Molecular Mechanisms of Reconsolidation-Dependent Memory Updating
Abstract
1. Introduction
2. Rediscovery of Reconsolidation
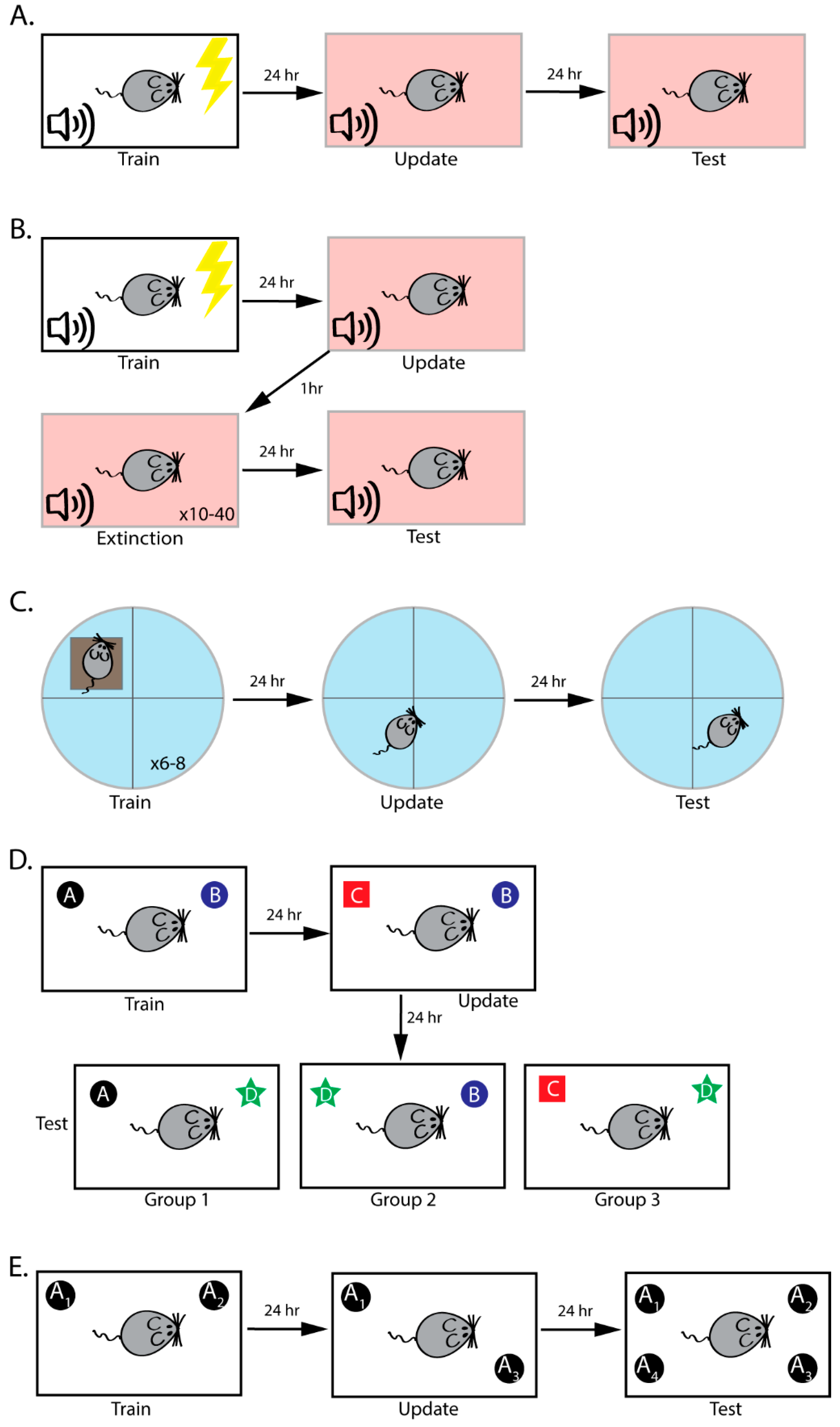
3. Behavioral Paradigms to Study Reconsolidation-Dependent Memory Updating
3.1. Fear Conditioning
3.2. Reconsolidation-Extinction
3.3. Morris Water Maze
3.4. Object Recognition Memory
3.5. Objects in Updated Locations
4. Molecular Mechanisms of the Reconsolidation-Dependent Memory Updating Process
4.1. Molecular Mechanisms of Memory Destabilization
4.2. Molecular Mechanisms of Memory Restabilization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AP5 | (2R)-amino-5-phosphonovaleric acid |
| BDNF | Brain-derived neurotrophic factor |
| BLA | Basolateral nucleus of the amygdala |
| CaMKII | Calcium/calmodulin-dependent kinase II |
| CI-AMPARs | Calcium-impermeable AMPARs |
| CNQX | 6-cyano-7-nitroquinoxaline-2,3-dione |
| CP-AMPARs | Calcium-permeable AMPA receptors |
| CPFE | Context pre-exposure facilitation effect |
| CR | Conditioned response |
| CREB | cAMP response element-binding protein |
| CS | Conditioned stimulus |
| DNQX | 6,7-dinitroquinoxaline-2,3-dione |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| mTOR | Mammalian target of rapamycin |
| MWM | Morris water maze |
| NMDA | N-Methyl-d-aspartic acid |
| ORM | Object recognition memory |
| OUL | Objects in updated locations |
| PTSD | Post-traumatic stress disorder |
| UCS | Unconditioned stimulus |
| Zif268 | Zinc-finger 268 protein |
References
- Lee, J.L.C. Reconsolidation: Maintaining memory relevance. Trends Neurosci. 2009, 32, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kwapis, J.L.; Alaghband, Y.; Keiser, A.A.; Dong, T.N.; Michael, C.M.; Rhee, D.; Shu, G.; Dang, R.T.; Matheos, D.P.; Wood, M.A. Aging mice show impaired memory updating in the novel OUL updating paradigm. Neuropsychopharmacology 2020, 45, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.C.; Nader, K.; Schiller, D. An update on memory reconsolidation updating. Trends Cogn. Sci. 2017, 21, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Alberini, C.M. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front. Behav. Neurosci. 2011, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Muravieva, E.V.; Alberini, C.M. Limited efficacy of propranolol on the reconsolidation of fear memories. Learn. Mem. 2010, 17, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Dȩbiec, J.; Ledoux, J.E. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 2004, 129, 267–272. [Google Scholar] [CrossRef]
- Tronel, S.; Alberini, C.M. Persistent Disruption of a Traumatic Memory by Postretrieval Inactivation of Glucocorticoid Receptors in the Amygdala. Biol. Psychiatry 2007, 62, 33–39. [Google Scholar] [CrossRef]
- Taubenfeld, S.M.; Riceberg, J.S.; New, A.S.; Alberini, C.M. Preclinical Assessment for Selectively Disrupting a Traumatic Memory via Postretrieval Inhibition of Glucocorticoid Receptors. Biol. Psychiatry 2009, 65, 249–257. [Google Scholar] [CrossRef][Green Version]
- Lee, J.L.C.; Di Ciano, P.; Thomas, K.L.; Everitt, B.J. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 2005, 47, 795–801. [Google Scholar] [CrossRef]
- Miller, C.A.; Marshall, J.F. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 2005, 47, 873–884. [Google Scholar] [CrossRef]
- Milekic, M.H.; Brown, S.D.; Castellini, C.; Alberini, C.M. Persistent disruption of an established morphine conditioned place preference. J. Neurosci. 2006, 26, 3010–3020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valjent, E.; Corbillé, A.G.; Bertran-Gonsalez, J.; Hervé, D.; Girault, J.A. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc. Natl. Acad. Sci. USA 2006, 103, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- Fricks-Gleason, A.N.; Marshall, J.F. Post-retrieval β-adrenergic receptor blockade: Effects on extinction and reconsolidation of cocaine-cue memories. Learn. Mem. 2008, 15, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.F.; Franklin, K.B.J. Reconsolidation of a morphine place preference: Impact of the strength and age of memory on disruption by propranolol and midazolam. Behav. Brain Res. 2010, 213, 201–207. [Google Scholar] [CrossRef]
