758–765. [Google Scholar] [CrossRef]
- Danila, M.D.; Privistirescu, A.; Duicu, O.M.; Ratiu, C.D.; Angoulvant, D.; Muntean, D.M.; Sturza, A. The effect of purinergic signaling via the P2Y11 receptor on vascular function in a rat model of acute inflammation. Mol. Cell Biochem. 2017, 431, 37–44. [Google Scholar] [CrossRef]
- Pendyala, S.; Usatyuk, P.V.; Gorshkova, I.A.; Garcia, J.G.; Natarajan, V. Regulation of NADPH oxidase in vascular endothelium: The role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid. Redox Signal. 2009, 11, 841–860. [Google Scholar] [CrossRef]
- Diaz-Vegas, A.; Campos, C.A.; Contreras-Ferrat, A.; Casas, M.; Buvinic, S.; Jaimovich, E.; Espinosa, A. ROS Production via P2Y1-PKC-NOX2 Is Triggered by Extracellular ATP after Electrical Stimulation of Skeletal Muscle Cells. PLoS ONE 2015, 10, e0129882. [Google Scholar] [CrossRef]
- Santillo, M.; Colantuoni, A.; Mondola, P.; Guida, B.; Damiano, S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Kalwa, H.; Sartoretto, J.L.; Martinelli, R.; Romero, N.; Steinhorn, B.S.; Tao, M.; Ozaki, C.K.; Carman, C.V.; Michel, T. Central role for hydrogen peroxide in P2Y1 ADP receptor-mediated cellular responses in vascular endothelium. Proc. Natl. Acad. Sci. USA 2014, 111, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- Riegel, A.K.; Faigle, M.; Zug, S.; Rosenberger, P.; Robaye, B.; Boeynaems, J.M.; Idzko, M.; Eltzschig, H.K. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 2011, 117, 2548–2555. [Google Scholar] [CrossRef]
- Agca, Y.; Qian, S.; Agca, C.; Seye, C.I. Direct Evidence for P2Y2 Receptor Involvement in Vascular Response to Injury. J. Vasc. Res. 2016, 53, 163–171. [Google Scholar] [CrossRef]
- Yang, Y.; Delalio, L.J.; Best, A.K.; Macal, E.; Milstein, J.; Donnelly, I.; Miller, A.M.; McBride, M.; Shu, X.; Koval, M.; et al. Endothelial Pannexin 1 Channels Control Inflammation by Regulating Intracellular Calcium. J. Immunol. 2020, 204, 2995–3007. [Google Scholar] [CrossRef]
- Gidlof, O.; Sathanoori, R.; Magistri, M.; Faghihi, M.A.; Wahlestedt, C.; Olde, B.; Erlinge, D. Extracellular Uridine Triphosphate and Adenosine Triphosphate Attenuate Endothelial Inflammation through miR-22-Mediated ICAM-1 Inhibition. J. Vasc. Res. 2015, 52, 71–80. [Google Scholar] [CrossRef]
- Horckmans, M.; Esfahani, H.; Beauloye, C.; Clouet, S.; di Pietrantonio, L.; Robaye, B.; Balligand, J.L.; Boeynaems, J.M.; Dessy, C.; Communi, D. Loss of mouse P2Y4 nucleotide receptor protects against myocardial infarction through endothelin-1 downregulation. J. Immunol. 2015, 194, 1874–1881. [Google Scholar] [CrossRef]
- Feliu, C.; Peyret, H.; Poitevin, G.; Cazaubon, Y.; Oszust, F.; Nguyen, P.; Millart, H.; Djerada, Z. Complementary Role of P2 and Adenosine Receptors in ATP Induced-Anti-Apoptotic Effects Against Hypoxic Injury of HUVECs. Int. J. Mol. Sci. 2019, 20, 1446. [Google Scholar] [CrossRef]
