Dnmt3a2/Dnmt3L Overexpression in the Dopaminergic System of Mice Increases Exercise Behavior through Signaling Changes in the Hypothalamus
Abstract
1. Introduction
2. Results
2.1. Key Findings
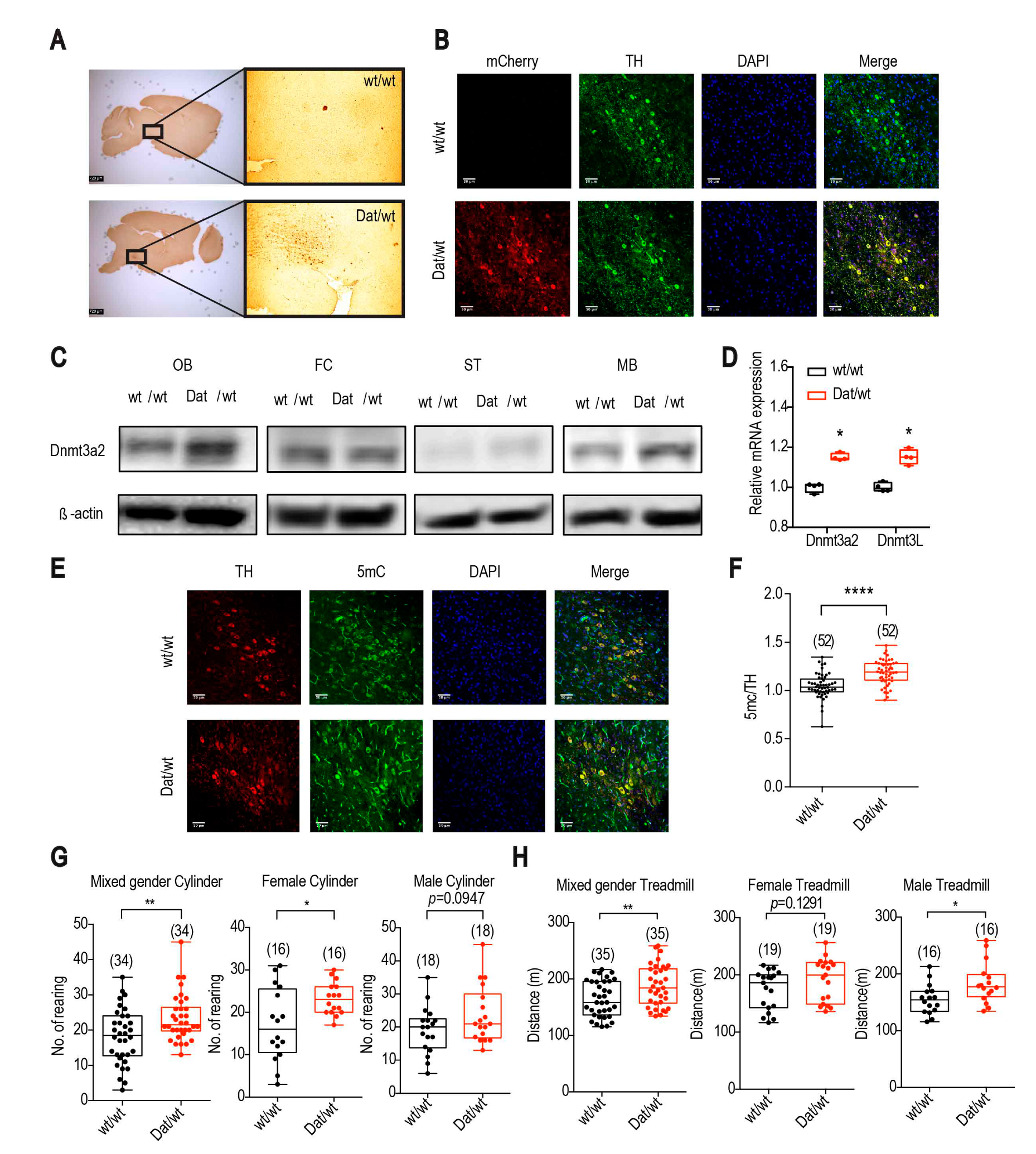
2.1.1. Dnmt3a2/3L Increases Spontaneous Activity and Exercise Performance of Mice with a Gender Preference
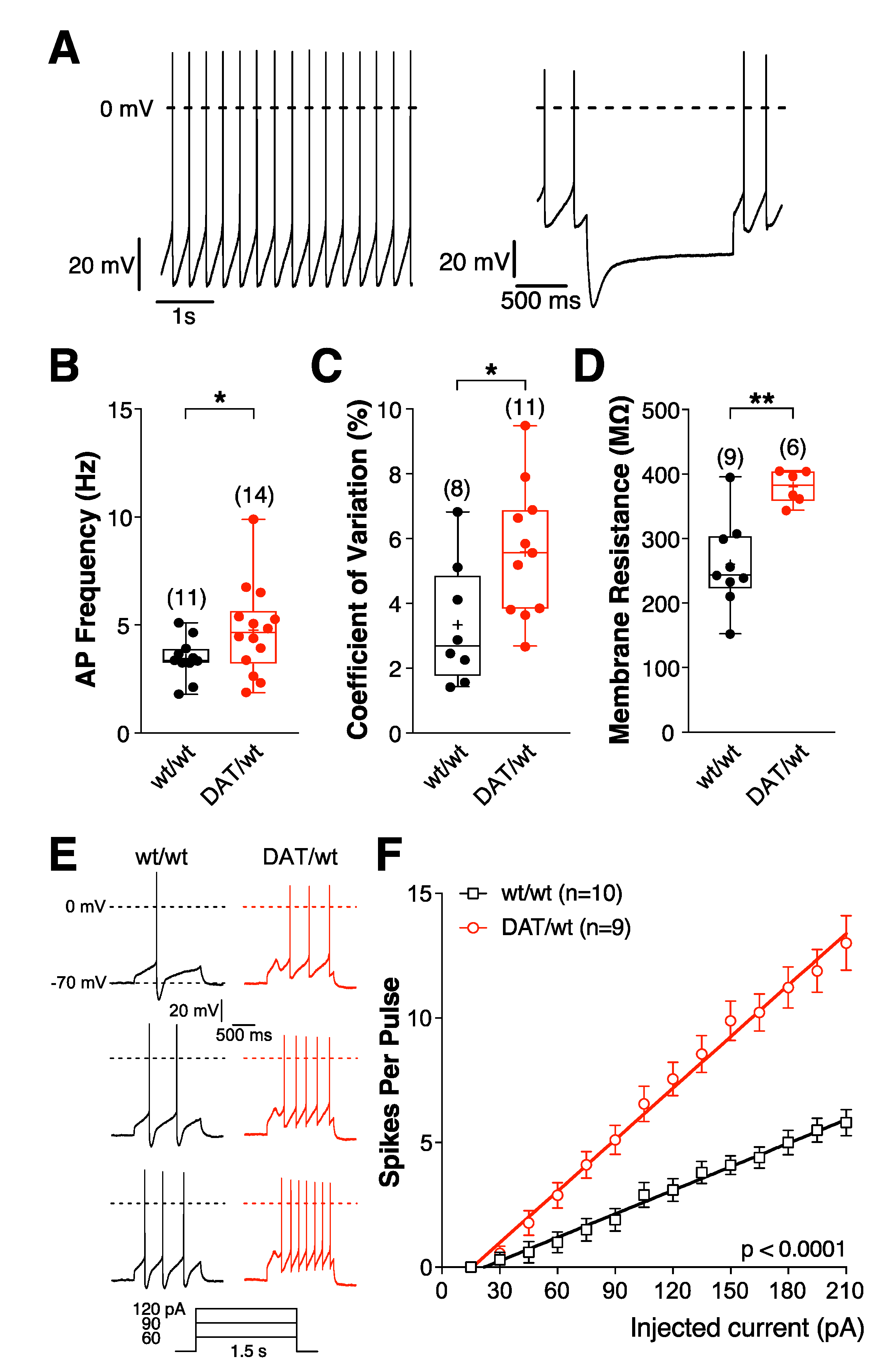
2.1.2. Dopaminergic Neurons from Dnmt3a2/3LDat/wt Animals Have a Higher Firing Frequency and Excitability
2.1.3. Dnmt3a2/3L Overexpression Decreased Dopamine Metabolites in the Striatum
2.1.4. Dnmt3a2/3L Overexpression Increased Dopamine Synthesis in Several Brain Areas, But Not in the Striatum
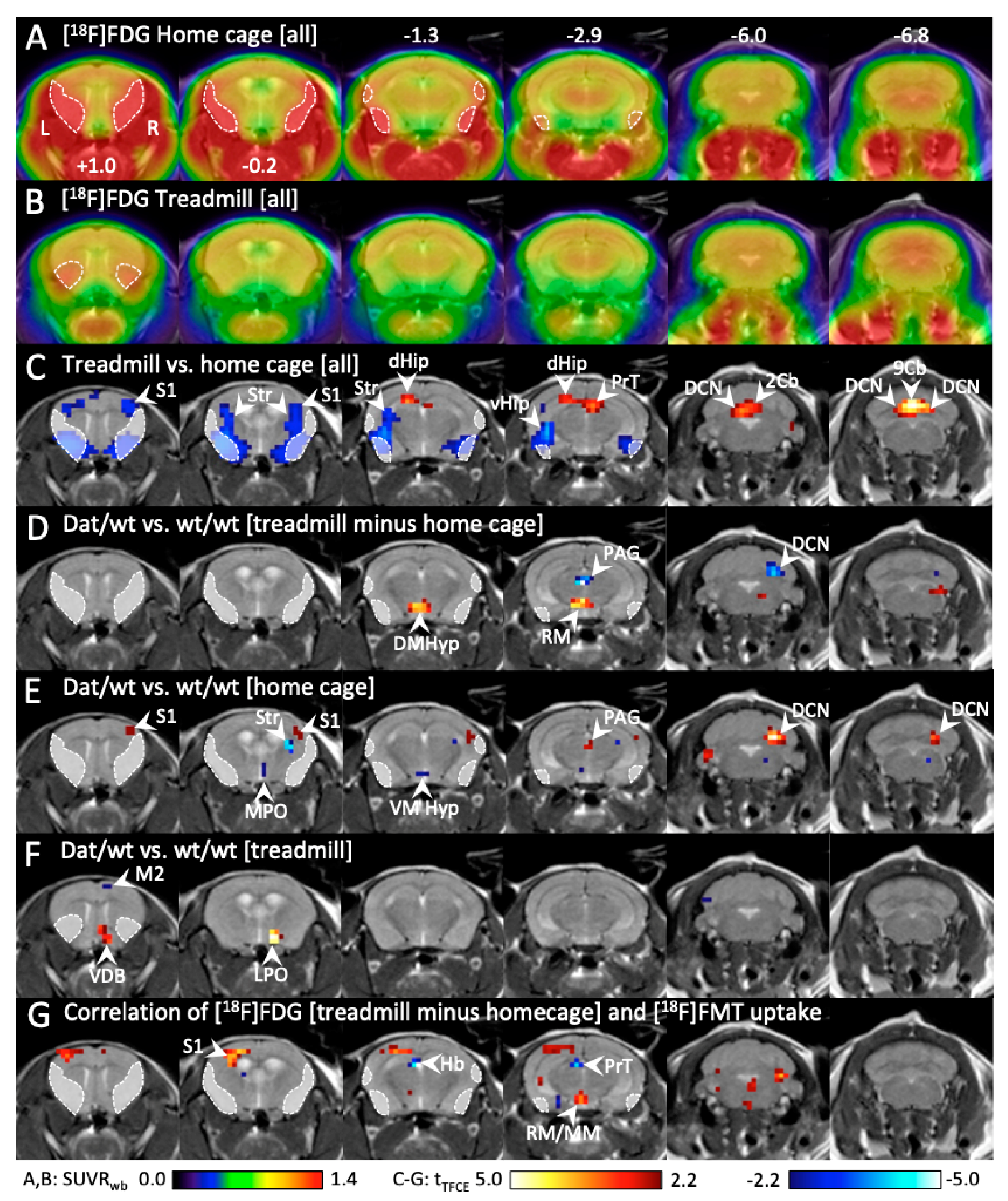
2.1.5. Hypothalamic Metabolic Activity Was Tightly Linked to Motor Behavior in Dnmt3a2/3LDat/wt Mice, and Correlated to Dopamine Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Transgenic Mice
4.2. Immunohistochemistry
4.3. DNA Methylation Level
4.4. Behavior and Metabolism
4.4.1. Cylinder Test
4.4.2. Open Field
4.4.3. Treadmill
4.4.4. Metabolic Cages
4.4.5. Electrophysiology
4.4.6. Tissue Metabolite Analysis
4.4.7. Positron Emission Tomography (PET)
4.4.8. Quantification and Statistical Analysis
Behavior and Metabolism
Electrophysiology
4.4.9. Tissue Metabolite Analysis
4.4.10. Positron Emission Tomography (PET)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DAT | Dopamine transporter |
| PET | Positron emission tomography |
| SNc | Substantia nigra pars compacta |
| DNMT | DNA methyltransferase |
| 3MT | 3-methoxytyramine |
| DOPAC | 3,4-dihydroxyphenyl acetic acid |
| HVA | Homovanillic acid |
| FMT | F-meta-tyrosine |
| OB | olfactory bulb |
| ST | striatum |
| MB | midbrain |
References
- Jones, M.J.; Goodman, S.J.; Kobor, M.S. DNA methylation and healthy human aging. Aging Cell 2015, 14, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Blanco, M.A.; Gu, L.; Sendinc, E.; Liu, J.; Aristizábal-Corrales, D.; Chih-Hung, H.; Aravind, L.; Chuan, H.; Yang, S. DNA methylation on N 6-adenine in C. elegans. Cell 2015, 161, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hellmann, I.; Stadler, B.M.; Ramos, L.; Svante, P.; Rebhan, M.; Schubeler, D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat. Genet. 2007, 39, 457. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, G.; Kreutz, M.R. Neuronal DNA Methyltransferases: Epigenetic Mediators between Synaptic Activity and Gene Expression? Neuroscience 2017, 24, 171–185. [Google Scholar] [CrossRef]
