Siloxanes—Versatile Materials for Surface Functionalisation and Graft Copolymers
Abstract
1. Introduction
2. Synthetic Routs for Producing Siloxanes
3. Functionalisation of Surfaces
3.1. Functionalisation with Polysiloxane Grafts
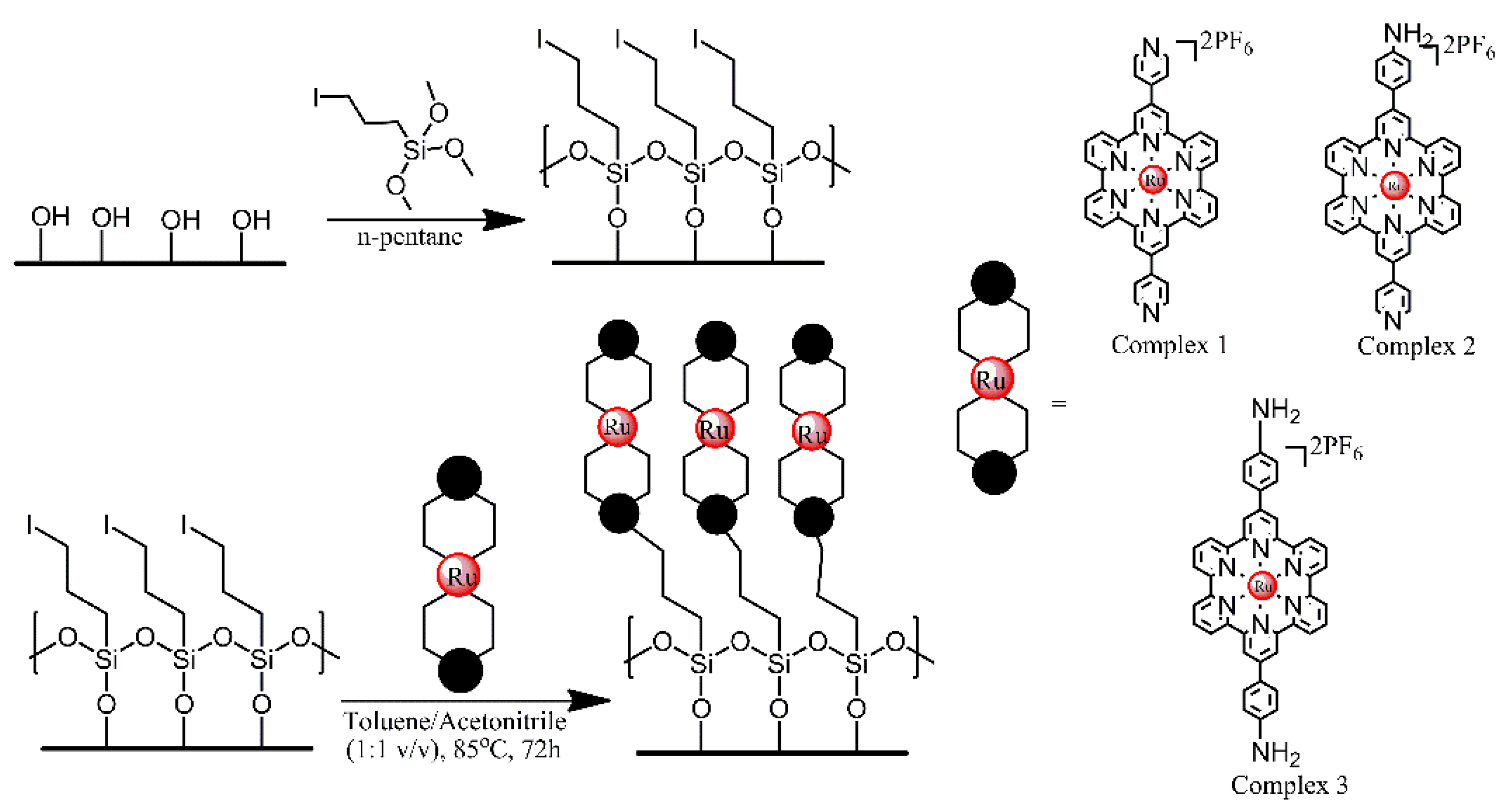
3.2. Functionalisation with Siloxanes
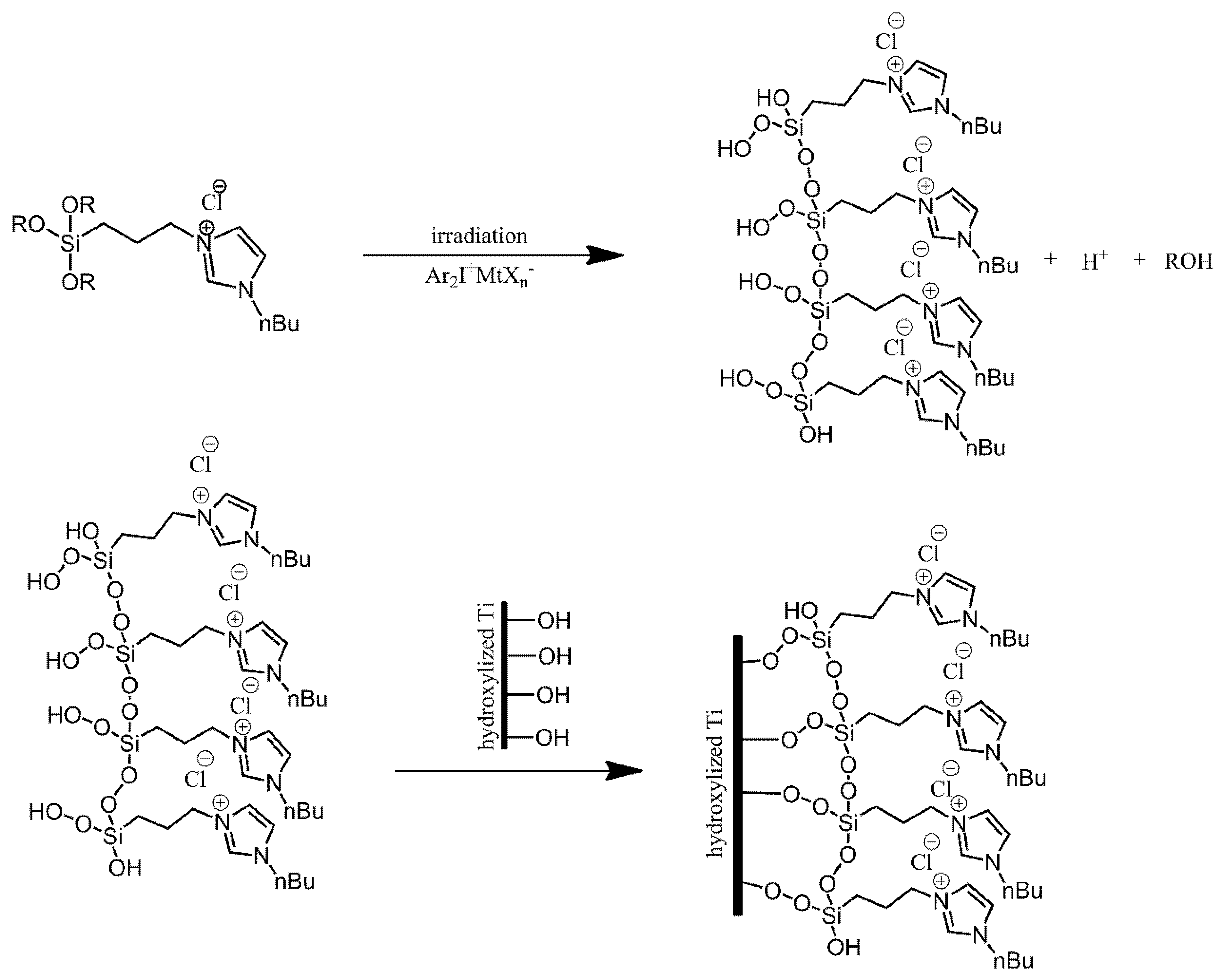
3.3. Functionalisation with Other Moieties via Siloxane Bonds
4. Graft Copolymers Bearing Polysiloxane Units
4.1. Polysiloxanes with Non-Polymer Grafts
4.2. Polysiloxane with Polymer Grafts
4.3. Polymers with Polysiloxane Grafts
4.4. Other Systems Bearing Siloxane Moieties
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1H NMR | proton nuclear magnetic resonance |
| 13C NMR | carbon 13 nuclear magnetic resonance |
| 29Si NMR | siloxane 29 nuclear magnetic resonance |
| AFM | atomic force microscopy |
| AIBN | Azobisisobutyronitrile |
| ATR-FTIR | attenuated total reflection Fourier transform infrared spectroscopy |
| BTA | benzene-1,3,5-tricarboxamide |
| CA | dynamic contact angle measurements |
| CAM | contact angle measurement |
| CAT | contact adhesion testing |
| CV | cyclic voltamperometry |
| DFLS | dynamic light scattering |
| DFT | density functional theory |
| DMA | dynamic mechanical analysis |
| DSC | differential scanning calorimetry |
| DTG | differential thermal gravimetry |
| EA | elementary analysis |
| EDX | energy-dispersive X-ray spectroscopy |
| EI MS | electrospray ionization mass spectroscopy |
| EIS | electrochemical impedance spectroscopy |
| FS | fluorescence spectroscopy |
| GPC | gel permeation chromatography |
| HRMS | high-resolution mass spectra |
| IR | infrared spectroscopy |
| IR RAS | infrared reflection-absorption spectroscopy |
| ITO | indium-tin oxide |
| MHS | methylhydrosiloxane |
| MW | molecular weight |
| PDI | polydispersity index |
| PDMS | poly(dimethylsiloxane) |
| PDMS-co-MHS | poly(dimethylsiloxane-co-methylhydrosiloxane) |
| PL | photoluminescence spectroscopy |
| POM | polarized optical microscopy |
| POSS | polyhedral oligomeric silsesquioxanes |
| PTFE | poly(tetrafluoroethylene) |
| PVAc | poly(vinyl acetate) |
| PXRD | powder X-ray Diffraction |
| RAFT | reversible addition-fragmentation chain transfer |
| RM | raman spectroscopy |
| SAXS | small angle X-ray scattering |
| SEC | size exclusion chromatography |
| SEM | scanning electron microscopy |
| TG | thermal gravimetry |
| UV-Vis | ultraviolet–visible spectroscopy |