- Strassheim, D.; Karoor, V.; Nijmeh, H.; Weston, P.; Lapel, M.; Schaack, J.; Sullivan, T.; Dempsey, E.C.; Stenmark, K.R.; Gerasimovskaya, E. c-Jun, Foxo3a, and c-Myc Transcription Factors are Key Regulators of ATP-Mediated Angiogenic Responses in Pulmonary Artery Vasa Vasorum Endothelial Cells. Cells 2020, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Danila, M.D.; Piollet, M.; Aburel, O.M.; Angoulvant, D.; Lefort, C.; Chadet, S.; Roger, S.; Muntean, M.D.; Ivanes, F. Modulation of P2Y11-related purinergic signaling in inflammation and cardio-metabolic diseases. Eur. J. Pharmacol. 2020, 876, 173060. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Liu, H.; Guo, S.; Wang, Y.; Zhang, H.; Qiao, Y. The antagonist of P2Y11 receptor NF157 ameliorates oxidized LDL-induced vascular endothelial inflammation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.Y.; McIntosh, K.A.; Hargrave, G.; Ho, K.H.; Paul, A.; Plevin, R. Inhibition of cytokine-mediated JNK signalling by purinergic P2Y11 receptors, a novel protective mechanism in endothelial cells. Cell Signal. 2018, 51, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Zylberg, J.; Ecke, D.; Fischer, B.; Reiser, G. Structure and ligand-binding site characteristics of the human P2Y11 nucleotide receptor deduced from computational modelling and mutational analysis. Biochem. J. 2007, 405, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Hapil, F.Z.; Copuroglu, F.E.; Ertosun, M.G.; Mert, U.; Ozes, D.; Ozes, O.N. Negative Regulation of TNFR1 Signaling Via PKA-Mediated Phosphorylation of TNFR1. J. Interferon Cytokine Res. 2020, 40, 225–235. [Google Scholar] [CrossRef]
- Avanzato, D.; Genova, T.; Fiorio Pla, A.; Bernardini, M.; Bianco, S.; Bussolati, B.; Mancardi, D.; Giraudo, E.; Maione, F.; Cassoni, P.; et al. Activation of P2 × 7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016, 6, 32602. [Google Scholar] [CrossRef]
- Avanzato, D.; Genova, T.; Fiorio Pla, A.; Bernardini, M.; Bianco, S.; Bussolati, B.; Mancardi, D.; Giraudo, E.; Maione, F.; Cassoni, P.; et al. Erratum: Activation of P2 × 7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016, 6, 35897. [Google Scholar] [CrossRef]
- Lezoualc’h, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP Sensor EPAC Proteins and Their Role in Cardiovascular Function and Disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef]
- Qian, S.; Hoggatt, A.; Jones-Hall, Y.L.; Ware, C.F.; Herring, P.; Seye, C.I. Deletion of P2Y2 receptor reveals a role for lymphotoxin-alpha in fatty streak formation. Vasc. Pharmacol. 2016, 85, 11–20. [Google Scholar] [CrossRef]
- Eun, S.Y.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. P2Y(2)R activation by nucleotides released from oxLDL-treated endothelial cells (ECs) mediates the interaction between ECs and immune cells through RAGE expression and reactive oxygen species production. Free Radic. Biol. Med. 2014, 69, 157–166. [Google Scholar] [CrossRef]
- Jin, H.; Ko, Y.S.; Park, S.W.; Kim, H.J. P2Y2R activation by ATP induces oxLDL-mediated inflammasome activation through modulation of mitochondrial damage in human endothelial cells. Free Radic. Biol. Med. 2019, 136, 109–117. [Google Scholar] [CrossRef]
- Chen, X.; Qian, S.; Hoggatt, A.; Tang, H.; Hacker, T.A.; Obukhov, A.G.; Herring, P.B.; Seye, C.I. Endothelial Cell-Specific Deletion of P2Y2 Receptor Promotes Plaque Stability in Atherosclerosis-Susceptible ApoE-Null Mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Stachon, P.; Peikert, A.; Michel, N.A.; Hergeth, S.; Marchini, T.; Wolf, D.; Dufner, B.; Hoppe, N.; Ayata, C.K.; Grimm, M.; et al. P2Y6 deficiency limits vascular inflammation and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Jiang, D.; You, D.; Zhang, L.; Liu, L.; Zhao, Q. Agonism of GPR120 prevents ox-LDL-induced attachment of monocytes to endothelial cells. Chem. Biol. Interact. 2020, 316, 108916. [Google Scholar] [CrossRef] [PubMed]
