The Beneficial Effects of Morusin, an Isoprene Flavonoid Isolated from the Root Bark of Morus
Abstract
:1. Introduction
2. Results
2.1. Chemical Properties of Morusin
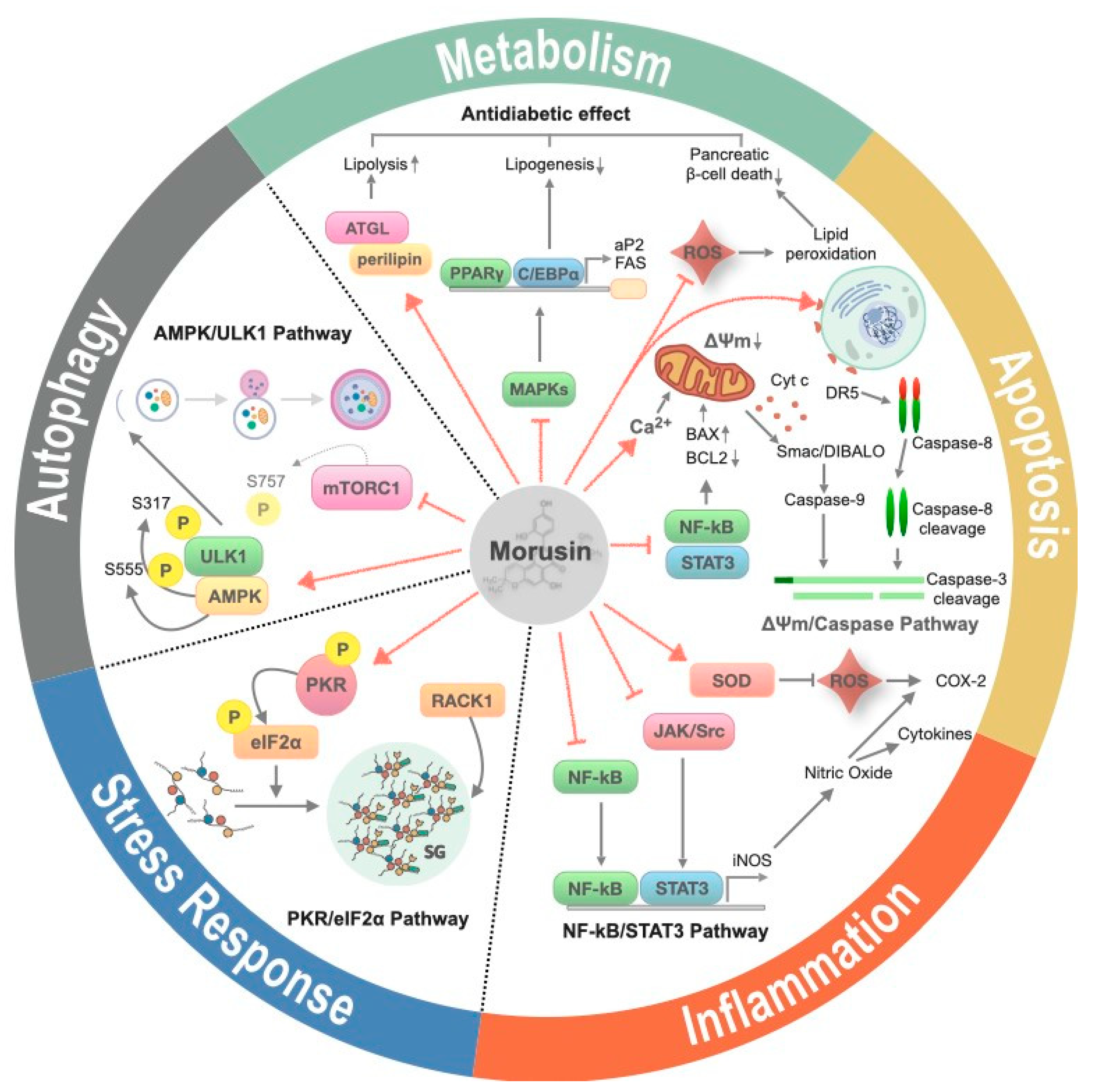
2.2. The Effects of Morusin Treatment on Cellular Processes
2.2.1. Inflammation
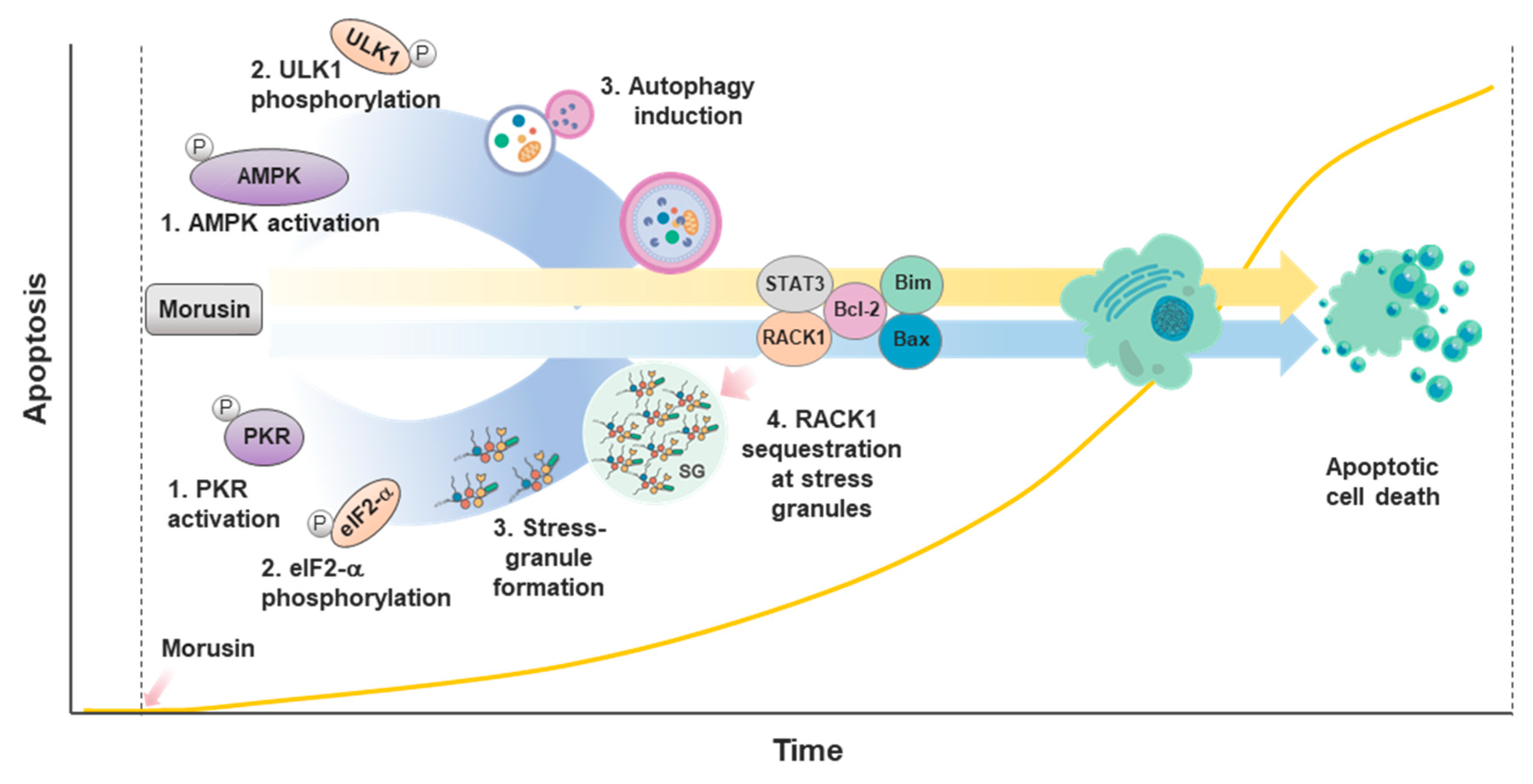
2.2.2. Apoptosis
2.2.3. Metabolism
2.2.4. Autophagy
2.2.5. Stress Response
2.3. Clinical Potential of Morusin Treatment in Pathophysiology
2.3.1. Inflammatory Diseases
2.3.2. Neurological Disorders
2.3.3. Diabetes
2.3.4. Cancer
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, S.; Wang, B.L.; Li, Y. Advances in the pharmacological study of Morus alba L. Yao Xue Xue Bao 2014, 49, 824–831. [Google Scholar] [PubMed]
- Nomura, T.; Fukai, T.; Matsumoto, J.; Ohmori, T. Constituents of the cultivated mulberry tree. Planta Med. 1982, 46, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Fukai, T.; Shimada, T.; Chen, I.S. Components of Root Bark of Morus australis. Planta Med. 1983, 49, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Fukai, T.; Hano, Y. Constituents of the Chinese crude drug “sang-bai-pi” (Morus root bark). Planta Med. 1983, 47, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Hano, Y.; Hirakura, K.; Nomura, T.; Terada, S.; Fukushima, K. Components of root bark of morus lhou1 1. Structures of two new natural diels-alder adducts, kuwanons N and o. Planta Med. 1984, 50, 127–130. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T.; Hano, Y.; Yoshizawa, S.; Suganuma, M.; Fujiki, H. Chemistry and anti-tumor promoting activity of Morus flavonoids. Prog. Clin. Biol. Res. 1988, 280, 267–281. [Google Scholar]
- Yan, J.; Ruan, J.; Huang, P.; Sun, F.; Zheng, D.; Zhang, Y.; Wang, T. The structure-activity relationship review of the main bioactive constituents of Morus genus plants. J. Nat. Med. 2020, 74, 331–340. [Google Scholar] [CrossRef]
- Cho, J.K.; Ryu, Y.B.; Curtis-Long, M.J.; Kim, J.Y.; Kim, D.; Lee, S.; Lee, W.S.; Park, K.H. Inhibition and structural reliability of prenylated flavones from the stem bark of Morus lhou on β-secretase (BACE-1). Bioorg. Med. Chem. Lett. 2011, 21, 2945–2948. [Google Scholar] [CrossRef]
- Syah, Y.M.; Juliawaty, L.D.; Achmad, S.A.; Hakim, E.H.; Takayama, H.; Said, I.M.; Latip, J. Phenolic constituents from the wood of Morus australis with cytotoxic activity. Z. Naturforsch. C J. Biosci. 2008, 63, 35–39. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Hakim, E.H.; Achmad, S.A.; Juliawaty, L.D.; Makmur, L.; Syah, Y.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Ghisalberti, E.L. Prenylated flavonoids and related compounds of the Indonesian Artocarpus (Moraceae). J. Nat. Med. 2006, 60, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.T.; Binh, P.T.; le Quynh, T.P.; Van Minh, C.; Huong, H.T.; Lee, J.J. Cytotoxic prenylated flavonoids from Morus alba. Fitoterapia 2010, 81, 1224–1227. [Google Scholar] [CrossRef]
