Effect of Urban Particulate Matter on Vocal Fold Fibrosis through the MAPK/NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Correlation between Airborne PM 10/2.5 Concentration and Acute Laryngitis/Tracheitis
2.2. PM Reduces the Viability of hVFF
2.3. PM Induces Pro-Inflammatory Cytokines in hVFF through Nuclear Factor-κB (NF-κB) Signaling
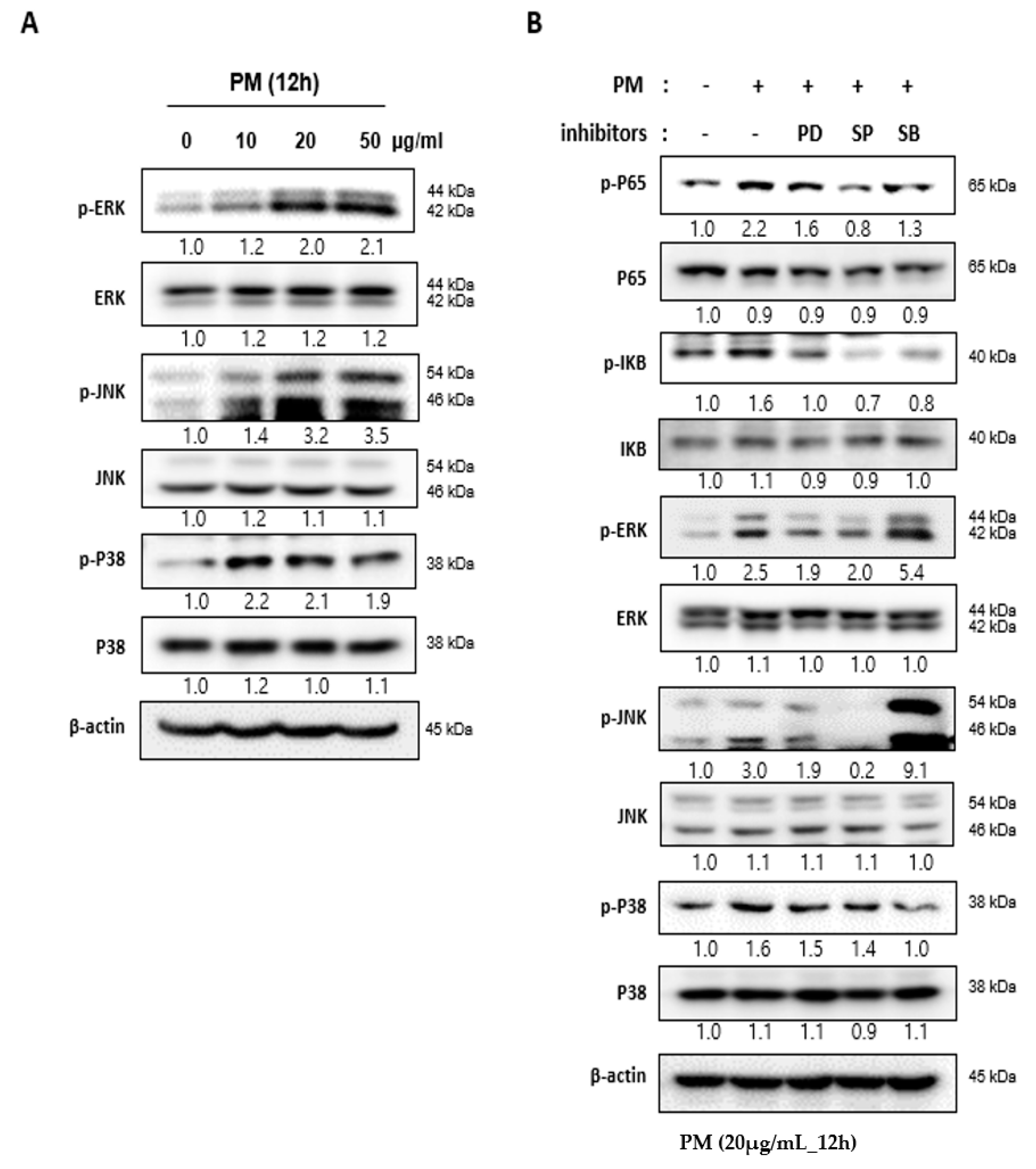
2.4. PM Induces NF-κB Signaling through the Mitogen-actiVated Protein Kinase (MAPK) Pathway
2.5. Reactive Oxygen Species (ROS) Induced by PM in hVFF Activate the MAPK Pathway
2.6. PM Induces the Differentiation of hVFF into Myofibroblasts
2.7. PM Induces Vocal Fold Mucosal Inflammation and Modulates ECM Deposition In Vivo
3. Discussion
4. Materials and Methods
4.1. Epidemiological Data Acquisition
4.2. Cell Culture
4.3. Cell Proliferation/Counting Assay
4.4. Cell Cytotoxic Assay
4.5. mRNA Isolation and Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.6. Western Blot Analysis
4.7. Analysis of ROS Production
4.8. Animal Model
4.9. Histological Analysis
4.10. Immunohistochemical Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PM | Particulate matter |
| VF | Vocal folds |
| hVFF | Human vocal fold fibroblasts |
| NF-κB | Nuclear factor-κB |
| mRNA | Messenger RNA |
| IL | Interleukin |
| IκBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha |
| MAPK | Mitogen-activated protein kinase |
| ROS | Reactive oxygen species |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| NAC | N-acetylcysteine |
| ECM | Extracellular matrix |
| TGF- β1 | Transforming growth factor β1 |
| Col1A1 | Alpha-1 type I collagen |
| α-SMA | α-smooth muscle actin |
| H&E | Hematoxylin and eosin |
| RT-PCR | Quantitative real-time reverse transcriptase polymerase chain reaction |
| cDNA | Complementary DNA |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
References
- Huang, Y.-C.T. Outdoor Air Pollution. J. Occup. Environ. Med. 2014, 56, S3–S7. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhou, Y.; Liu, S.; Chen, X.; Zou, W.; Zhao, D.; Li, X.; Pu, J.; Huang, L.; Chen, J.; et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: Results from a cross-sectional study in China. Thorax 2016, 72, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichenthal, S.; Lavigne, E.; Evans, G.J.; Pollitt, K.J.G.; Burnett, R.T. Fine Particulate Matter and Emergency Room Visits for Respiratory Illness. Effect Modification by Oxidative Potential. Am. J. Respir. Crit. Care Med. 2016, 194, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fajersztajn, L.; Veras, M.M.; Barrozo, L.; Saldiva, P.H.N. Air pollution: A potentially modifiable risk factor for lung cancer. Nat. Rev. Cancer 2013, 13, 674–678. [Google Scholar] [CrossRef]
- Lee, D.C.; Choi, H.; Oh, J.-M.; Hong, Y.; Jeong, S.H.; Kim, C.-S.; Kim, D.-K.; Cho, W.-K.; Kim, S.W.; Kim, S.W.; et al. The effect of urban particulate matter on cultured human nasal fibroblasts. Int. Forum Allergy Rhinol. 2018, 8, 993–1000. [Google Scholar] [CrossRef]
- Fujii, T.; Hayashi, S.; Hogg, J.C.; Vincent, R.; Van Eeden, S.F. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Boil. 2001, 25, 265–271. [Google Scholar] [CrossRef]
- De Grove, K.C.; Provoost, S.; Brusselle, G.G.; Joos, G.F.; Maes, T. Insights in particulate matter-induced allergic airway inflammation: Focus on the epithelium. Clin. Exp. Allergy 2018, 48, 773–786. [Google Scholar] [CrossRef]