- Johansen, J.P.; Cain, C.K.; Ostroff, L.E.; Ledoux, J.E. Molecular mechanisms of fear learning and memory. Cell 2011, 147, 509–524. [Google Scholar] [CrossRef]
- Nader, K.; Hardt, O. A single standard for memory: The case for reconsolidation. Nat. Rev. Neurosci. 2009, 10, 224–234. [Google Scholar] [CrossRef]
- McGaugh, J.L. Time-dependent processes in memory storage. Science 1966, 153, 1351–1358. [Google Scholar] [CrossRef]
- Hupbach, A.; Gomez, L.; Hardt, O.; Nadel, R. A Subtle Reminder Triggers Integration of New Information. Learn. Mem. 2007, 14, 47–53. [Google Scholar] [CrossRef]
- Lee, J.L.C. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat. Neurosci. 2008, 11, 1264–1266. [Google Scholar] [CrossRef]
- Díaz-Mataix, L.; Ruiz Martinez, R.C.; Schafe, G.E.; Ledoux, J.E.; Doyère, V. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Curr. Biol. 2013, 23, 467–472. [Google Scholar] [CrossRef]
- Kwapis, J.L.; Jarome, T.J.; Ferrara, N.C.; Helmstetter, F.J. Updating Procedures Can Reorganize the Neural Circuit Supporting a Fear Memory. Neuropsychopharmacology 2017, 42, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Jarome, T.J.; Ferrara, N.C.; Kwapis, J.L.; Helmstetter, F.J. Contextual Information Drives the Reconsolidation-Dependent Updating of Retrieved Fear Memories. Neuropsychopharmacology 2015, 40, 3044–3052. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tronel, S.; Milekic, M.H.; Alberini, C.M. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLoS Biol. 2005, 3, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.D.; Tucci, M.C.; DaCosta-Furtado, M. Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learn. Mem. 2009, 16, 545–553. [Google Scholar] [CrossRef]
- Misanin, J.R.; Miller, R.R.; Lewis, D.J. Retrograde Amnesia Produced by Electroconvulsive Shock after Reactivation of a Consolidated Memory Trace. Science 1968, 160, 554–555. [Google Scholar] [CrossRef]
- Nader, K.; Schafe, G.E.; Le Doux, J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000, 406, 722–726. [Google Scholar] [CrossRef]
- Nader, K. Memory traces unbound. Trends Neurosci. 2003, 26, 65–72. [Google Scholar] [CrossRef]
- Eisenberg, M.; Kobilo, T.; Berman, D.E.; Dudai, Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science 2003, 301, 1102–1104. [Google Scholar] [CrossRef]
- Scholl, C.; Kübert, N.; Muenz, T.S.; Rössler, W. CaMKII knockdown affects both early and late phases of olfactory long-term memory in the honeybee. J. Exp. Biol. 2015, 218, 3788–3796. [Google Scholar] [CrossRef]
- Stollhoff, N.; Menzel, R.; Eisenhardt, D. Spontaneous recovery from extinction depends on the reconsolidation of the acquisition memory in an appetitive learning paradigm in the honeybee (Apis mellifera). J. Neurosci. 2005, 25, 4485–4492. [Google Scholar] [CrossRef]
- Rose, J.K.; Rankin, C.H. Blocking memory reconsolidation reverses memory-associated changes in glutamate receptor expression. J. Neurosci. 2006, 26, 11582–11587. [Google Scholar] [CrossRef] [PubMed]
- Sangha, S.; Scheibenstock, A.; Lukowiak, K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J. Neurosci. 2003, 23, 8034–8040. [Google Scholar] [CrossRef]
- Pedreira, M.E.; Pérez-Cuesta, L.M.; Maldonado, H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 2004, 11, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Anokhin, K.V.; Tiunova, A.A.; Rose, S.P.R. Reminder effects—Reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur. J. Neurosci. 2002, 15, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, J.H.; Lee, N.; Lee, H.R.; Kim, J.I.; Yu, N.K.; Choi, S.L.; Lee, S.H.; Kim, H.; Kaang, B.K. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 2008, 319, 1253–1256. [Google Scholar] [CrossRef]