- Bahramann, E. The pathology of testicular neoplasms. Z. Urol. Nephrol. 1976, 69, 91–97. [Google Scholar] [PubMed]
- Park, H.J.; Min, T.R.; Chi, G.Y.; Choi, Y.H.; Park, S.H. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018, 505, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Parant, M.R.; Vial, H.J. Rapid and serial determination of protein kinase C activity and of the associated [3H]PDBu binding using a 96-well microtiter plate and a cell harvester. Anal. Biochem. 1990, 184, 283–290. [Google Scholar] [CrossRef]
- Shi, X.; Yang, S.; Zhang, G.; Song, Y.; Su, D.; Liu, Y.; Guo, F.; Shan, L.; Cai, J. The different metabolism of morusin in various species and its potent inhibition against UDP-glucuronosyltransferase (UGT) and cytochrome p450 (CYP450) enzymes. Xenobiotica 2016, 46, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Liu, W.; Liang, Z.; Han, W.; Li, J.; Ye, L.; Liu, M.; Cai, Z.; Zhao, J.; Chen, Y.; et al. UGT-mediated metabolism plays a dominant role in the pharmacokinetic behavior and the disposition of morusin in vivo and in vitro. J. Pharm. Biomed. Anal. 2018, 154, 339–353. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, W.S.; Kim, Y.S.; Curtis-Long, M.J.; Lee, B.W.; Ryu, Y.B.; Park, K.H. Isolation of cholinesterase-inhibiting flavonoids from Morus lhou. J. Agric. Food Chem. 2011, 59, 4589–4596. [Google Scholar] [CrossRef]
- Reddy, G.R.; Ueda, N.; Hada, T.; Sackeyfio, A.C.; Yamamoto, S.; Hano, Y.; Aida, M.; Nomura, T. A prenylflavone, artonin E, as arachidonate 5-lipoxygenase inhibitor. Biochem. Pharmacol. 1991, 41, 115–118. [Google Scholar] [CrossRef]
- Shi, X.; Mackie, B.; Zhang, G.; Yang, S.; Song, Y.; Su, D.; Liu, Y.; Shan, L. Identification of the Metabolic Enzyme Involved Morusin Metabolism and Characterization of Its Metabolites by Ultraperformance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UPLC/Q-TOF-MS/MS). Evid. Based Complement. Altern. Med. eCAM 2016, 2016, 9240103. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Mohamed, M.S.; Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Formulation, characterization and evaluation of morusin loaded niosomes for potentiation of anticancer therapy. RSC Adv. 2018, 8, 32621–32636. [Google Scholar] [CrossRef] [Green Version]