| VT-IR | variable temperature infrared spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
References
- Guerret-Legras, L.; Maillot, B.; Audibert, J.F.; Dubacheva, G.V.; Galmiche, L.; Lang, P.; Miomandre, F. Electrofluorochromism of Surface-Confined Tetrazines Investigated on the Monolayer Scale. J. Phys. Chem. C 2019, 123, 29255–29261. [Google Scholar] [CrossRef]
- Cavoue, T.; Bounou Abassi, H.; Vayssade, M.; Nguyen Van Nhien, A.; Kang, I.K.; Kwon, G.W.; Pourceau, G.; Dubot, P.; Abbad Andaloussi, S.; Versace, D.L. Imidazolium-based titanium substrates against bacterial colonization. Biomater. Sci. 2017, 5, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Chu, F.; Hu, J.; Li, C. Liquid Polydimethylsiloxane Grafting to Enable Dendrite-Free Li Plating for Highly Reversible Li-Metal Batteries. Adv. Funct. Mater. 2019, 29, 1902220. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, X.; Yin, Y.; Wang, C. Temperature induced color changing cotton fabricated via grafting epoxy modified thermochromic capsules. Cellulose 2019, 26, 5745–5756. [Google Scholar] [CrossRef]
- Wu, B.; Ye, L.; Zhao, X. Construction of robust siloxane coating for urea-formaldehyde foam and durable hydrophobic mechanism. Compos. Part A Appl. Sci. Manuf. 2019, 122, 96–106. [Google Scholar] [CrossRef]
- Li, L.; Chi, X.; Gai, F.; Zhou, H.; Zhang, F.; Zhao Kent, Z. Synthesis of novel pyridinium N-chloramine precursors and its antimicrobial application on cotton fabrics. J. Appl. Polym. Sci. 2017, 134, 45323. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Fu, Z.; Geng, J.; Hua, J. Preparation and properties of 1,2-polybutadiene grafting with poly(1,3-butadiene)-block-(dimethylsiloxane). Polym. Adv. Technol. 2019, 30, 1663–1672. [Google Scholar] [CrossRef]
- Yi, L.; Xu, K.; Xia, G.; Li, J.; Li, W.; Cai, Y. New protein-resistant surfaces of amphiphilic graft copolymers containing hydrophilic poly(ethylene glycol) and low surface energy fluorosiloxane side-chains. Appl. Surf. Sci. 2019, 480, 923–933. [Google Scholar] [CrossRef]
- Ślęczkowski, M.L.; Meijer, E.W.; Palmans, A.R.A. Cooperative Folding of Linear Poly(dimethyl siloxane)s via Supramolecular Interactions. Macromol. Rapid Commun. 2017, 38, 1700566. [Google Scholar] [CrossRef]