- Rao-Ruiz, P.; Rotaru, D.C.; Van Der Loo, R.J.; Mansvelder, H.D.; Stiedl, O.; Smit, A.B.; Spijker, S. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat. Neurosci. 2011, 14, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Josselyn, S.A.; Frankland, P.W.; Masushige, S.; Silva, A.J.; Kida, S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004, 24, 4787–4795. [Google Scholar] [CrossRef]
- Trouche, S.; Bontempi, B.; Roullet, P.; Rampon, C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc. Natl. Acad. Sci. USA 2009, 106, 5919–5924. [Google Scholar] [CrossRef]
- Von Hertzen, L.S.J.; Giese, K.P. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J. Neurosci. 2005, 25, 1935–1942. [Google Scholar] [CrossRef]
- Wiltgen, B.J.; Zhou, M.; Cai, Y.; Balaji, J.; Karlsson, M.G.; Parivash, S.N.; Li, W.; Silva, A.J. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr. Biol. 2010, 20, 1336–1344. [Google Scholar] [CrossRef]
- Wright, D.S.; Bodinayake, K.K.; Kwapis, J.L. Investigating Memory Updating in Mice Using the Objects in Updated Locations Task. Curr. Protoc. Neurosci. 2020, 91, e87. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.L.; Huganir, R.L. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 2010, 330, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Vigil, F.A.; Mizuno, K.; Lucchesi, W.; Valls-Comamala, V.; Giese, K.P. Prevention of long-term memory loss after retrieval by an endogenous CaMKII inhibitor. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bozon, B.; Davis, S.; Laroche, S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 2003, 40, 695–701. [Google Scholar] [CrossRef]
- Cao, X.; Wang, H.; Mei, B.; An, S.; Yin, L.; Wang, L.P.; Tsien, J.Z. Inducible and Selective Erasure of Memories in the Mouse Brain via Chemical-Genetic Manipulation. Neuron 2008, 60, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Costanzi, M.; Cannas, S.; Saraulli, D.; Rossi-Arnaud, C.; Cestari, V. Extinction after retrieval: Effects on the associative and nonassociative components of remote contextual fear memory. Learn. Mem. 2011, 18, 508–518. [Google Scholar] [CrossRef]
- Gräff, J.; Joseph, N.F.; Horn, M.E.; Samiei, A.; Meng, J.; Seo, J.; Rei, D.; Bero, A.W.; Phan, T.X.; Wagner, F.; et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 2014, 156, 261–276. [Google Scholar] [CrossRef]
- Khalaf, O.; Resch, S.; Dixsaut, L.; Gorden, V.; Glauser, L.; Gräff, J. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 2018, 1242, 1239–1242. [Google Scholar] [CrossRef]
- Kida, S.; Josselyn, S.A.; De Ortiz, S.P.; Kogan, J.H.; Chevere, I.; Masushige, S.; Silva, A.J. CREB required for the stability of new and reactivated fear memories. Nat. Neurosci. 2002, 5, 348–355. [Google Scholar] [CrossRef]
- Kim, R.; Moki, R.; Kida, S. Molecular mechanisms for the destabilization and restabilization of reactivated spatial memory in the Morris water maze. Mol. Brain 2011, 4, 9. [Google Scholar] [CrossRef]
- Lattal, K.M.; Abel, T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc. Natl. Acad. Sci. USA 2004, 101, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Chalkia, A.; Schroyens, N.; Leng, L.; Vanhasbroeck, N.; Zenses, A.K.; Van Oudenhove, L.; Beckers, T. No persistent attenuation of fear memories in humans: A registered replication of the reactivation-extinction effect. Cortex 2020, 129, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Schiller, D.; Monfils, M.H.; Raio, C.M.; Johnson, D.C.; Ledoux, J.E.; Phelps, E.A. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 2010, 463, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Sevenster, D.; Beckers, T.; Kindt, M. Prediction error demarcates the transition from retrieval, to reconsolidation, to new learning. Learn. Mem. 2014, 21, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Sevenster, D.; Beckers, T.; Kindt, M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol. Learn. Mem. 2012, 97, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Sevenster, D.; Beckers, T.; Kindt, M. Prediction Error Governs Pharmacologically Induced Amnesia for Learned Fear. Science 2013, 339, 830–833. [Google Scholar] [CrossRef]
- Walker, M.P.; Brakefield, T.; Hobson, J.A.; Stickgold, R. Dissociable stages of human memory consolidation and reconsolidation. Nature 2003, 425, 616–620. [Google Scholar] [CrossRef]
- Zeng, X.X.; Du, J.; Zhuang, C.Q.; Zhang, J.H.; Jia, Y.L.; Zheng, X.F. Unconditioned stimulus revaluation to promote conditioned fear extinction in the memory reconsolidation window. PLoS ONE 2014, 9, e101589. [Google Scholar] [CrossRef]
- Dȩbiec, J.; Doyère, V.; Nader, K.; LeDoux, J.E. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl. Acad. Sci. USA 2006, 103, 3428–3433. [Google Scholar] [CrossRef]
- Debiec, J.; LeDoux, J.E.; Nader, K. Cellular and systems reconsolidation in the hippocampus. Neuron 2002, 36, 527–538. [Google Scholar] [CrossRef]
- Duvarci, S.; Nader, K.; LeDoux, J.E. Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur. J. Neurosci. 2005, 21, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Duvarci, S.; Nader, K.; Ledoux, J.E. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learn. Mem. 2008, 15, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Exton-McGuinness, M.T.J.; Patton, R.C.; Sacco, L.B.; Lee, J.L.C. Reconsolidation of a well-learned instrumental memory. Learn. Mem. 2014, 21, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.C.; Jarome, T.J.; Cullen, P.K.; Orsi, S.A.; Kwapis, J.L.; Trask, S.; Pullins, S.E.; Helmstetter, F.J. GluR2 endocytosis-dependent protein degradation in the amygdala mediates memory updating. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Goltseker, K.; Bolotin, L.; Barak, S. Counterconditioning during Reconsolidation Prevents Relapse of Cocaine Memories. Neuropsychopharmacology 2017, 42, 716–726. [Google Scholar] [CrossRef]
- Gruest, N.; Richer, P.; Hars, B. Memory consolidation and reconsolidation in the rat pup require protein synthesis. J. Neurosci. 2004, 24, 10488–10492. [Google Scholar] [CrossRef]
- Hall, J.; Thomas, K.L.; Everitt, B.J. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur. J. Neurosci. 2001, 13, 1453–1458. [Google Scholar] [CrossRef]
- Haubrich, J.; Crestani, A.P.; Cassini, L.F.; Santana, F.; Sierra, R.O.; De Oliveira Alvares, L.; Quillfeldt, J.A. Reconsolidation allows fear memory to be updated to a less aversive level through the incorporation of appetitive information. Neuropsychopharmacology 2015, 40, 315–326. [Google Scholar] [CrossRef]
- Ishii, D.; Matsuzawa, D.; Matsuda, S.; Tomizawa, H.; Sutoh, C.; Shimizu, E. No erasure effect of retrieval-extinction trial on fear memory in the hippocampus-independent and dependent paradigms. Neurosci. Lett. 2012, 523, 76–81. [Google Scholar] [CrossRef]
- Jarome, T.J.; Ferrara, N.C.; Kwapis, J.L.; Helmstetter, F.J. CaMKII regulates proteasome phosphorylation and activity and promotes memory destabilization following retrieval. Neurobiol. Learn. Mem. 2016, 128, 103–109. [Google Scholar] [CrossRef]
- Jarome, T.J.; Werner, C.T.; Kwapis, J.L.; Helmstetter, F.J. Activity Dependent Protein Degradation Is Critical for the Formation and Stability of Fear Memory in the Amygdala. PLoS ONE 2011, 6, e24349. [Google Scholar] [CrossRef]
