Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat
Abstract
1. Introduction
2. Results
2.1. Molecular Analysis of Gene-Modified UCBC
2.2. Morphometric Analysis of Infarct Area
2.3. Immunofluorescent Study of Brain
2.3.1. Cellular Stress and Apoptosis Proteins
2.3.2. Neuroglia Cells
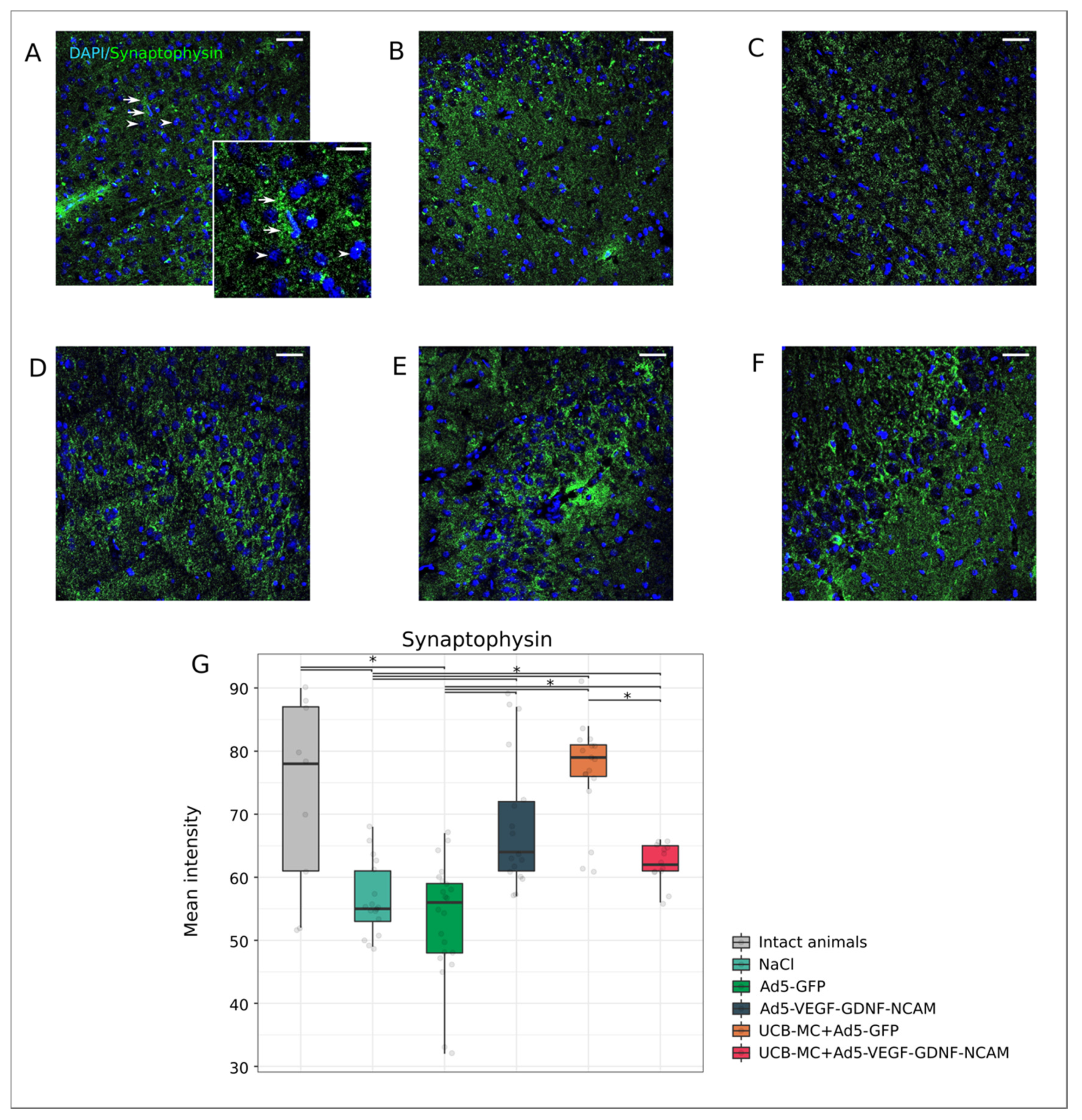
2.3.3. Synaptic Proteins
2.4. Expression of Recombinant Molecules in the Rat Brain after Intrathecal Adenoviral-Mediated Delivery of Transgenes
2.5. Expression of Recombinant Molecules in Rat Brain after Intrathecal UCB-MC-Mediated Delivery of Transgenes
2.6. Multiplex Analysis of Cytokines, Chemokines and Growth Factors in Blood (Serum) and Cerebrospinal Fluid (Liquor) Samples of Experimental and Intact Animals
3. Discussion
4. Materials and Methods
4.1. Preparation and Molecular Analysis of Gene-Modified UCB-MC
4.2. Animals and Treatments
4.3. Morphometric and Immunofluorescent Analysis of the Brain
4.4. Multiplex Cytokine Analysis of the Cerebrospinal Fluid
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karlupia, N.; Manley, N.C.; Prasad, K.; Schäfer, R.; Steinberg, G.K. Intraarterial transplantation of human umbilical cord blood mononuclear cells is more efficacious and safer compared with umbilical cord mesenchymal stromal cells in a rodent stroke model. Stem Cell Res. Ther. 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Rhim, T.; Lee, M. Targeted delivery of growth factors in ischemic stroke animal models. Expert Opin. Drug Deliv. 2016, 13, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Deguchi, K.; Nagotani, S.; Kamiya, T.; Abe, K. Gene and Stem Cell Therapy in Ischemic Stroke. Cell Transplant. 2009, 18, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Ooboshi, H. Gene Therapy as a Novel Pharmaceutical Intervention for Stroke. Curr. Pharm. Des. 2011, 17, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Zhang, Y.; Wu, F.; Min, W.P.; Minev, B.; Zhang, M.; Luo, X.L.; Ramos, F.; Ichim, T.E.; Riordan, N.H.; et al. Safety evaluation of allogeneic umbilical cord blood mononuclear cell therapy for degenerative conditions. J. Transl. Med. 2010, 8, 75. [Google Scholar] [CrossRef]
- Harris, D.T.; Rogers, I. Umbilical cord blood: A unique source of pluripotent stem cells for regenerative medicine. Curr. Stem Cell Res. Ther. 2007, 2, 301–309. [Google Scholar] [CrossRef]
- Arien-Zakay, H.; Lazarovici, P.; Nagler, A. Tissue regeneration potential in human umbilical cord blood. Best Pract. Res. Clin. Haematol. 2010, 23, 291–303. [Google Scholar] [CrossRef]
- Arien-Zakay, H.; Lecht, S.; Nagler, A.; Lazarovici, P. Human umbilical cord blood stem cells: Rational for use as a neuroprotectant in ischemic brain disease. Int. J. Mol. Sci. 2010, 11, 3513–3528. [Google Scholar] [CrossRef]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef]
- Kögler, G.; Sensken, S.; Airey, J.A.; Trapp, T.; Müschen, M.; Feldhahn, N.; Liedtke, S.; Sorg, R.V.; Fischer, J.; Rosenbaum, C.; et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 2004, 200, 123–135. [Google Scholar] [CrossRef]