- Shimojima, A.; Kuroda, K. Alkoxy- and Silanol-Functionalized Cage-Type Oligosiloxanes as Molecular Building Blocks to Construct Nanoporous Materials. Molecules 2020, 25, 524. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, Y.; Liu, M.; Zhang, Y.; Yin, Y.; Gutowski, W.S.; Deng, P.; Zheng, C. Improving the Damping Properties of Nanocomposites by Monodispersed Hybrid POSS Nanoparticles: Preparation and Mechanisms. Polymers (Basel) 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Gueye, M.; Ammar-Merah, S.; Nowak, S.; Decorse, P.; Chevillot-Biraud, A.; Perrière, L.; Couzinie, J.P.; Guillot, I.; Dirras, G. Study of the stability under in vitro physiological conditions of surface silanized equimolar HfNbTaTiZr high-entropy alloy: A first step toward bio-implant applications. Surf. Coat. Technol. 2020, 385, 125374. [Google Scholar] [CrossRef]
- Mondal, P.C.; Singh, V.; Jeyachandran, Y.L.; Zharnikov, M. Opto-Electroactive Amino- and Pyridyl-Terminated Monolayers of RuII-Terpyridyl Complexes and Their Usage as Hg2+ Sensors. J. Phys. Chem. C 2019, 123, 6121–6129. [Google Scholar] [CrossRef]
- Zhou, Q.; Yan, S.; Han, C.C.; Xie, P.; Zhang, R. Promising Functional Materials Based on Ladder Polysiloxanes. Adv. Mater. 2008, 20, 2970–2976. [Google Scholar] [CrossRef]
- He, S.; Sun, G.; Cheng, X.; Dai, H.; Chen, X. Nanoporous SiO2 grafted aramid fibers with low thermal conductivity. Compos. Sci. Technol. 2017, 146, 91–98. [Google Scholar] [CrossRef]
- Zhao, D.; He, J.; Zheng, N.; Huang, Y. Improved atomic oxygen erosion resistance of the carbon fibre–epoxy interface with polyhedral oligomeric silsesquioxane. High Perform. Polym. 2020, 32, 095400831989682. [Google Scholar] [CrossRef]
- Borges-Muñoz, A.C.; Miller, D.P.; Zurek, E.; Colón, L.A. Silanization of superficially porous silica particles with p-aminophenyltrimethoxysilane. Microchem. J. 2019, 147, 263–268. [Google Scholar] [CrossRef]
- Ren, Z.; Yan, S. Polysiloxanes for optoelectronic applications. Prog. Mater. Sci. 2016, 83, 383–416. [Google Scholar] [CrossRef]
- González Calderón, J.A.; Contreras López, D.; Pérez, E.; Vallejo Montesinos, J. Polysiloxanes as polymer matrices in biomedical engineering: Their interesting properties as the reason for the use in medical sciences. Polym. Bull. 2020, 77, 2749–2817. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Purkayastha, A.; Baruah, J.B. Review: Synthetic methodologies in siloxanes. Appl. Organomet. Chem. 2004, 18, 166–175. [Google Scholar] [CrossRef]
- Kuciński, K.; Hreczycho, G. Catalytic Formation of Silicon–Heteroatom (N, P, O, S) Bonds. ChemCatChem 2017, 9, 1868–1885. [Google Scholar] [CrossRef]