- Tseng, T.-H.; Chuang, S.-K.; Hu, C.-C.; Chang, C.-F.; Huang, Y.-C.; Lin, C.-W.; Lee, Y.-J. The synthesis of morusin as a potent antitumor agent. Tetrahedron 2010, 66, 1335–1340. [Google Scholar] [CrossRef]
- Nitya Bankupalli, S.K.G.; Shaik, M. Comparative modeling of sodium- and chloride-dependent GABA transporter 1 and docking studies with natural compounds. J. Appl. Biol. Biotechnol. 2020, 8, 12–21. [Google Scholar]
- Jin, S.E.; Ha, H.; Shin, H.K.; Seo, C.S. Anti-Allergic and Anti-Inflammatory Effects of Kuwanon G and Morusin on MC/9 Mast Cells and HaCaT Keratinocytes. Molecules 2019, 24, 265. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Ryu, J.; Park, S.H.; Woo, E.R.; Kim, A.R.; Lee, S.K.; Kim, Y.S.; Kim, J.O.; Hong, J.H.; Lee, C.J. Effects of Morus alba L. and Natural Products Including Morusin on In Vivo Secretion and In Vitro Production of Airway MUC5AC Mucin. Tuberc. Respir. Dis. 2014, 77, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; He, W.; Zhang, H.; He, L.; Wang, Y.; Zhang, T.; Peng, J.; Sun, P.; Qian, Y. Morusin Ameliorates IL-1β-Induced Chondrocyte Inflammation and Osteoarthritis via NF-κB Signal Pathway. Drug Des. Dev. Ther. 2020, 14, 1227–1240. [Google Scholar] [CrossRef] [Green Version]
- Cheon, B.S.; Kim, Y.H.; Son, K.S.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of prenylated flavonoids and biflavonoids on lipopolysaccharide-induced nitric oxide production from the mouse macrophage cell line RAW 264.7. Planta Med. 2000, 66, 596–600. [Google Scholar] [CrossRef]
- Vochyanova, Z.; Pokorna, M.; Rotrekl, D.; Smekal, V.; Fictum, P.; Suchy, P.; Gajdziok, J.; Smejkal, K.; Hosek, J. Prenylated flavonoid morusin protects against TNBS-induced colitis in rats. PLoS ONE 2017, 12, e0182464. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, J.; Chen, J.; Zhou, L.; Wang, H.; Chen, J.; Xu, Z.; Zhu, S.; Liu, W.; Yu, R.; et al. Morusin alleviates mycoplasma pneumonia via the inhibition of Wnt/beta-catenin and NF-kappaB signaling. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.G.; Matsuzaki, K.; Takamatsu, S.; Kitanaka, S. Inhibitory effects of constituents from Morus alba var. multicaulis on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells. Molecules 2011, 16, 6010–6022. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; da Lyu, H.; Koo, U.; Nam, K.W.; Hong, S.S.; Kim, K.O.; Kim, K.H.; Lee, D.; Mar, W. Protection of prenylated flavonoids from Mori Cortex Radicis (Moraceae) against nitric oxide-induced cell death in neuroblastoma SH-SY5Y cells. Arch. Pharm. Res. 2012, 35, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-kappaB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.L.; Lai, D.Y.; Lee, Y.J.; Chen, N.F.; Tseng, T.H. Antitumor progression potential of morusin suppressing STAT3 and NFkappaB in human hepatoma SK-Hep1 cells. Toxicol. Lett. 2015, 232, 490–498. [Google Scholar] [CrossRef]