- Cui, D.; Xu, X. DNA Methyltransferases, DNA Methylation, and Age-Associated Cognitive Function. Int. J. Mol. Sci. 2018, 19, 1315. [Google Scholar] [CrossRef]
- Chen, T.; Ueda, Y.; Xie, S.; Li, E. A novel Dnmt3a isoform produced from an alternative promoter localizes to euchromatin and its expression correlates with active de novo methylation. J. Biol. Chem. 2002, 277, 38746–38754. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 132–146. [Google Scholar] [CrossRef]
- Suetake, I.; Shinozaki, F.; Miyagawa, J.; Takeshima, H.; Tajima, S. DNMT3L stimulates the DNA methylation activity of Dnmt3a and Dnmt3b through a direct interaction. J. Biol. Chem. 2004, 279, 27816–27823. [Google Scholar] [CrossRef]
- Nimura, K.; Ishida, C.; Koriyama, H.; Hata, K.; Yamanaka, S.; Li, E.; Ura, K.; Kaneda, Y. Dnmt3a2 targets endogenous Dnmt3L to ES cell chromatin and induces regional DNA methylation. Genes Cells 2006, 11, 1225–1237. [Google Scholar] [CrossRef]
- Oliveira, A.M.M.; Hemstedt, T.J.; Bading, H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat. Neurosci. 2012, 15, 1111. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.M.; Hemstedt, T.J.; Freitag, H.E.; Bading, H. Dnmt3a2: a hub for enhancing cognitive functions. Mol. Psychiatry 2016, 21, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Karaca, K.G.; Kupke, J.; Brito, D.V.C.; Zeuch, B.; Thome, C.; Weichenhan, D.; Lutsik, P.; Plass, C.; Oliveira, A.M.M. Neuronal ensemble-specific DNA methylation strengthens engram stability. Nat. Commun. 2020, 11, 1–13. [Google Scholar]
- Feng, J.; Zhou, Y.; Campbell, S.L.; Le, T.; Li, E.; Sweatt, J.D.; Silva, A.J.; Fan, G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 2010, 13, 423–430. [Google Scholar] [CrossRef]
- Morris, M.J.; Adachi, M.; Na, E.S.; Monteggia, L.M. Selective role for DNMT3a in learning and memory. Neurobiol. Learn. Mem. 2014, 115, 30–37. [Google Scholar] [CrossRef]