- Jarome, T.J.; Kwapis, J.L.; Werner, C.T.; Parsons, R.G.; Gafford, G.M.; Helmstetter, F.J. The timing of multiple retrieval events can alter GluR1 phosphorylation and the requirement for protein synthesis in fear memory reconsolidation. Learn. Mem. 2012, 19, 300–306. [Google Scholar] [CrossRef][Green Version]
- Jones, C.E.; Monfils, M.H. Post-retrieval extinction in adolescence prevents return of juvenile fear. Learn. Mem. 2016, 23, 567–575. [Google Scholar] [CrossRef]
- Lee, H.J.; Haberman, R.P.; Roquet, R.F.; Monfils, M.-H. Extinction and Retrieval + Extinction of Conditioned Fear Differentially Activate Medial Prefrontal Cortex and Amygdala in Rats. Front. Behav. Neurosci. 2016, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.C. Memory reconsolidation mediates the updating of hippocampal memory content. Front. Behav. Neurosci. 2010, 4, 168. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Everitt, B.J.; Thomas, K.L. Independent Cellular Processes for Hippocampal Memory Consolidation and Reconsolidation. Science 2004, 304, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Luyten, L.; Beckers, T. A preregistered, direct replication attempt of the retrieval-extinction effect in cued fear conditioning in rats. Neurobiol. Learn. Mem. 2017, 144, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Maddox, S.A.; Schafe, G.E. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn. Mem. 2011, 18, 579–593. [Google Scholar] [CrossRef]
- Maddox, S.A.; Watts, C.S.; Doyère, V.; Schafe, G.E. A Naturally-Occurring Histone Acetyltransferase Inhibitor Derived from Garcinia indica Impairs Newly Acquired and Reactivated Fear Memories. PLoS ONE 2013, 8, e54463. [Google Scholar] [CrossRef]
- Monfils, M.H.; Cowansage, K.K.; Klann, E.; Ledoux, J.E. Extinction-Reconsolidation boundaries: Key to persistent attenuation of fear memories. Science 2009, 324, 951–955. [Google Scholar] [CrossRef]
- Morris, R.G.M.; Inglis, J.; Ainge, J.A.; Olverman, H.J.; Tulloch, J.; Dudai, Y.; Kelly, P.A.T. Memory Reconsolidation: Sensitivity of Spatial Memory to Inhibition of Protein Synthesis in Dorsal Hippocampus during Encoding and Retrieval. Neuron 2006, 50, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.G.; Gafford, G.M.; Helmstetter, F.J. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J. Neurosci. 2006, 26, 12977–12983. [Google Scholar] [CrossRef] [PubMed]
- Przybyslawski, J.; Sara, S.J. Reconsolidation of memory after its reactivation. Behav. Brain Res. 1997, 84, 241–246. [Google Scholar] [CrossRef]
- Richardson, R.; Riccio, D.C.; Jamis, M.; Cabosky, J.; Skoczen, T. Modification of reactivated memory through “counterconditioning”. Am. J. Psychol. 1982, 95, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.F.; Berridge, K.C. Instant transformation of learned repulsion into motivational “wanting”. Curr. Biol. 2013, 23, 282–289. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, C.J.; Garcia-DeLaTorre, P.; Benavidez, E.; Ballesteros, M.A.; Bermudez-Rattoni, F. Intrahippocampal anisomycin infusions disrupt previously consolidated spatial memory only when memory is updated. Neurobiol. Learn. Mem. 2008, 89, 352–359. [Google Scholar] [CrossRef]
- Rossato, J.I.; Bevilaqua, L.R.M.; Medina, J.H.; Izquierdo, I.; Cammarota, M. Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn. Mem. 2006, 13, 431–440. [Google Scholar] [CrossRef]
- Rossato, J.I.; Köhler, C.A.; Radiske, A.; Lima, R.H.; Bevilaqua, L.R.M.; Cammarota, M. State-dependent effect of dopamine D1/D5 receptors inactivation on memory destabilization and reconsolidation. Behav. Brain Res. 2015, 285, 194–199. [Google Scholar] [CrossRef]
- Rossato, J.I.; Bevilaqua, L.R.M.; Myskiw, J.C.; Medina, J.H.; Izquierdo, I.; Cammarota, M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn. Mem. 2007, 14, 36–46. [Google Scholar] [CrossRef]