- Lee, J.C.; Won, S.J.; Chao, C.L.; Wu, F.L.; Liu, H.S.; Ling, P.; Lin, C.N.; Su, C.L. Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells. Biochem. Biophys. Res. Commun. 2008, 372, 236–242. [Google Scholar] [CrossRef]
- Lim, S.L.; Park, S.Y.; Kang, S.; Park, D.; Kim, S.H.; Um, J.Y.; Jang, H.J.; Lee, J.H.; Jeong, C.H.; Jang, J.H.; et al. Morusin induces cell death through inactivating STAT3 signaling in prostate cancer cells. Am. J. Cancer Res. 2015, 5, 289–299. [Google Scholar]
- Xue, J.; Li, R.; Zhao, X.; Ma, C.; Lv, X.; Liu, L.; Liu, P. Morusin induces paraptosis-like cell death through mitochondrial calcium overload and dysfunction in epithelial ovarian cancer. Chem. Biol. Interact. 2018, 283, 59–74. [Google Scholar] [CrossRef]
- Park, D.; Ha, I.J.; Park, S.Y.; Choi, M.; Lim, S.L.; Kim, S.H.; Lee, J.H.; Ahn, K.S.; Yun, M.; Lee, S.G. Morusin Induces TRAIL Sensitization by Regulating EGFR and DR5 in Human Glioblastoma Cells. J. Nat. Prod. 2016, 79, 317–323. [Google Scholar] [CrossRef]
- Guo, H.; Liu, C.; Yang, L.; Dong, L.; Wang, L.; Wang, Q.; Li, H.; Zhang, J.; Lin, P.; Wang, X. Morusin inhibits glioblastoma stem cell growth in vitro and in vivo through stemness attenuation, adipocyte transdifferentiation and apoptosis induction. Mol. Carcinog. 2016, 55, 77–89. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Yang, L.; Dong, L.; Lin, C.; Zhang, J.; Lin, P.; Wang, X. Morusin inhibits human cervical cancer stem cell growth and migration through attenuation of NF-κB activity and apoptosis induction. Mol. Cell. Biochem. 2013, 379, 7–18. [Google Scholar] [CrossRef]
- Chi, Y.S.; Jong, H.G.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: Cyclooxygenases and lipoxygenases. Biochem. Pharmacol. 2001, 62, 1185–1191. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.S.; Hu, C.C.; Wang, C.J.; Lee, Y.J.; Chung, W.C.; Tseng, T.H. Involvement of the antioxidative property of morusin in blocking phorbol ester-induced malignant transformation of JB6 P(+) mouse epidermal cells. Chem. Biol. Interact. 2017, 264, 34–42. [Google Scholar] [CrossRef]
- Wan, L.Z.; Ma, B.; Zhang, Y.Q. Preparation of morusin from Ramulus mori and its effects on mice with transplanted H22 hepatocarcinoma. Biofactors 2014, 40, 636–645. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Sun, Z.; Li, H.; Wang, Q.; Yi, C.; Wang, X. Morusin shows potent antitumor activity for human hepatocellular carcinoma in vitro and in vivo through apoptosis induction and angiogenesis inhibition. Drug Des. Dev. Ther. 2017, 11, 1789–1802. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Kim, J.H.; Oh, E.Y.; Nam, D.; Lee, S.G.; Lee, J.; Kim, S.H.; Shim, B.S.; Ahn, K.S. Blockage of STAT3 Signaling Pathway by Morusin Induces Apoptosis and Inhibits Invasion in Human Pancreatic Tumor Cells. Pancreas 2016, 45, 409–419. [Google Scholar] [CrossRef]