- Gluckman, E. Ten years of cord blood transplantation: From bench to bedside. Br. J. Haematol. 2009, 147, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Coelho, P.M.; Rosado-de-Castro, P.H.; Barbosa da Fonseca, L.M.; Mendez-Otero, R. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic–ischemic encephalopathy. Pediatr. Res. 2012, 71, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, G.; Toren, A.; Nagler, A. Transplantation and Other Uses of Human Umbilical Cord Blood and Stem Cells. Curr. Pharm. Des. 2007, 13, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Labopin, M.; Sanz, G.; Arcese, W.; Schwerdtfeger, R.; Bosi, A.; Jacobsen, N.; Ruutu, T.; De Lima, M.; Finke, J.; et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N. Engl. J. Med. 2004, 351, 2276–2285. [Google Scholar] [CrossRef]
- Balassa, K.; Rocha, V. Anticancer cellular immunotherapies derived from umbilical cord blood. Expert Opin. Biol. Ther. 2018, 18, 121–134. [Google Scholar] [CrossRef]
- Arien-Zakay, H.; Lecht, S.; Bercu, M.M.; Tabakman, R.; Kohen, R.; Galski, H.; Nagler, A.; Lazarovici, P. Neuroprotection by cord blood neural progenitors involves antioxidants, neurotrophic and angiogenic factors. Exp. Neurol. 2009, 216, 83–94. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Pabon, M.M.; Cole, M.J.; Hudson, C.E.; Sanberg, P.R.; Willing, A.E.; Bickford, P.C.; Gemma, C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008, 9, 22. [Google Scholar] [CrossRef]
- Dasari, V.R.; Spomar, D.G.; Li, L.; Gujrati, M.; Rao, J.S.; Dinh, D.H. Umbilical cord blood stem cell mediated downregulation of Fas improves functional recovery of rats after spinal cord injury. Neurochem. Res. 2008, 33, 134–149. [Google Scholar] [CrossRef]
- Schira, J.; Gasis, M.; Estrada, V.; Hendricks, M.; Schmitz, C.; Trapp, T.; Kruse, F.; Kögler, G.; Wernet, P.; Hartung, H.P.; et al. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain 2012, 135, 431–446. [Google Scholar] [CrossRef]
- Xiao, J.; Nan, Z.; Motooka, Y.; Low, W.C. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005, 14, 722–733. [Google Scholar] [CrossRef]
- Ikeda, Y.; Fukuda, N.; Wada, M.; Matsumoto, T.; Satomi, A.; Yokoyama, S.-I.; Saito, S.; Matsumoto, K.; Kanmatsuse, K.; Mugishima, H. Development of angiogenic cell and gene therapy by transplantation of umbilical cord blood with vascular endothelial growth factor gene. Hypertens. Res. 2004, 27, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.K.; Hung, H.F.; Shyu, K.G.; Wang, B.W.; Sheu, J.R.; Liang, Y.J.; Chang, C.C.; Kuan, P. Combined cord blood stem cells and gene therapy enhances angiogenesis and improves cardiac performance in mouse after acute myocardial infarction. Eur. J. Clin. Investig. 2005, 35, 677–686. [Google Scholar] [CrossRef]