- Fortună, M.E.; Ignat, M.; Asandulesa, M.; Rotaru, R.; Pricop, L.; Harabagiu, V. Improved Physico-chemical Properties of Mesoporous Carbon by Functionalization with Aminopropyl-polydimethylsiloxane (AP-PDMS). J. Inorg. Organomet. Polym. Mater. 2018, 28, 2275–2287. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M.; Dong, W.; Le, Z.; Zhang, D.; Henderson, I.M. A 29Si, 1H, and 13C Solid-State NMR Study on the Surface Species of Various Depolymerized Organosiloxanes at Silica Surface. Nanoscale Res. Lett. 2019, 14, 160. [Google Scholar] [CrossRef]
- Klonos, P.A.; Goncharuk, O.V.; Pakhlov, E.M.; Sternik, D.; Derylo-Marczewska, A.; Kyritsis, A.; Gun’Ko, V.M.; Pissis, P. Morphology, Molecular Dynamics, and Interfacial Phenomena in Systems Based on Silica Modified by Grafting Polydimethylsiloxane Chains and Physically Adsorbed Polydimethylsiloxane. Macromolecules 2019, 52, 2863–2877. [Google Scholar] [CrossRef]
- Wang, B.; Ye, Z.; Tang, Y.; Han, Y.; Lin, Q.; Liu, H.; Chen, H.; Nan, K. Fabrication of nonfouling, bactericidal, and bacteria corpse release multifunctional surface through surface-initiated RAFT polymerization. Int. J. Nanomed. 2016, 12, 111–125. [Google Scholar] [CrossRef]
- Nowacka, M.; Rygała, A.; Kręgiel, D.; Kowalewska, A. Poly(silsesquioxanes) and poly(siloxanes) grafted with N-acetylcysteine for eradicating mature bacterial biofilms in water environment. Colloids Surf. B Biointerfaces 2018, 172, 627–634. [Google Scholar] [CrossRef]
- Lemonier, S.; Marty, J.; Fitremann, J. Polysiloxanes Modified by Thiol-Ene Reaction and Their Interaction with Gold Nanoparticles. Helv. Chim. Acta 2019, 102, e1900180. [Google Scholar] [CrossRef]
- Maksym, P.; Tarnacka, M.; Bielas, R.; Hachuła, B.; Zajac, A.; Szpecht, A.; Smiglak, M.; Kaminski, K.; Paluch, M. Structure-property relationships of tailored imidazolium- and pyrrolidinium-based poly(ionic liquid)s. Solid-like vs. gel-like systems. Polymer (Guildf) 2020, 192, 122262. [Google Scholar] [CrossRef]
- Ge, C.; Guo, Y.; Ma, X.; Hou, B. Functionalization of epoxy resin and the performance of the resultant magnesium-rich primer. E Polymers 2017, 17, 137–148. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Y.; Zhai, Y.; Wang, W.; Du, Z.; Qin, J. Polyglycerol modified polysiloxane surfactants: Their adsorption and aggregation behavior in aqueous solution. J. Ind. Eng. Chem. 2017, 47, 121–127. [Google Scholar] [CrossRef]
- Hu, H.; Liu, G.; Wang, J. Preparation and comparison of NP-GLIDE, SLIPS, superhydrophobic, and other coatings from identical precursors at different mixing ratios. J. Mater. Chem. A 2019, 7, 1519–1528. [Google Scholar] [CrossRef]