- SenBanerjee, S.; Lin, Z.; Atkins, G.B.; Greif, D.M.; Rao, R.M.; Kumar, A.; Feinberg, M.W.; Chen, Z.; Simon, D.I.; Luscinskas, F.W.; et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 2004, 199, 1305–1315. [Google Scholar] [CrossRef]
- Ding, L.; Ma, W.; Littmann, T.; Camp, R.; Shen, J. The P2Y(2) nucleotide receptor mediates tissue factor expression in human coronary artery endothelial cells. J. Biol. Chem. 2011, 286, 27027–27038. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, C.; Roy, S.; Shen, J. Purinergic P2Y2 Receptor Control of Tissue Factor Transcription in Human Coronary Artery Endothelial Cells: NEW AP-1 TRANSCRIPTION FACTOR SITE AND NEGATIVE REGULATOR. J. Biol. Chem. 2016, 291, 1553–1563. [Google Scholar] [CrossRef]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Musher, D.M.; Wells, G.A.; Chirinos, J.A.; Chen, L.; Fine, M.J. Cardiac complications in patients with community-acquired pneumonia: Incidence, timing, risk factors, and association with short-term mortality. Circulation 2012, 125, 773–781. [Google Scholar] [CrossRef]
- Katzan, I.L.; Cebul, R.D.; Husak, S.H.; Dawson, N.V.; Baker, D.W. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology 2003, 60, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Katzan, I.L.; Dawson, N.V.; Thomas, C.L.; Votruba, M.E.; Cebul, R.D. The cost of pneumonia after acute stroke. Neurology 2007, 68, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Ramakrishna, H.; Augoustides, J.G. Recent advances in chronic thromboembolic pulmonary hypertension. J. Cardiothorac. Vasc. Anesth. 2011, 25, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef]
- Wang, Z.; Ginnan, R.; Abdullaev, I.F.; Trebak, M.; Vincent, P.A.; Singer, H.A. Calcium/Calmodulin-dependent protein kinase II delta 6 (CaMKIIdelta6) and RhoA involvement in thrombin-induced endothelial barrier dysfunction. J. Biol. Chem. 2010, 285, 21303–21312. [Google Scholar] [CrossRef]
- Eckle, T.; Faigle, M.; Grenz, A.; Laucher, S.; Thompson, L.F.; Eltzschig, H.K. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 2008, 111, 2024–2035. [Google Scholar] [CrossRef]
- Kovacs-Kasa, A.; Kim, K.M.; Cherian-Shaw, M.; Black, S.M.; Fulton, D.J.; Verin, A.D. Extracellular adenosine-induced Rac1 activation in pulmonary endothelium: Molecular mechanisms and barrier-protective role. J. Cell. Physiol. 2018, 233, 5736–5746. [Google Scholar] [CrossRef]
- Umapathy, N.S.; Fan, Z.; Zemskov, E.A.; Alieva, I.B.; Black, S.M.; Verin, A.D. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vasc. Pharmacol. 2010, 52, 199–206. [Google Scholar] [CrossRef]
- Umapathy, S.N.; Kaczmarek, E.; Fatteh, N.; Burns, N.; Lucas, R.; Stenmark, K.R.; Verin, A.D.; Gerasimovskaya, E.V. Adenosine A1 receptors promote vasa vasorum endothelial cell barrier integrity via Gi and Akt-dependent actin cytoskeleton remodeling. PLoS ONE 2013, 8, e59733. [Google Scholar] [CrossRef]
- Verin, A.D.; Batori, R.; Kovacs-Kasa, A.; Cherian-Shaw, M.; Kumar, S.; Czikora, I.; Karoor, V.; Strassheim, D.; Stenmark, K.R.; Gerasimovskaya, E. Extracellular adenosine enhances pulmonary artery vasa vasorum endothelium cell barrier function via the Gi/ELMO1/Rac1/PKA-dependent signaling mechanisms. Am. J. Physiol. Cell Physiol. 2020, 319, C183–C193. [Google Scholar] [CrossRef]