- Jay, T.M. Dopamine: A potential substrate for synaptic plasticity and memory mechanisms. Prog. Neurobiol. 2003, 69, 375–390. [Google Scholar] [CrossRef]
- Krashia, P.; Martini, A.; Nobili, A.; Aversa, D.; D’Amelio, M.; Berretta, N.; Guatteo, E.; Mercuri, N.B. On the properties of identified dopaminergic neurons in the mouse substantia nigra and ventral tegmental area. Eur. J. Neurosci. 2017, 45, 92–105. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Figge, D.A.; Jaunarajs, K.L.E.; Standaert, D.G. Dynamic DNA methylation regulates levodopa-induced dyskinesia. J. Neurosci. 2016, 36, 6514–6524. [Google Scholar] [CrossRef]
- Hara, S.; Takano, T.; Fujikawa, T.; Yamada, M.; Wakai, T.; Kono, T.; Obata, Y. Forced expression of DNA methyltransferases during oocyte growth accelerates the establishment of methylation imprints but not functional genomic imprinting. Hum. Mol. Genet. 2014, 23, 3853–3864. [Google Scholar] [CrossRef]
- Ekstrand, M.I.; Terzioglu, M.; Galter, D.; Zhu, S.; Hofstetter, C.; Lindqvist, E.; Thams, S.; Bergstrand, A.; Hansson, F.S.; Trifunovic, A. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Guzman, J.N.; Sánchez-Padilla, J.; Chan, C.S.; Surmeier, D.J. Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 2009, 29, 11011–11019. [Google Scholar] [CrossRef]
- Manley, L.D.; Kuczenski, R.; Segal, D.S.; Young, S.J.; Groves, P.M. Effects of frequency and pattern of medial forebrain bundle stimulation on caudate dialysate dopamine and serotonin. J. Neurochem. 1992, 58, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.H.; Murrin, L.C.; Walters, J.R. Central dopaminergic neurons: Effects of alterations in impulse flow on the accumulation of dihydroxyphenylacetic acid. Eur. J. Pharmacol. 1976, 36, 163–171. [Google Scholar] [CrossRef]
- Klanker, M.; Feenstra, M.; Willuhn, I.; Denys, D. Deep brain stimulation of the medial forebrain bundle elevates striatal dopamine concentration without affecting spontaneous or reward-induced phasic release. Neuroscience 2017, 364, 82–92. [Google Scholar] [CrossRef]
- Cachope, R.; Cheer, J.F. Local control of striatal dopamine release. Front. Behav. Neurosci. 2014, 8, 188. [Google Scholar] [CrossRef]