- Islamov, R.R.; Sokolov, M.E.; Bashirov, F.V.; Fadeev, F.O.; Shmarov, M.M.; Naroditskiy, B.S.; Povysheva, T.V.; Shaymardanova, G.F.; Yakupov, R.A.; Chelyshev, Y.A.; et al. A pilot study of cell-mediated gene therapy for spinal cord injury in mini pigs. Neurosci. Lett. 2017, 644, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Izmailov, A.A.; Povysheva, T.V.; Bashirov, F.V.; Sokolov, M.E.; Fadeev, F.O.; Garifulin, R.R.; Naroditsky, B.S.; Logunov, D.Y.; Salafutdinov, I.I.; Chelyshev, Y.A.; et al. Spinal Cord Molecular and Cellular Changes Induced by Adenoviral Vector- and Cell-Mediated Triple Gene Therapy after Severe Contusion. Front. Pharmacol. 2017, 8, 813. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.E.; Bashirov, F.V.; Markosyan, V.A.; Povysheva, T.V.; Fadeev, F.O.; Izmailov, A.A.; Kuztetsov, M.S.; Safiullov, Z.Z.; Shmarov, M.M.; Naroditskyi, B.S.; et al. Triple-Gene Therapy for Stroke: A Proof-of-Concept in Vivo Study in Rats. Front. Pharmacol. 2018, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tator, C.H. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Dev. Neurosci. 2013, 31, 701–713. [Google Scholar] [CrossRef]
- Razavi, S.; Ghasemi, N.; Mardani, M.; Salehi, H. Remyelination improvement after neurotrophic factors secreting cells transplantation in rat spinal cord injury. Iran. J. Basic Med. Sci. 2017, 20, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Safiullov, Z.Z.; Garanina, E.E.; Izmailov, A.A.; Garifulin, R.R.; Fedotova, V.Y.; Salafutdinov, I.I.; Rizvanov, A.A.; Islamov, R.R. Homing and survivability of genetically modified mononuclear umbilical cord blood cells after transplantation into transgenic G93A mice with amyotrophic lateral sclerosis. Genes Cells 2015, X, 1–4. [Google Scholar]
- Franklin, T.B.; Krueger-Naug, A.M.; Clarke, D.B.; Arrigo, A.-P.; Currie, R.W. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int. J. Hyperth. 2005, 21, 379–392. [Google Scholar] [CrossRef]
- Barreto, G.E.; White, R.; Ouyang, Y.; Xu, L.G.; Giffard, R. Astrocytes: Targets for Neuroprotection in Stroke. Cent. Nerv. Syst. Agents Med. Chem. 2012, 11, 164–173. [Google Scholar] [CrossRef]
- Dewar, D.; Underhill, S.M.; Goldberg, M.P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Hu, R.; Csernansky, C.A.; Hsu, C.Y.; Choi, D.W. Very delayed infarction after mild focal cerebral ischemia: A role for apoptosis? J. Cereb. Blood Flow Metab. 1996, 16, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.; Pelt, J.L.; Benton, R.L.; Howard, R.M.; Tsoulfas, P.; Ping, P.; Xu, X.-M.; Whittemore, S.R. Gene delivery to the spinal cord: Comparison between lentiviral, adenoviral, and retroviral vector delivery systems. J. Neurosci. Res. 2006, 84, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.J.; Housley, G.D. Evaluation of Gene Therapy as an Intervention Strategy to Treat Brain Injury from Stroke. Front. Mol. Neurosci. 2016, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Shirakura, M.; Inoue, M.; Fujikawa, S.; Washizawa, K.; Komaba, S.; Maeda, M.; Watabe, K.; Yoshikawa, Y.; Hasegawa, M. Postischemic administration of Sendai virus vector carrying neurotrophic factor genes prevents delayed neuronal death in gerbils. Gene Ther. 2004, 11, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Mätlik, K.; Abo-Ramadan, U.; Harvey, B.K.; Arumäe, U.; Airavaara, M. AAV-mediated targeting of gene expression to the peri-infarct region in rat cortical stroke model. J. Neurosci. Methods 2014, 236, 107–113. [Google Scholar] [CrossRef]