- Kolosova, I.A.; Mirzapoiazova, T.; Adyshev, D.; Usatyuk, P.; Romer, L.H.; Jacobson, J.R.; Natarajan, V.; Pearse, D.B.; Garcia, J.G.; Verin, A.D. Signaling pathways involved in adenosine triphosphate-induced endothelial cell barrier enhancement. Circ. Res. 2005, 97, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.; Bobalova, J.; Mendoza, M.G.; Lew, C.; Mutafova-Yambolieva, V.N. Release of beta-nicotinamide adenine dinucleotide upon stimulation of postganglionic nerve terminals in blood vessels and urinary bladder. J. Biol. Chem. 2004, 279, 48893–48903. [Google Scholar] [CrossRef] [PubMed]
- Billington, R.A.; Bruzzone, S.; De Flora, A.; Genazzani, A.A.; Koch-Nolte, F.; Ziegler, M.; Zocchi, E. Emerging functions of extracellular pyridine nucleotides. Mol. Med. 2006, 12, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Niere, M. NAD+ surfaces again. Biochem. J. 2004, 382, e5–e6. [Google Scholar] [CrossRef]
- Koch-Nolte, F.; Fischer, S.; Haag, F.; Ziegler, M. Compartmentation of NAD+-dependent signalling. FEBS Lett. 2011, 585, 1651–1656. [Google Scholar] [CrossRef]
- Horckmans, M.; Robaye, B.; Leon-Gomicronmez, E.; Lantz, N.; Unger, P.; Dol-Gleizes, F.; Clouet, S.; Cammarata, D.; Schaeffer, P.; Savi, P.; et al. P2Y(4) nucleotide receptor: A novel actor in post-natal cardiac development. Angiogenesis 2012, 15, 349–360. [Google Scholar] [CrossRef]
- Visovatti, S.H.; Hyman, M.C.; Goonewardena, S.N.; Anyanwu, A.C.; Kanthi, Y.; Robichaud, P.; Wang, J.; Petrovic-Djergovic, D.; Rattan, R.; Burant, C.F.; et al. Purinergic dysregulation in pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H286–H298. [Google Scholar] [CrossRef]
- Rumjahn, S.M.; Yokdang, N.; Baldwin, K.A.; Thai, J.; Buxton, I.L. Purinergic regulation of vascular endothelial growth factor signaling in angiogenesis. Br. J. Cancer 2009, 100, 1465–1470. [Google Scholar] [CrossRef]
- Roedersheimer, M.; Nijmeh, H.; Burns, N.; Sidiakova, A.A.; Stenmark, K.R.; Gerasimovskaya, E.V. Complementary effects of extracellular nucleotides and platelet-derived extracts on angiogenesis of vasa vasorum endothelial cells in vitro and subcutaneous Matrigel plugs in vivo. Vasc. Cell 2011, 3, 4. [Google Scholar] [CrossRef]
- Davie, N.J.; Crossno, J.T., Jr.; Frid, M.G.; Hofmeister, S.E.; Reeves, J.T.; Hyde, D.M.; Carpenter, T.C.; Brunetti, J.A.; McNiece, I.K.; Stenmark, K.R. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: Contribution of progenitor cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L668–L678. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, L.O.; Rodriguez-Porcel, M.; Holmes, D.R., Jr.; Richardson, D.M.; Ritman, E.L.; Lerman, A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc. Res. 2001, 51, 762–766. [Google Scholar] [CrossRef]
- Davie, N.J.; Gerasimovskaya, E.V.; Hofmeister, S.E.; Richman, A.P.; Jones, P.L.; Reeves, J.T.; Stenmark, K.R. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: A process mediated by hypoxia and endothelin-1. Am. J. Pathol. 2006, 168, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Langheinrich, A.C.; Kampschulte, M.; Buch, T.; Bohle, R.M. Vasa vasorum and atherosclerosis—Quid novi? Thromb. Haemost. 2007, 97, 873–879. [Google Scholar] [PubMed]