- Simon, A.R.; Rai, U.; Fanburg, B.L.; Cochran, B.H. Activation of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. 1998, 275, C1640–C1652. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Kim, E.O.; Kim, S.H.; Lee, J.H.; Ahn, K.S.; Yun, M.; Lee, S.G. Morusin induces apoptosis by regulating expression of Bax and Survivin in human breast cancer cells. Oncol. Lett. 2017, 13, 4558–4562. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.L.; Lv, Y.; Wang, S.; Zhang, Y.Q. Morusin suppresses A549 cell migration and induces cell apoptosis by downregulating the expression of COX--2 and VEGF genes. Oncol. Rep. 2018, 40, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Luo, J.; Luo, X.; Jia, W.; Fang, Z.; Yi, S.; Li, L. Morusin exerts anti-cancer activity in renal cell carcinoma by disturbing MAPK signaling pathways. Ann. Transl. Med. 2020, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weng, Q.; Chen, J.; Han, J. Morusin inhibited human osteosarcoma via PI3K-AKT signaling pathway. Curr. Pharm. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Roy, S.; Nicholson, D.W. Cross-talk in cell death signaling. J. Exp. Med. 2000, 192, F21–F25. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Singab, A.N.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef]
- Abd El-Mawla, A.M.; Mohamed, K.M.; Mostafa, A.M. Induction of Biologically Active Flavonoids in Cell Cultures of Morus nigra and Testing their Hypoglycemic Efficacy. Sci. Pharm. 2011, 79, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.D.; Ge, G.B.; Weng, Z.M.; Dai, Z.R.; Leng, Y.H.; Ding, L.L.; Jin, L.L.; Yu, Y.; Cao, Y.F.; Hou, J. Natural constituents from Cortex Mori Radicis as new pancreatic lipase inhibitors. Bioorg. Chem. 2018, 80, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Fromenty, B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol. Res. Perspect. 2016, 4, e00211. [Google Scholar] [CrossRef] [PubMed]