- Hermann, D.M.; Kilic, E.; Kügler, S.; Isenmann, S.; Bähr, M. Adenovirus-mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice. Neurobiol. Dis. 2001, 8, 655–666. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Yu, Z.; Yu, Z.; Yang, Z.; Zhao, H.; Liu, L.; Zhao, J. rAAV-mediated delivery of brain-derived neurotrophic factor promotes neurite outgrowth and protects neurodegeneration in focal ischemic model. Int. J. Clin. Exp. Pathol. 2011, 4, 496–504. [Google Scholar]
- Arvidsson, A.; Kirik, D.; Lundberg, C.; Mandel, R.J.; Andsberg, G.; Kokaia, Z.; Lindvall, O. Elevated GDNF levels following viral vector-mediated gene transfer can increase neuronal death after stroke in rats. Neurobiol. Dis. 2003, 14, 542–556. [Google Scholar] [CrossRef]
- Andsberg, G.; Kokaia, Z.; Klein, R.L.; Muzyczka, N.; Lindvall, O.; Mandel, R.J. Neuropathological and behavioral consequences of adeno-associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiol. Dis. 2002, 9, 187–204. [Google Scholar] [CrossRef]
- Sondell, M.; Lundborg, G.; Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 1999, 19, 5731–5740. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.R.; Chintalgattu, V.; Pak, E.S.; Katwa, L.C.; Murashov, A.K. Induction of VEGF and its Flt-1 receptor after sciatic nerve crush injury. Neuroreport 2004, 15, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, J.M.; Mani, N.; Silverman, W.F.; Krum, J.M. Patterns of brain angiogenesis after vascular endothelial growth factor administration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 7086–7091. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Neff, T.; Blau, C.A. Marrow sensitization to 5-fluorouracil using the ligands for Flt-3 and c-Kit. Exp. Hematol. 1999, 27, 520–525. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, J.-P.; Tzeng, S.-F. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J. Neurosci. Res. 2002, 69, 397–405. [Google Scholar] [CrossRef]
- Iannotti, C.; Li, H.; Yan, P.; Lu, X.; Wirthlin, L.; Xu, X.M. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp. Neurol. 2003, 183, 379–393. [Google Scholar] [CrossRef]
- Drury-Stewart, D.; Song, M.; Mohamad, O.; Guo, Y.; Gu, X.; Chen, D.; Wei, L. Highly efficient differentiation of neural precursors from human embryonic stem cells and benefits of transplantation after ischemic stroke in mice. Stem Cell Res. Ther. 2013, 4, 93. [Google Scholar] [CrossRef]
- Yuan, T.; Liao, W.; Feng, N.-H.; Lou, Y.-L.; Niu, X.; Zhang, A.-J.; Wang, Y.; Deng, Z.-F. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem Cell Res. Ther. 2013, 4, 73. [Google Scholar] [CrossRef]
- He, B.; Yao, Q.; Liang, Z.; Lin, J.; Xie, Y.; Li, S.; Wu, G.; Yang, Z.; Xu, P. The Dose of Intravenously Transplanted Bone Marrow Stromal Cells Determines the Therapeutic Effect on Vascular Remodeling in a Rat Model of Ischemic Stroke. Cell Transplant. 2016, 25, 2173–2185. [Google Scholar] [CrossRef]