- Da Silva, W.C.; Bonini, J.S.; Bevilaqua, L.R.M.; Medina, J.H.; Izquierdo, I.; Cammarota, M. Inhibition of mRNA Synthesis in the Hippocampus Impairs Consolidation and Reconsolidation of Spatial Memory. Hippocampus 2008, 18, 29–39. [Google Scholar] [CrossRef]
- Tedesco, V.; Roquet, R.F.; DeMis, J.; Chiamulera, C.; Monfils, M.H. Extinction, applied after retrieval of auditory fear memory, selectively increases zinc-finger protein 268 and phosphorylated ribosomal protein S6 expression in prefrontal cortex and lateral amygdala. Neurobiol. Learn. Mem. 2014, 115, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Torquatto, K.I.; Menegolla, A.P.; Popik, B.; Casagrande, M.A.; de Oliveira Alvares, L. Role of calcium-permeable AMPA receptors in memory consolidation, retrieval and updating. Neuropharmacology 2019, 144, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Tronson, N.C.; Wiseman, S.L.; Olausson, P.; Taylor, J.R. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat. Neurosci. 2006, 9, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.D.; Tucci, M.C.; Jacklin, D.L.; Reid, J.M.; Newsome, J. On the dynamic nature of the engram: Evidence for circuit-level reorganization of object memory traces following reactivation. J. Neurosci. 2011, 31, 17719–17728. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Frankland, P.W.; Sekeres, M.; Fogel, S.; Moscovitch, M. Changes in context-specificity during memory reconsolidation: Selective effects of hippocampal lesions. Learn. Mem. 2009, 16, 722–729. [Google Scholar] [CrossRef]
- Wang, S.H.; Ostlund, S.B.; Nader, K.; Balleine, B.W. Consolidation and reconsolidation of incentive learning in the amygdala. J. Neurosci. 2005, 25, 830–835. [Google Scholar] [CrossRef]
- Winters, B.D.; Forwood, S.E.; Cowell, R.A.; Saksida, L.M.; Bussey, T.J. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J. Neurosci. 2004, 24, 5901–5908. [Google Scholar] [CrossRef]
- Hong, I.; Kim, J.; Kim, J.; Lee, S.; Ko, H.G.; Nader, K.; Kaang, B.K.; Tsien, R.W.; Choi, S. AMPA receptor exchange underlies transient memory destabilization on retrieval. Proc. Natl. Acad. Sci. USA 2013, 110, 8218–8223. [Google Scholar] [CrossRef]
- Ben Mamou, C.; Gamache, K.; Nader, K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat. Neurosci. 2006, 9, 1237–1239. [Google Scholar] [CrossRef]
- Duvarci, S.; Nader, K. Characterization of fear memory reconsolidation. J. Neurosci. 2004, 24, 9269–9275. [Google Scholar] [CrossRef]
- Milekic, M.H.; Alberini, C.M. Temporally graded requirement for protein synthesis following memory reactivation. Neuron 2002, 36, 521–525. [Google Scholar] [CrossRef]
- Monsey, M.S.; Ota, K.T.; Akingbade, I.F.; Hong, E.S.; Schafe, G.E. Epigenetic Alterations Are Critical for Fear Memory Consolidation and Synaptic Plasticity in the Lateral Amygdala. PLoS ONE 2011, 6, e19958. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.M.; Malvaez, M.; Kramar, E.; Matheos, D.P.; Arrizon, A.; Cabrera, S.M.; Lynch, G.; Greene, R.W.; Wood, M.A. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology 2011, 36, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Cogan, E.S.; Shapses, M.A.; Robinson, T.E.; Tronson, N.C. Disrupting reconsolidation: Memory erasure or blunting of emotional/motivational value? Neuropsychopharmacology 2019, 44, 399–407. [Google Scholar] [CrossRef]
- Bouton, M.E.; King, D.A. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. J. Exp. Psychol. Anim. Behav. Process. 1983, 9, 248–265. [Google Scholar] [CrossRef]
- Hemstedt, T.J.; Lattal, K.M.; Wood, M.A. Reconsolidation and extinction: Using epigenetic signatures to challenge conventional wisdom. Neurobiol. Learn. Mem. 2017, 142, 55–65. [Google Scholar] [CrossRef]
- Chalkia, A.; Van Oudenhove, L.; Beckers, T. Preventing the return of fear in humans using reconsolidation update mechanisms: A verification report of Schiller et al. (2010). Cortex 2020, 129, 510–525. [Google Scholar] [CrossRef]