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and Roles in Cancer, Diabetes and Atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Park, J.W.; Kang, M.J.; Choi, Y.W.; Kim, K.M.; et al. Morusin Functions as a Lipogenesis Inhibitor as Well as a Lipolysis Stimulator in Differentiated 3T3-L1 and Primary Adipocytes. Molecules 2018, 23, 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, Q.; Dong, L.; Liu, C.; Sun, Z.; Gao, L.; Wang, X. Morusin suppresses breast cancer cell growth in vitro and in vivo through C/EBPβ and PPARγ mediated lipoapoptosis. J. Exp. Clin. Cancer Res. 2015, 34, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Li, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Liu, J.; Li, H. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharmacother. 2019, 119, 109415. [Google Scholar] [CrossRef]
- Cho, S.W.; Na, W.; Choi, M.; Kang, S.J.; Lee, S.G.; Choi, C.Y. Autophagy inhibits cell death induced by the anti-cancer drug morusin. Am. J. Cancer Res. 2017, 7, 518–530. [Google Scholar]
- Fujikake, N.; Shin, M.; Shimizu, S. Association Between Autophagy and Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 255. [Google Scholar] [CrossRef] [Green Version]
- Buchan, J.R.; Parker, R. Eukaryotic stress granules: The ins and outs of translation. Mol. Cell 2009, 36, 932–941. [Google Scholar] [CrossRef] [Green Version]
- Wolozin, B.; Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Protter, D.S.W.; Parker, R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yague, E.; Raguz, S. Escape from stress granule sequestration: Another way to drug resistance? Biochem. Soc. Trans. 2010, 38, 1537–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.J.; Choi, D.W.; Cho, S.W.; Han, J.; Yang, S.; Choi, C.Y. Stress Granule Formation Attenuates RACK1-Mediated Apoptotic Cell Death Induced by Morusin. Int. J. Mol. Sci. 2020, 21, 5360. [Google Scholar] [CrossRef]
- Zuo, G.Y.; Yang, C.X.; Han, J.; Li, Y.Q.; Wang, G.C. Synergism of prenylflavonoids from Morus alba root bark against clinical MRSA isolates. Phytomedicine 2018, 39, 93–99. [Google Scholar] [CrossRef]
- Pang, D.; Liao, S.; Wang, W.; Mu, L.; Li, E.; Shen, W.; Liu, F.; Zou, Y. Destruction of the cell membrane and inhibition of cell phosphatidic acid biosynthesis in Staphylococcus aureus: An explanation for the antibacterial mechanism of morusin. Food Funct. 2019, 10, 6438–6446. [Google Scholar] [CrossRef]
- Wu, S.C.; Han, F.; Song, M.R.; Chen, S.; Li, Q.; Zhang, Q.; Zhu, K.; Shen, J.Z. Natural Flavones from Morus alba against Methicillin-Resistant Staphylococcus aureus via Targeting the Proton Motive Force and Membrane Permeability. J. Agric. Food Chem. 2019, 67, 10222–10234. [Google Scholar] [CrossRef]
- Syahdi, R.R.; Mun’im, A.; Suhartanto, H.; Yanuar, A. Virtual screening of Indonesian herbal database as HIV-1 reverse transcriptase inhibitor. Bioinformation 2012, 8, 1206–1210. [Google Scholar] [CrossRef]
- Singh, S.; Florez, H. Coronavirus disease 2019 drug discovery through molecular docking. F1000Research 2020, 9, 502. [Google Scholar] [CrossRef]