- Mulligan-Kehoe, M.J. The vasa vasorum in diseased and nondiseased arteries. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H295–H305. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Douglas, I.S.; Nicolls, M. Angiogenesis in chronic lung disease. Chest 2007, 131, 874–879. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K.; Voelkel, N.F. Endothelial cells and pulmonary arterial hypertension: Apoptosis, proliferation, interaction and transdifferentiation. Respir. Res. 2009, 10, 95. [Google Scholar] [CrossRef]
- Nijmeh, H.; Balasubramaniam, V.; Burns, N.; Ahmad, A.; Stenmark, K.R.; Gerasimovskaya, E.V. High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L661–L671. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Yeager, M.E.; El Kasmi, K.C.; Nozik-Grayck, E.; Gerasimovskaya, E.V.; Li, M.; Riddle, S.R.; Frid, M.G. The adventitia: Essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 2013, 75, 23–47. [Google Scholar] [CrossRef]
- Billaud, M.; Hill, J.C.; Richards, T.D.; Gleason, T.G.; Phillippi, J.A. Medial Hypoxia and Adventitial Vasa Vasorum Remodeling in Human Ascending Aortic Aneurysm. Front. Cardiovasc. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Mulligan-Kehoe, M.J.; Simons, M. Vasa vasorum in normal and diseased arteries. Circulation 2014, 129, 2557–2566. [Google Scholar] [CrossRef]
- Numano, F. Vasa vasoritis, vasculitis and atherosclerosis. Int. J. Cardiol. 2000, 75 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef]
- Hamaoka-Okamoto, A.; Suzuki, C.; Yahata, T.; Ikeda, K.; Nagi-Miura, N.; Ohno, N.; Arai, Y.; Tanaka, H.; Takamatsu, T.; Hamaoka, K. The involvement of the vasa vasorum in the development of vasculitis in animal model of Kawasaki disease. Pediatr. Rheumatol. Online J. 2014, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, J.; Hasebe, N. Role of the vasa vasorum and vascular resident stem cells in atherosclerosis. Biomed. Res. Int. 2014, 2014, 701571. [Google Scholar] [CrossRef]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Shi, G.P. Vasa vasorum in atherosclerosis and clinical significance. Int. J. Mol. Sci. 2015, 16, 11574–11608. [Google Scholar] [CrossRef]
- Mitzner, W.; Wagner, E.M. Vascular remodeling in the circulations of the lung. J. Appl. Physiol. 2004, 97, 1999–2004. [Google Scholar] [CrossRef][Green Version]
- Gerasimovskaya, E.V.; Woodward, H.N.; Tucker, D.A.; Stenmark, K.R. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis 2008, 11, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.L.; Boyle, J.P.; Roberts, J.A.; Challiss, R.A.; Gubby, S.E.; Boarder, M.R. Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen-activated protein kinase. Br. J. Pharmacol. 1997, 122, 935–941. [Google Scholar] [CrossRef]
- Cha, S.H.; Hahn, T.W.; Sekine, T.; Lee, K.H.; Endou, H. Purinoceptor-mediated calcium mobilization and cellular proliferation in cultured bovine corneal endothelial cells. Jpn. J. Pharmacol. 2000, 82, 181–187. [Google Scholar] [CrossRef][Green Version]