- Chan, W.Y.M.; Leung, H.T.; Westbrook, R.F.; McNally, G.P. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn. Mem. 2010, 17, 512–521. [Google Scholar] [CrossRef]
- Chan, W.Y.M. The Effects of Retrieval-Extinction Training on the Restoration of Pavlovian Conditioned Fear. Ph.D. Thesis, University of New South Wales, New South Wales, Australia, January 2014. [Google Scholar]
- Rescorla, R.A.; Heth, C.D. Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1975, 1, 88–96. [Google Scholar] [CrossRef]
- Quirk, G.J. Memory for Extinction of Conditioned Fear Is Long-lasting and Persists Following Spontaneous Recovery. Learn. Mem. 2002, 9, 402–407. [Google Scholar] [CrossRef]
- Delamater, A.R. Experimental extinction in Pavlovian conditioning: Behavioural and neuroscience perspectives. Q. J. Exp. Psychol. Sect. B 2004, 57, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yeh, S.H.; Lu, H.Y.; Gean, P.W. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J. Neurosci. 2003, 23, 8310–8317. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lee, C.C.; Gean, P.W. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol. Pharmacol. 2003, 63, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Auber, A.; Tedesco, V.; Jones, C.E.; Monfils, M.H.; Chiamulera, C. Post-retrieval extinction as reconsolidation interference: Methodological issues or boundary conditions? Psychopharmacology 2013, 226, 631–647. [Google Scholar] [CrossRef]
- Morris, R.G.M. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981, 12, 239–260. [Google Scholar] [CrossRef]
- Harrison, F.E.; Hosseini, A.H.; McDonald, M.P. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 2009, 198, 247–251. [Google Scholar] [CrossRef]
- Vogel-Ciernia, A.; Wood, M.A. Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci. 2014, 69, 8–31. [Google Scholar] [CrossRef]
- Alberini, C.M.; Ledoux, J.E. Memory reconsolidation. Curr. Biol. 2013, 23, R746–R750. [Google Scholar] [CrossRef]
- Tronson, N.C.; Taylor, J.R. Molecular mechanisms of memory reconsolidation. Nat. Rev. Neurosci. 2007, 8, 262–275. [Google Scholar] [CrossRef]
- Jarome, T.J.; Helmstetter, F.J. Protein degradation and protein synthesis in long-term memory formation. Front. Mol. Neurosci. 2014, 7, 61. [Google Scholar] [CrossRef]
- Vigil, F.A.; Giese, K.P. Calcium/calmodulin-dependent kinase II and memory destabilization: A new role in memory maintenance. J. Neurochem. 2018, 147, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singh, S.; Shukla, S. Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson’s disease. J. Exp. Neurosci. 2018, 12, 1179069518779829. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Schuman, E.M. Regulation and function of local protein synthesis in neuronal dendrites. Trends Biochem. Sci. 2002, 27, 506–513. [Google Scholar] [CrossRef]
- Takei, N.; Inamura, N.; Kawamura, M.; Namba, H.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci. 2004, 24, 9760–9769. [Google Scholar] [CrossRef]
- Kwapis, J.L.; Wood, M.A. Epigenetic mechanisms in fear conditioning: Implications for treating post-traumatic stress disorder. Trends Neurosci. 2014, 37, 706–720. [Google Scholar] [CrossRef]
| Paradigm | Pros | Cons | Sources |
|---|---|---|---|
| Fear Conditioning | Stimuli presentation controlled Number of pairings controlled Pairing timing controlled Behavior is robust, predictable, and measurable Cross-species Model for human diseases | Strong memory resistant to updating Older memories harder to update Unable to determine if reaction is to original or updated memory Cannot assess both memories in single session | [5,6,7,9,12,19,20,21,22,26,28,33,35,36,39,40,42,43,45,49,51,54,55,56,59,60,61,64,67,68,70,71,75,76,78,79,82,84,92,93,95,98,99,100,105,106] |