- DeJesus, O.T.; Sunderland, J.J.; Nickles, J.R.; Mukherjee, J.; Appelman, E.H. Synthesis of radiofluorinated analogs of m-tyrosine as potential L-dopa tracers via direct reaction with acetylhypofluorite. Int. J. Radiat. Appl. Instrument. Part A. Appl. Radiat. Isot. 1990, 41, 433–437. [Google Scholar] [CrossRef]
- Jordan, S.; Bankiewicz, K.S.; Eberling, J.L.; VanBrocklin, H.F.; O’neil, J.P.; Jagust, W.J. An in vivo microdialysis study of striatal 6-[18 F] fluoro-l-m-tyrosine metabolism. Neurochem. Res. 1998, 23, 513–517. [Google Scholar] [CrossRef]
- DeJesus, O.T.; Endres, C.J.; Shelton, S.E.; Nickles, R.J.; Holden, J.E. Evaluation of fluorinated m-tyrosine analogs as PET imaging agents of dopamine nerve terminals: comparison with 6-fluoroDOPA. J. Nucl. Med. 1997, 38, 630–636. [Google Scholar]
- Hadjiconstantinou, M.; Neff, N.H. Enhancing aromatic L-amino acid decarboxylase activity: implications for L-DOPA treatment in Parkinson’s disease. CNS Neurosci. Ther. 2008, 14, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.D.; DeJesus, O.T.; Pyzalski, R.W.; Malischke, L.; Roberts, A.D.; Shelton, S.E.; Uno, H.; Houser, W.D.; Nickles, R.J.; Holden, J.E. Localization of trapping of 6-[18F] fluoro-L-m-tyrosine, an aromatic L-amino acid decarboxylase tracer for PET. Synapse 1999, 34, 111–123. [Google Scholar] [CrossRef]
- Ugrumov, M. V Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance. J. Chem. Neuroanat. 2009, 38, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.A.; Dunn, A.R.; Lohr, K.M.; Alter, S.P.; Cliburn, R.A.; Guillot, T.S.; Miller, G.W. Selective enhancement of dopamine release in the ventral pallidum of methamphetamine-sensitized mice. ACS Chem. Neurosci. 2016, 7, 1364–1373. [Google Scholar] [CrossRef]
- Moore, K.E. Interactions between prolactin and dopaminergic neurons. Biol. Reprod. 1987, 36, 47–58. [Google Scholar] [CrossRef]
- Tovar, S.; Diéguez, C. Prolactin and energy homeostasis: pathophysiological mechanisms and therapeutic considerations. Endocrinology 2014, 155, 659–662. [Google Scholar] [CrossRef]
- Hackney, A.C.; Davis, H.C.; Lane, A.R. Growth hormone-insulin-like growth factor axis, thyroid axis, prolactin, and exercise. Sports Endocrinol. 2016, 47, 1–11. [Google Scholar]