- Roy, N.; Merrill, R.M.; Gray, S.D.; Smith, E.M. Voice Disorders in the General Population: Prevalence, Risk Factors, and Occupational Impact. Laryngoscope 2005, 115, 1988–1995. [Google Scholar] [CrossRef]
- Bhattacharyya, N. The prevalence of voice problems among adults in the United States. Laryngoscope 2014, 124, 2359–2362. [Google Scholar] [CrossRef]
- Cohen, S.M.; Kim, J.; Roy, N.; Asche, C.; Courey, M. Direct health care costs of laryngeal diseases and disorders. Laryngoscope 2012, 122, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Tateya, I.; Tateya, T.; Sohn, J.-H.; Bless, D.M. Histological Effect of Basic Fibroblast Growth Factor on Chronic Vocal Fold Scarring in a Rat Model. Clin. Exp. Otorhinolaryngol. 2016, 9, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Heisey, D.; Bless, D.M.; Ford, C.N. Effect of growth factors on hyaluronan production by canine vocal fold fibroblasts. Ann. Otol. Rhinol. Laryngol. 2003, 112, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, N.; Bing, R.; Kraja, I.; Branski, R.C. Mesenchymal stem cells have antifibrotic effects on transforming growth factor-beta1-stimulated vocal fold fibroblasts. Laryngoscope 2017, 127, E35–E41. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Holme, J.A.; Schwarze, P.E. Activation of Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter: Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viatour, P.; Merville, M.-P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Jin, Y.; An, Z.; Liu, Y.; Samet, J.M.; Wu, W. Inflammatory cell signaling following exposures to particulate matter and ozone. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2016, 1860, 2826–2834. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Dhouib, I.E.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A minireview on N -acetylcysteine: An old drug with new approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef]
- Border, W.A.; Noble, N.A. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994, 331, 1286–1292. [Google Scholar] [PubMed]
- Won, H.-R.; Song, E.H.; Won, J.E.; Lee, H.Y.; Kang, S.U.; Shin, Y.S.; Kim, C.-H. Liquid-type non-thermal atmospheric plasma ameliorates vocal fold scarring by modulating vocal fold fibroblast. Exp. Boil. Med. 2019, 244, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.; Tateya, I.; Tateya, T.; Muñoz-Del-Río, A.; Bless, D.M. Immediate inflammatory response and scar formation in wounded vocal folds. Ann. Otol. Rhinol. Laryngol. 2006, 115, 921–929. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air Pollution and Cardiovascular Disease. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Air pollution and airway disease. Clin. Exp. Allergy 2011, 41, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Girguis, M.S.; Strickland, M.J.; Hu, X.; Liu, Y.; Bartell, S.; Vieira, V.M. Maternal exposure to traffic-related air pollution and birth defects in Massachusetts. Environ. Res. 2016, 146, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Garcidueñas, L.; Solt, A.C.; Henríquez-Roldán, C.; Torres-Jardon, R.; Nuse, B.; Herritt, L.; Villarreal-Calderón, R.; Osnaya, N.; Stone, I.; García, R.; et al. Long-term Air Pollution Exposure Is Associated with Neuroinflammation, an Altered Innate Immune Response, Disruption of the Blood-Brain Barrier, Ultrafine Particulate Deposition, and Accumulation of Amyloid β-42 and α-Synuclein in Children and Young Adults. Toxicol. Pathol. 2008, 36, 289–310. [Google Scholar] [CrossRef]