- Gupta, G.; Chellappan, D.K.; Agarwal, M.; Ashwathanarayana, M.; Nammi, S.; Pabreja, K.; Dua, K. Pharmacological Evaluation of the Recuperative Effect of Morusin Against Aluminium Trichloride (AlCl3)-Induced Memory Impairment in Rats. Cent. Nerv. Syst. Agents Med. Chem. 2017, 17, 196–200. [Google Scholar] [CrossRef]
- Kuk, E.B.; Jo, A.R.; Oh, S.I.; Sohn, H.S.; Seong, S.H.; Roy, A.; Choi, J.S.; Jung, H.A. Anti-Alzheimer’s disease activity of compounds from the root bark of Morus alba L. Arch. Pharm. Res. 2017, 40, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Borah, K.; Sharma, S.; Silla, Y. Structural bioinformatics-based identification of putative plant based lead compounds for Alzheimer Disease Therapy. Comput. Biol. Chem. 2019, 78, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.I.; Ahn, Y.H. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes 2004, 53 (Suppl. 1), S60–S65. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Badana, A.K.; Murali, M.G.; Shailender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Mohamed, M.S.; Mizuki, T.; Maekawa, T.; Sakthi Kumar, D. Chlorotoxin modified morusin-PLGA nanoparticles for targeted glioblastoma therapy. J. Mater. Chem. B 2019, 7, 5896–5919. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, D.; Mao, J.; Ke, X.X.; Zhang, R.; Yin, C.; Gao, N.; Cui, H. Morusin inhibits cell proliferation and tumor growth by down-regulating c-Myc in human gastric cancer. Oncotarget 2017, 8, 57187–57200. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Zhang, Y.; Yan, X.; Yan, F.; Sun, Y.; Zeng, J.; Waigel, S.; Yin, Y.; Fraig, M.M.; Egilmez, N.K.; et al. Circulating Adipose Fatty Acid Binding Protein Is a New Link Underlying Obesity-Associated Breast/Mammary Tumor Development. Cell Metab. 2018, 28, 689–705.e685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Organ | Cell Type | Effects on Cellular Processes | System | Reference |
|---|---|---|---|---|
| Liver | LO2 HepG2 Hep3B | Apoptosis induction & angiogenesis inhibition
| in vitro in vivo | [47] |
| Liver | SK-Hep1 | Anti-tumor progression through suppressing STAT3 and NF-kB
| in vitro in vivo | [34] |
| Lung | A549 | Apoptosis induction & cell migration suppression
| in vitro | [52] |
| Lung | H1299 H460 H292 | Apoptosis induction by suppression of EGFR/STAT3 activation
| in vitro | [14] |
| Breast | MCF-10A 4T1 MCF-7 | Suppression of cancer cell growth through C/EBPβ- and PPARγ-mediated lipoapoptosis
| in vitro in vivo | [65] |
| Breast | MCF-10A MCF-7 MDA-MB231 | Apoptosis induction
| in vitro | [51] |