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 2016, 6, 123–148. [Google Scholar] [CrossRef]
- Korchounov, A.; Meyer, M.F.; Krasnianski, M. Postsynaptic nigrostriatal dopamine receptors and their role in movement regulation. J. Neural Transm. 2010, 117, 1359–1369. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Smith, C.B.; Davidsen, L.; Savaki, H.; Sokoloff, L.; Mata, M.; Fink, D.J.; Gainer, H. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 1979, 205, 723–725. [Google Scholar] [CrossRef]
- Raichle, M.E.; Mintun, M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 2006, 29, 449–476. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Behar, K.L.; Rothman, D.L. Cellular origin of [18F] FDG-PET imaging signals during Ceftriaxone-stimulated glutamate uptake: astrocytes and neurons. Neuroscience 2018, 24, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-García, J.M.; Da Guarda, S.N.F.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Mariën, P. Consensus paper: roles of the cerebellum in motor control—The diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- Li, J.-Y.; Kuo, T.B.J.; Hsieh, I.-T.; Yang, C.C.H. Changes in hippocampal theta rhythm and their correlations with speed during different phases of voluntary wheel running in rats. Neuroscience 2012, 213, 54–61. [Google Scholar] [CrossRef]
- Friend, D.M.; Kravitz, A. V Working together: Basal ganglia pathways in action selection. Trends Neurosci. 2014, 37, 301–303. [Google Scholar] [CrossRef]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central fatigue. Sport. Med. 2006, 36, 881–909. [Google Scholar] [CrossRef]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical exercise-induced fatigue: the role of serotonergic and dopaminergic systems. Braz. J. Med. Biol. Res. 2017, 50. [Google Scholar] [CrossRef]
- Martyn, C.N.; Osmond, C.; Edwardson, J.A.; Barker, D.J.P.; Harris, E.C.; Lacey, R.F. Geographical relation between Alzheimer’s disease and aluminium in drinking water. Lancet 1989, 333, 59–62. [Google Scholar] [CrossRef]
- Fleming, S.M.; Ekhator, O.R.; Ghisays, V. Assessment of sensorimotor function in mouse models of Parkinson’s disease. J. Vis. Exp. JoVE 2013, 76, e50303. [Google Scholar] [CrossRef]