- Sun, Q.; Yue, P.; Deiuliis, J.A.; Lumeng, C.N.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.; Lu, B.; Parthasarathy, S.; et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009, 119, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Sundaresan, A.S.; Hirsch, A.G.; Storm, M.; Tan, B.K.; Kennedy, T.L.; Greene, J.S.; Kern, R.C.; Schwartz, B.S. Occupational and environmental risk factors for chronic rhinosinusitis: A systematic review. Int. Forum Allergy Rhinol. 2015, 5, 996–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Du, L.; Sun, W.; Yu, Z.; He, F.; Chen, J.; Li, X.; Li, X.; Yu, L.; Chen, D. Maternal exposure to fine particulate air pollution induces epithelial-to-mesenchymal transition resulting in postnatal pulmonary dysfunction mediated by transforming growth factor-β/Smad3 signaling. Toxicol. Lett. 2017, 267, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.T.; Saravia, J.S.; Jin, N.; Giaimo, J.D.; Chustz, R.E.; Mahne, S.; Kelley, M.A.; Hebert, V.Y.; Dellinger, B.; Dugas, T.R.; et al. Radical-Containing Ultrafine Particulate Matter Initiates Epithelial-to-Mesenchymal Transitions in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Boil. 2013, 48, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012, 261, 121–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Øvrevik, J.; Refsnes, M.; Låg, M.; Brinchmann, B.; Schwarze, P.E.; Holme, J.A. Triggering Mechanisms and Inflammatory Effects of Combustion Exhaust Particles with Implication for Carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2017, 121, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, S.H.; Schauer, J.J.; Antkiewicz, D.S.; Shafer, M.M.; Kadhim, A.K. ROS production and gene expression in alveolar macrophages exposed to PM2.5 from Baghdad, Iraq: Seasonal trends and impact of chemical composition. Sci. Total. Environ. 2016, 543, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Orr, B.; Holl, M.B. Nanoparticle Interactions with Biological Membranes. Nanotoxicology 2007, 40, 99–113. [Google Scholar] [CrossRef]
- Beamer, C.A.; Holian, A. Silica suppresses Toll-like receptor ligand-induced dendritic cell activation. FASEB J. 2008, 22, 2053–2063. [Google Scholar] [CrossRef]
- Rui, W.; Guan, L.; Zhang, F.; Zhang, W.; Ding, W. PM 2.5 -induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-?B-dependent pathway. J. Appl. Toxicol. 2015, 36, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chiang, I.T.; Moreno-Vinasco, L.; Lang, G.D.; Pendyala, S.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; Chillrud, S.N.; Natarajan, V.; et al. Particulate Matter Disrupts Human Lung Endothelial Barrier Integrity via ROS- and p38 MAPK–Dependent Pathways. Am. J. Respir. Cell Mol. Boil. 2009, 42, 442–449. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, X.; Shen, Z.; Cao, L.; Cao, Y. Airborne fine particulate matter causes murine bronchial hyperreactivity via MAPK pathway-mediated M3muscarinic receptor upregulation. Environ. Toxicol. 2016, 32, 371–381. [Google Scholar] [CrossRef]
- Hanson, S.E.; Kim, J.; Dds, B.H.Q.J.; Bradley, B.; Breunig, M.J.; Hematti, P.; Thibeault, S.L. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. Laryngoscope 2010, 120, 546–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, M.J.; Lee, S.C.; Park, J.H.; Park, K.N.; Kim, H.K.; Lee, S.W. Regenerative Efficacy of Fibroblast Growth Factor for the Treatment of Aged Vocal Fold: From Animal Model to Clinical Application. Clin. Otolaryngol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, Q. Fine particulate matter air pollution and atherosclerosis: Mechanistic insights. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2016, 1860, 2863–2868. [Google Scholar] [CrossRef]