- Van Daele, P.; Van Coevorden, A.; Roger, P.P.; Boeynaems, J.M. Effects of adenine nucleotides on the proliferation of aortic endothelial cells. Circ. Res. 1992, 70, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Oike, M.; Karashima, Y.; Ito, Y. Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J. Physiol. 2004, 558, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sokabe, T.; Ohura, N.; Nakatsuka, H.; Kamiya, A.; Ando, J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H793–H803. [Google Scholar] [CrossRef]
- Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc. Res. 2009, 82, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Waterfield, M.D. Signaling by distinct classes of phosphoinositide 3-kinases. Exp. Cell Res. 1999, 253, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Guba, M.; von Breitenbuch, P.; Steinbauer, M.; Koehl, G.; Flegel, S.; Hornung, M.; Bruns, C.J.; Zuelke, C.; Farkas, S.; Anthuber, M.; et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat. Med. 2002, 8, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.H.; Zheng, J.Z.; Aoki, M.; Vogt, P.K. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1749–1753. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef]
- Wang, L.; Jacobsen, S.E.; Bengtsson, A.; Erlinge, D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004, 5, 16. [Google Scholar] [CrossRef]
- Phung, T.L.; Hochman, M.; Mihm, M.C. Current knowledge of the pathogenesis of infantile hemangiomas. Arch. Facial Plast. Surg. 2005, 7, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Marteau, F.; Communi, D.; Boeynaems, J.M.; Suarez Gonzalez, N. Involvement of multiple P2Y receptors and signaling pathways in the action of adenine nucleotides diphosphates on human monocyte-derived dendritic cells. J. Leukoc. Biol. 2004, 76, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Seye, C.I.; Kong, Q.; Erb, L.; Garrad, R.C.; Krugh, B.; Wang, M.; Turner, J.T.; Sturek, M.; Gonzalez, F.A.; Weisman, G.A. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation 2002, 106, 2720–2726. [Google Scholar] [CrossRef]
- Seye, C.I.; Yu, N.; Gonzalez, F.A.; Erb, L.; Weisman, G.A. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J. Biol. Chem. 2004, 279, 35679–35686. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strassheim, D.; Verin, A.; Batori, R.; Nijmeh, H.; Burns, N.; Kovacs-Kasa, A.; Umapathy, N.S.; Kotamarthi, J.; Gokhale, Y.S.; Karoor, V.; et al. P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 6855. https://doi.org/10.3390/ijms21186855
Strassheim D, Verin A, Batori R, Nijmeh H, Burns N, Kovacs-Kasa A, Umapathy NS, Kotamarthi J, Gokhale YS, Karoor V, et al. P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases. International Journal of Molecular Sciences. 2020; 21(18):6855. https://doi.org/10.3390/ijms21186855
Chicago/Turabian StyleStrassheim, Derek, Alexander Verin, Robert Batori, Hala Nijmeh, Nana Burns, Anita Kovacs-Kasa, Nagavedi S. Umapathy, Janavi Kotamarthi, Yash S. Gokhale, Vijaya Karoor, and et al. 2020. "P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases" International Journal of Molecular Sciences 21, no. 18: 6855. https://doi.org/10.3390/ijms21186855
APA StyleStrassheim, D., Verin, A., Batori, R., Nijmeh, H., Burns, N., Kovacs-Kasa, A., Umapathy, N. S., Kotamarthi, J., Gokhale, Y. S., Karoor, V., Stenmark, K. R., & Gerasimovskaya, E. (2020). P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases. International Journal of Molecular Sciences, 21(18), 6855. https://doi.org/10.3390/ijms21186855