| Reconsolidation-Extinction | More permanent extinction Cross-species Model for treating human diseases | Does not persistently attenuate memories Remote memories more resistant | [46,47,48,52,53,58,69,72,73,74,77,80,91,107,108,109] |
| Morris Water Maze | Relies on hippocampus Well-studied spatial task | Stressful for animal Less appropriate for older animals Multiple potential confounding variables- stress hormones, exercise | [38,50,81,86,87,90,92] |
| Object Recognition Memory | Non-emotional and low stress Can look at original and updated memories independently | Animals must be in separate groups Relies on a less studied brain region, the perirhinal cortex | [44,45,88,89] |
| Objects in Updated Locations | Relies on hippocampus Can look at original and updated memories independently Non-emotional and low stress Able to identify age-related impairments | New, and therefore not as well-studied as other paradigms Only tested on mice | [2,41] |
| Destabilization | |||
|---|---|---|---|
| Process | Manipulation | Memory Effect | Sources |
| Protein degradation | Proteasome inhibitor | Prevented pharmacological-induced amnesia | [35,71,75] |
| CP-AMPARs | Pharmacological inhibition | Did not prevent pharmacological-induced amnesia | [99] |
| Prevented updating | [31,42,92] | ||
| Synthetic GluA2 causing inhibition | [36,98] | ||
| Pharmacological inhibition of GluA2 | [64] | ||
| Performed multiple retrieval events | Affected GluA1 phosphorylation | [72,80] | |
| Pre-exposed animal to retrieval context to prevent memory updating | No increase in GluA2 subunits following retrieval session and induced amnesia | [22] | |
| NMDARs | Pharmacological inhibition | Prevented updating | [24,50,63,68,83] |
| Prevented pharmacological-induced amnesia | [99] | ||
| CaMKII | RNAi/Plasmid knockdown | Prevented updating | [29,43] |
| Pharmacological inhibition | [29,70] | ||
| Chemical-genetic overexpression | [45] | ||
| Plasmid overexpression | High overexpression prevented updating Low overexpression did not prevent updating | [43] | |
| Dopaminergic | Pharmacological inhibition | Prevented pharmacological-induced amnesia | [88] |
| Restabilization | |||
| Protein synthesis | PKA pharmacological inhibition | Prevented updating | [93] |
| Heat shock | [31] | ||
| Pharmacological inhibition | [11,12,19,26,32,34,37,50,59,60,61,66,72,86,87,89,94,96,100,101] | ||
| Prevented updating for a limited time | [51] | ||
| Updating not inhibited due to boundary conditions | [28,37,101] | ||
| mRNA/Transcription | Fear conditioning | Increase in CREB transcription in amygdala and not the hippocampus | [67] |
| mRNA pharmacological inhibitor | Did not prevent updating | [82] | |
| Prevented updating | [32,62,90] | ||
| CREB transgenic mice | [49,50] | ||
| Zif268 inhibition using ASO | [9,19,75,76] | ||
| Zif268 mutant mice | [44] | ||
| Epigenetic mechanisms | HDAC2 pharmacological inhibition | Enhances reconsolidation | [78,102] |
| HAT pharmacological inhibition | Impairs reconsolidation | [79] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellfy, L.; Kwapis, J.L. Molecular Mechanisms of Reconsolidation-Dependent Memory Updating. Int. J. Mol. Sci. 2020, 21, 6580. https://doi.org/10.3390/ijms21186580
Bellfy L, Kwapis JL. Molecular Mechanisms of Reconsolidation-Dependent Memory Updating. International Journal of Molecular Sciences. 2020; 21(18):6580. https://doi.org/10.3390/ijms21186580
Chicago/Turabian StyleBellfy, Lauren, and Janine L. Kwapis. 2020. "Molecular Mechanisms of Reconsolidation-Dependent Memory Updating" International Journal of Molecular Sciences 21, no. 18: 6580. https://doi.org/10.3390/ijms21186580
APA StyleBellfy, L., & Kwapis, J. L. (2020). Molecular Mechanisms of Reconsolidation-Dependent Memory Updating. International Journal of Molecular Sciences, 21(18), 6580. https://doi.org/10.3390/ijms21186580