| Brain | U87 GI-1 HCN-1A | Morusin-loaded nanoparticles for targeted glioblastoma therapy
| in vitro | [89] |
| Brain | WJ1 WJ2 | Inhibition of glioblastoma stem cell growth through stemness attenuation, adipocyte transdifferentiation
| in vitro in vivo | [39] |
| Brain | U251MG LN18 U87MG | TRAIL sensitization by regulating EGFR and DR5 in human glioblastoma cells
| in vitro | [38] |
| Skin | JB6 P+ | Blocking TPA-induced malignant transformation of mouse epidermal cells
| in vitro | [45] |
| Stomach | MKN45 SGC7901 | Inhibition of cell proliferation and tumor growth by down-regulating c-Myc
|
in vitro in vivo | [90] |
| Pancreas | AsPC-1 BxPC-3 MIAPaCa-2 PANC-1 | Apoptosis induction and inhibition of invasion by blockage of STAT3 signaling pathway
| in vitro | [48] |
| Bone | U2OS HOS | Inhibition of human osteosarcoma via PI3K-AKT signaling pathway
| in vitro | [55] |
| Ovary | A2780 SKOV-3 HO-8910 | Paraptosis-like cell death induction through mitochondrial calcium overload and dysfunction
| in vitro in vivo | [37] |
| Prostate | DU145 PC-3 LNCaP RWPE-1 | Cell death induction through inactivating STAT3 signaling
| in vitro | [36] |
| Cervix | HeLa | Apoptosis induction & inhibition of human cervical cancer stem cell growth and migration through attenuation of NF-κB activity
| in vitro | [40] |
| Kidney | 769-P 786-O OSRC-2 | Anti-cancer activity by disturbing MAPK signaling pathways
| in vitro in vivo | [54] |
| Liver Spleen | H22 | Inhibition of transplanted H22 hepatocarcinoma
| in vitro in vivo | [46] |
| Colon Liver Breast | HT-29 Hep3B MCF-7 | Apoptosis induction & suppression of NF-κB activity
| in vitro | [35] |
| Cervix Breast Bone | HeLa U2OS ZR75B | Attenuation of RACK1-mediated apoptotic cell death by stress granule (SG) formation
| in vitro | [74] |
| Cervix Breast Bone Colon | HeLa MCF-7 U2OS HCT116 | Autophagy induction inhibits cell death
| in vitro | [68] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.W.; Cho, S.W.; Lee, S.-G.; Choi, C.Y. The Beneficial Effects of Morusin, an Isoprene Flavonoid Isolated from the Root Bark of Morus. Int. J. Mol. Sci. 2020, 21, 6541. https://doi.org/10.3390/ijms21186541
Choi DW, Cho SW, Lee S-G, Choi CY. The Beneficial Effects of Morusin, an Isoprene Flavonoid Isolated from the Root Bark of Morus. International Journal of Molecular Sciences. 2020; 21(18):6541. https://doi.org/10.3390/ijms21186541
Chicago/Turabian StyleChoi, Dong Wook, Sang Woo Cho, Seok-Geun Lee, and Cheol Yong Choi. 2020. "The Beneficial Effects of Morusin, an Isoprene Flavonoid Isolated from the Root Bark of Morus" International Journal of Molecular Sciences 21, no. 18: 6541. https://doi.org/10.3390/ijms21186541