- Zhu, H.; Poon, W.; Liu, Y.; Leung, G.K.-K.; Wong, Y.; Feng, Y.; Ng, S.C.P.; Tsang, K.S.; Sun, D.T.F.; Yeung, D.K.; et al. Phase III Clinical Trial Assessing Safety and Efficacy of Umbilical Cord Blood Mononuclear Cell Transplant Therapy of Chronic Complete Spinal Cord Injury. Cell Transplant. 2016, 25, 1925–1943. [Google Scholar] [CrossRef]
- Nomura, T.; Honmou, O.; Harada, K.; Houkin, K.; Hamada, H.; Kocsis, J.D. IV Infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 2005, 136, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Honmou, O.; Harada, K.; Nakamura, K.; Houkin, K.; Hamada, H.; Kocsis, J.D. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 2006, 129, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, H.; Liao, J.; Yi, Y.; Wang, G.; Tong, L.; Ge, J. Regulation of naotai recipe on the expression of HIF-lα/VEGF signaling pathway in cerebral ischemia/reperfusion rats. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chin. J. Integr. Tradit. West. Med. 2014, 34, 1225–1230. [Google Scholar]
- Wang, X.-L.; Zhao, Y.-S.; Hu, M.-Y.; Sun, Y.-Q.; Chen, Y.-X.; Bi, X.-H. Umbilical cord blood cells regulate endogenous neural stem cell proliferation via hedgehog signaling in hypoxic ischemic neonatal rats. Brain Res. 2013, 1518, 26–35. [Google Scholar] [CrossRef]
- Chernykh, E.R.; Shevela, E.Y.; Starostina, N.M.; Morozov, S.A.; Davydova, M.N.; Menyaeva, E.V.; Ostanin, A.A. Safety and Therapeutic Potential of M2 Macrophages in Stroke Treatment. Cell Transplant. 2016, 25, 1461–1471. [Google Scholar] [CrossRef]
- Hernández, J.; Torres-Espín, A.; Navarro, X. Adult stem cell transplants for spinal cord injury repair: Current state in preclinical research. Curr. Stem Cell Res. Ther. 2011, 6, 273–287. [Google Scholar] [CrossRef]
- Islamov, R.R.; Rizvanov, A.A.; Mukhamedyarov, M.A.; Salafutdinov, I.I.; Garanina, E.E.; Fedotova, V.Y.; Solovyeva, V.V.; Mukhamedshina, Y.O.; Safiullov, Z.Z.; Izmailov, A.A.; et al. Symptomatic improvement, increased life-span and sustained cell homing in amyotrophic lateral sclerosis after transplantation of human umbilical cord blood cells genetically modified with adeno-viral vectors expressing a neuro-protective factor and a neur. Curr. Gene Ther. 2015, 15, 266–276. [Google Scholar] [CrossRef]
- Park, D.-H.; Lee, J.-H.; Borlongan, C.V.; Sanberg, P.R.; Chung, Y.-G.; Cho, T.-H. Transplantation of Umbilical Cord Blood Stem Cells for Treating Spinal Cord Injury. Stem Cell Rev. Rep. 2011, 7, 181–194. [Google Scholar] [CrossRef]
- Islamov, R.R.; Bashirov, F.V.; Sokolov, M.E.; Izmailov, A.A.; Fadeev, F.O.; Markosyan, V.A.; Davleeva, M.A.; Zubkova, O.V.; Smarov, M.M.; Logunov, D.Y.; et al. Gene-modified leucoconcentrate for personalized ex vivo gene therapy in a mini pig model of moderate spinal cord injury. Neural Regen. Res. 2020, 16, 357–361. [Google Scholar] [CrossRef]










| Groups | Preparation for Animals Treatment | Number of Animals |
|---|---|---|
| Control | 20 µL 0.9% NaCl intrathecally injected 4 days before ischemic stroke modelling | 5 |