- Shen, K.; Hu, H.; Wang, J.; Liu, G. Synthesis and dynamic de-wetting properties of poly(arylene ether sulfone)-graft-poly(dimethyl siloxane). Polymer (Guildf) 2017, 132, 198–205. [Google Scholar] [CrossRef]
- Satti, A.J.; Ressia, J.A.; Molinari, E.; Ciolino, A.E.; Vallés, E.M. Metallocenic polyolefin composites with siloxane polymer additives. Radiat. Phys. Chem. 2018, 151, 205–210. [Google Scholar] [CrossRef]
- Sethi, S.K.; Shankar, U.; Manik, G. Fabrication and characterization of non-fluoro based transparent easy-clean coating formulations optimized from molecular dynamics simulation. Prog. Org. Coat. 2019, 136, 105306. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Anisimov, A.A.; Peregudov, A.S.; Dubovik, A.S.; Orlov, V.N.; Malakhova, Y.N.; Stupnikov, A.A.; Buzin, M.I.; Nikiforova, G.G.; Vasil’ev, V.G.; et al. Star-shaped siloxane polymers with various cyclic cores: Synthesis and properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1233–1246. [Google Scholar] [CrossRef]
- Träger, A.; Klein, G.; Carrick, C.; Pettersson, T.; Johansson, M.; Wågberg, L.; Pendergraph, S.A.; Carlmark, A. Macroscopic cellulose probes for the measurement of polymer grafted surfaces. Cellulose 2019, 26, 1467–1477. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.; Miao, D.; Ning, X. Fabrication of Durably Superhydrophobic Cotton Fabrics by Atmospheric Pressure Plasma Treatment with a Siloxane Precursor. Polymers (Basel) 2018, 10, 460. [Google Scholar] [CrossRef]
- Huber, R.O.; Beebe, J.M.; Smith, P.B.; Howell, B.A.; Ahn, D. Facile Synthesis of Thermoresponsive Poly(NIPAAm- g-PDMS) Copolymers Using Room Temperature Alkylborane Chemistry. Macromolecules 2018, 51, 4259–4268. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Han, X.; Li, F.; Li, Z.; Yang, Q. Preparation, Surface Activities, and Biodegradability of a Bola-Type Collagen Hydrolysate-Based Siloxane Surfactant. J. Surfactants Deterg. 2018, 21, 313–322. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Tabasum, S.; Aftab, W.; Shahid, M.; Zuber, M. Structural elucidation and biological aptitude of modified hydroxyethylcellulose-polydimethyl siloxane based polyurethanes. Int. J. Biol. Macromol. 2020, 150, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Meazzini, I.; Behrendt, J.M.; Turner, M.L.; Evans, R.C. Targeted β-Phase Formation in Poly(fluorene)-Ureasil Grafted Organic-Inorganic Hybrids. Macromolecules 2017, 50, 4235–4243. [Google Scholar] [CrossRef]