- Hess, M.E.; Hess, S.; Meyer, K.D.; Verhagen, L.A.W.; Koch, L.; Brönneke, H.S.; Dietrich, M.O.; Jordan, S.D.; Saletore, Y.; Elemento, O. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 2013, 16, 1042–1048. [Google Scholar] [CrossRef]
- Ortner, N.J.; Bock, G.; Dougalis, A.; Kharitonova, M.; Duda, J.; Hess, S.; Tuluc, P.; Pomberger, T.; Stefanova, N.; Pitterl, F. Lower affinity of isradipine for L-type Ca2+ channels during substantia nigra dopamine neuron-like activity: implications for neuroprotection in Parkinson’s disease. J. Neurosci. 2017, 37, 6761–6777. [Google Scholar] [CrossRef]
- Wong, J.-M.T.; Malec, P.A.; Mabrouk, O.S.; Ro, J.; Dus, M.; Kennedy, R.T. Benzoyl chloride derivatization with liquid chromatography–mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J. Chromatogr. A 2016, 1446, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Leahy, R.M.; Cherry, S.R.; Chatziioannou, A.; Farquhar, T.H. High-resolution 3D Bayesian image reconstruction using the microPET small-animal scanner. Phys. Med. Biol. 1998, 43, 1001. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Nichols, T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef]
- Jia, D.; Jurkowska, R.Z.; Zhang, X.; Jeltsch, A.; Cheng, X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature 2007, 449, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gottschalk, W.; Chow, A.; Wilson, R.I.; Schnell, E.; Zang, K.; Wang, D.; Nicoll, R.A.; Lu, B.; Reichardt, L.F. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J. Neurosci. 2020, 20, 6888–6897. [Google Scholar] [CrossRef]
- Dodt, H.-U. Infrared-interference videomicroscopy of living brain slices. In Optical Imaging of Brain Function and Metabolism; Springer: Boston, MA, USA, 1993; pp. 245–249. [Google Scholar]
- Ungless, M.A.; Whistler, J.L.; Malenka, R.C.; Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 2001, 411, 583–587. [Google Scholar] [CrossRef]
- Richards, C.D.; Shiroyama, T.; Kitai, S.T. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience 1997, 80, 545–557. [Google Scholar] [CrossRef]