- Loxham, M. Harmful effects of particulate air pollution: Identifying the culprits. Respirology 2014, 20, 7–8. [Google Scholar] [CrossRef]
- Park, M.; Lee, J.S.; Park, M.K. The Effects of Air Pollutants on the Prevalence of Common Ear, Nose, and Throat Diseases in South Korea: A National Population-Based Study. Clin. Exp. Otorhinolaryngol. 2019, 12, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Dobashi, K.; Akiyama, M.; Naruse, I.; Nakazawa, T.; Mori, M. Effects of N-acetylcysteine and ambroxol on the production of IL-12 and IL-10 in human alveolar macrophages. Respiration 2000, 67, 662–671. [Google Scholar] [CrossRef]
- He, D.; Behar, S.; Roberts, J.E.; Lim, H.W. The effect of L-cysteine and N-acetylcysteine on porphyrin/heme biosynthetic pathway in cells treated with 5-aminolevulinic acid and exposed to radiation. Photodermatol. Photoimmunol. Photomed. 1996, 12, 194–199. [Google Scholar] [CrossRef]
- Sio, T.T.; Blanchard, M.J.; Novotny, P.J.; Patel, S.H.; Rwigema, J.-C.M.; Pederson, L.D.; McGee, L.A.; Gamez, M.E.; Seeger, G.R.; Martenson, J.A.; et al. N-Acetylcysteine Rinse for Thick Secretion and Mucositis of Head and Neck Chemoradiotherapy (Alliance MC13C2). Mayo Clin. Proc. 2019, 94, 1814–1824. [Google Scholar] [CrossRef]
- Demirel, C.; Kilciksiz, S.; Evirgen-Ayhan, S.; Gurgul, S.; Erdal, N. The preventive effect of N-acetylcysteine on radiation-induced dermatitis in a rat model. J. B.U.ON. Off. J. Balk. Union Oncol. 2010, 15, 577–582. [Google Scholar]
- Air Korea. Available online: https://www.airkorea.or.kr/eng (accessed on 10 September 2020).
- Healthcare Bigdata Hub. Available online: https://opendata.hira.or.kr/home.do (accessed on 10 September 2020).
- Thibeault, S.L.; Li, W.; Bartley, S. A method for identification of vocal fold lamina propria fibroblasts in culture. Otolaryngol. Neck Surg. 2008, 139, 816–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www-s.nist.gov/srmors/view_cert.cfm?srm=1648A (accessed on 10 September 2020).







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, H.-R.; Jung, S.-N.; Yeo, M.-K.; Yi, S.; Liu, L.; Lim, M.A.; Oh, C.; Kang, Y.E.; Chang, J.W.; Rha, K.S.; et al. Effect of Urban Particulate Matter on Vocal Fold Fibrosis through the MAPK/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6643. https://doi.org/10.3390/ijms21186643
Won H-R, Jung S-N, Yeo M-K, Yi S, Liu L, Lim MA, Oh C, Kang YE, Chang JW, Rha KS, et al. Effect of Urban Particulate Matter on Vocal Fold Fibrosis through the MAPK/NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2020; 21(18):6643. https://doi.org/10.3390/ijms21186643
Chicago/Turabian StyleWon, Ho-Ryun, Seung-Nam Jung, Min-Kyung Yeo, Shinae Yi, Lihua Liu, Mi Ae Lim, Chan Oh, Yea Eun Kang, Jae Won Chang, Ki Sang Rha, and et al. 2020. "Effect of Urban Particulate Matter on Vocal Fold Fibrosis through the MAPK/NF-κB Signaling Pathway" International Journal of Molecular Sciences 21, no. 18: 6643. https://doi.org/10.3390/ijms21186643
APA StyleWon, H.-R., Jung, S.-N., Yeo, M.-K., Yi, S., Liu, L., Lim, M. A., Oh, C., Kang, Y. E., Chang, J. W., Rha, K. S., & Koo, B. S. (2020). Effect of Urban Particulate Matter on Vocal Fold Fibrosis through the MAPK/NF-κB Signaling Pathway. International Journal of Molecular Sciences, 21(18), 6643. https://doi.org/10.3390/ijms21186643