- White, B.T.; Long, T.E. Advances in Polymeric Materials for Electromechanical Devices. Macromol. Rapid Commun. 2019, 40, 1800521. [Google Scholar] [CrossRef]

















| Ref. | Structural | Properties Confirmed by* |
|---|---|---|
| [1] | IR, 1H NMR | CV, FS |
| [2] | ATR-FTIR, IRRAS, XPS | CAM |
| [3] | XPS | EIS |
| [4] | IR, XPS | SEM, UV-Vis, TG, DTG |
| [5] | IR, XPS | |
| [6] | 1H, 13C-NMR, IR | SEM |
| [7] | IR, GPC-MALLS, EA | |
| [8] | 1H NMR, XPS, GPC | CAM, AFM |
| [9] | VT-IR 1H 13C NMR | CD, SAXS, POM, DSC, DLS |
| [11] | IR, 1H 13C NMR, TEM, SEM | DMA |
| [12] | XPS | SEM, hydrophilicity by CA. |
| [13] | 1H NMR, XPS, EI-MS | UV−Vis, CV |
| [15] | IR, XPS | TG-DSC, SEM |
| [16] | IR, XPS | SEM |
| [23] | IR, 1H NMR, EDX | DSC, SEM, TEM, TG, DTG, Dielectric Conductivity, N2-Sorption Measurements |
| [24] | 1H, 13C, 29Si NMR | |
| [25] | IR | DSC, SEM |
| [26] | XPS, GPC | AFM |
| [27] | 1H, 13C, 29Si NMR, SEC | CAM, TGA, DSC, DLS |
| [28] | SEC, 1H, 13C, 29Si NMR | |
| [29] | 1H NMR, IR, RM, SEC | DSC |
| [30] | IR, GPC | DSC |
| [31] | IR, 1H, 29Si NMR, | |
| [32] | 1H NMR, SEC | |
| [33] | 1H NMR, SEC | DSC |
| [34] | IR, SEC | |
| [35] | IR, | DSC, TGA, CA, UV-Vis (Transparency) |
| [36] | 1H, 13C, 29Si NMR, IR GPC HRMS | |
| [37] | IR, 1H NMR, SEC | CAT |
| [38] | IR | SEM, EDS, CAT |
| [39] | 1H, 13C, 29Si NMR, SEC | DSC, AFM |
| [40] | IR, 1H NMR | Particle size |
| [41] | IR and solid-state 1H NMR | |
| [42] | 1H NMR and solid-state 13C, 29Si NMR | TGA, PXRD, UV/Vis, PL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glosz, K.; Stolarczyk, A.; Jarosz, T. Siloxanes—Versatile Materials for Surface Functionalisation and Graft Copolymers. Int. J. Mol. Sci. 2020, 21, 6387. https://doi.org/10.3390/ijms21176387
Glosz K, Stolarczyk A, Jarosz T. Siloxanes—Versatile Materials for Surface Functionalisation and Graft Copolymers. International Journal of Molecular Sciences. 2020; 21(17):6387. https://doi.org/10.3390/ijms21176387
Chicago/Turabian StyleGlosz, Karolina, Agnieszka Stolarczyk, and Tomasz Jarosz. 2020. "Siloxanes—Versatile Materials for Surface Functionalisation and Graft Copolymers" International Journal of Molecular Sciences 21, no. 17: 6387. https://doi.org/10.3390/ijms21176387
APA StyleGlosz, K., Stolarczyk, A., & Jarosz, T. (2020). Siloxanes—Versatile Materials for Surface Functionalisation and Graft Copolymers. International Journal of Molecular Sciences, 21(17), 6387. https://doi.org/10.3390/ijms21176387