- Lacey, M.G.; Mercuri, N.B.; North, R.A. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J. Neurosci. 1989, 9, 1233–1241. [Google Scholar] [CrossRef]
- Horn, R.; Marty, A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988, 92, 145–159. [Google Scholar] [CrossRef]
- Akaike, N.; Harata, N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn. J. Physiol. 1994, 44, 433–473. [Google Scholar] [CrossRef] [PubMed]
- Lindau, M.; Fernandez, J.M. A patch-clamp study of histamine-secreting cells. J. Gen. Physiol. 1986, 88, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Kyrozis, A.; Reichling, D.B. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J. Neurosci. Methods 1995, 57, 27–35. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, D.; Mesaros, A.; Burdeos, G.; Voigt, I.; Giavalisco, P.; Hinze, Y.; Purrio, M.; Neumaier, B.; Drzezga, A.; Obata, Y.; et al. Dnmt3a2/Dnmt3L Overexpression in the Dopaminergic System of Mice Increases Exercise Behavior through Signaling Changes in the Hypothalamus. Int. J. Mol. Sci. 2020, 21, 6297. https://doi.org/10.3390/ijms21176297
Cui D, Mesaros A, Burdeos G, Voigt I, Giavalisco P, Hinze Y, Purrio M, Neumaier B, Drzezga A, Obata Y, et al. Dnmt3a2/Dnmt3L Overexpression in the Dopaminergic System of Mice Increases Exercise Behavior through Signaling Changes in the Hypothalamus. International Journal of Molecular Sciences. 2020; 21(17):6297. https://doi.org/10.3390/ijms21176297
Chicago/Turabian StyleCui, Di, Andrea Mesaros, Gregor Burdeos, Ingo Voigt, Patrick Giavalisco, Yvonne Hinze, Martin Purrio, Bernd Neumaier, Alexander Drzezga, Yayoi Obata, and et al. 2020. "Dnmt3a2/Dnmt3L Overexpression in the Dopaminergic System of Mice Increases Exercise Behavior through Signaling Changes in the Hypothalamus" International Journal of Molecular Sciences 21, no. 17: 6297. https://doi.org/10.3390/ijms21176297
APA StyleCui, D., Mesaros, A., Burdeos, G., Voigt, I., Giavalisco, P., Hinze, Y., Purrio, M., Neumaier, B., Drzezga, A., Obata, Y., Endepols, H., & Xu, X. (2020). Dnmt3a2/Dnmt3L Overexpression in the Dopaminergic System of Mice Increases Exercise Behavior through Signaling Changes in the Hypothalamus. International Journal of Molecular Sciences, 21(17), 6297. https://doi.org/10.3390/ijms21176297