| Ad5-GFP | Ad5 carrying gfp in 20 µL of saline intrathecally injected 4 days before ischemic stroke modelling | 6 |
| Ad5-VEGF+Ad5-GDNF+Ad5-NCAM | Mixture of the three Ad5 carrying vegf165, gdnf and ncam1 in 20 µL of saline intrathecally injected 4 days before ischemic stroke modelling | 6 |
| UCBC+Ad5-GFP | 2 × 106 UCBC transduced with Ad5 carrying gfp in 20 µL of saline intrathecally injected 3 days before ischemic stroke modelling | 6 |
| UCBC+Ad5-VEGF-GDNF-NCAM | 2 × 106 UCBC simultaneously transduced with three Ad5 carrying vegf165, gdnf and ncam1 in 20 µL of saline intrathecally injected 3 days before ischemic stroke modelling | 6 |
| Antibody against: | Host | Dilution | Source |
|---|---|---|---|
| Caspase3 | Rabbit | 1:200 | Abcam |
| Glial cell-derived neurotrophic factor (GDNF) | Rabbit | 1:100 | Santa Cruz |
| Glial fibrillary acidic protein (GFAP) | Mouse | 1:200 | Santa Cruz |
| Ionized calcium binding adaptor molecule 1 (Iba1) | Rabbit | 1:150 | Biocare Medical |
| Human Nuclear Antigen (HNA) | Mouse | 1:150 | Millipore |
| Heat shock protein 70 kDa (Hsp70) | Rabbit | 1:200 | Abcam |
| Oligodendrocyte transcription factor 2 (Olig2) | Rabbit | 1:100 | Santa Cruz |
| Neural cell adhesion molecule (NCAM) | Rabbit | 1:100 | Santa Cruz |
| Postsynaptic density protein 95 kDa (PSD95) | Rabbit | 1:200 | Abcam |
| Synaptophysin | Rabbit | 1:100 | Abcam |
| Vascular endothelial growth factor (VEGF) | Goat | 1:300 | Sigma |
| Rabbit IgG conjugated with Alexa 647 | Donkey | 1:200 | Invitrogen |
| Mouse IgG conjugated with Alexa 488 | Donkey | 1:200 | Invitrogen |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markosyan, V.; Safiullov, Z.; Izmailov, A.; Fadeev, F.; Sokolov, M.; Kuznetsov, M.; Trofimov, D.; Kim, E.; Kundakchyan, G.; Gibadullin, A.; et al. Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat. Int. J. Mol. Sci. 2020, 21, 6858. https://doi.org/10.3390/ijms21186858
Markosyan V, Safiullov Z, Izmailov A, Fadeev F, Sokolov M, Kuznetsov M, Trofimov D, Kim E, Kundakchyan G, Gibadullin A, et al. Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat. International Journal of Molecular Sciences. 2020; 21(18):6858. https://doi.org/10.3390/ijms21186858
Chicago/Turabian StyleMarkosyan, Vage, Zufar Safiullov, Andrei Izmailov, Filip Fadeev, Mikhail Sokolov, Maksim Kuznetsov, Dmitry Trofimov, Evgeny Kim, Grayr Kundakchyan, Airat Gibadullin, and et al. 2020. "Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat" International Journal of Molecular Sciences 21, no. 18: 6858. https://doi.org/10.3390/ijms21186858
APA StyleMarkosyan, V., Safiullov, Z., Izmailov, A., Fadeev, F., Sokolov, M., Kuznetsov, M., Trofimov, D., Kim, E., Kundakchyan, G., Gibadullin, A., Salafutdinov, I., Nurullin, L., Bashirov, F., & Islamov, R. (2020). Preventive Triple Gene Therapy Reduces the Negative Consequences of Ischemia-Induced Brain Injury after Modelling Stroke in a Rat. International Journal of Molecular Sciences, 21(18), 6858. https://doi.org/10.3390/ijms21186858






