Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes
Abstract
1. Introduction
2. Results
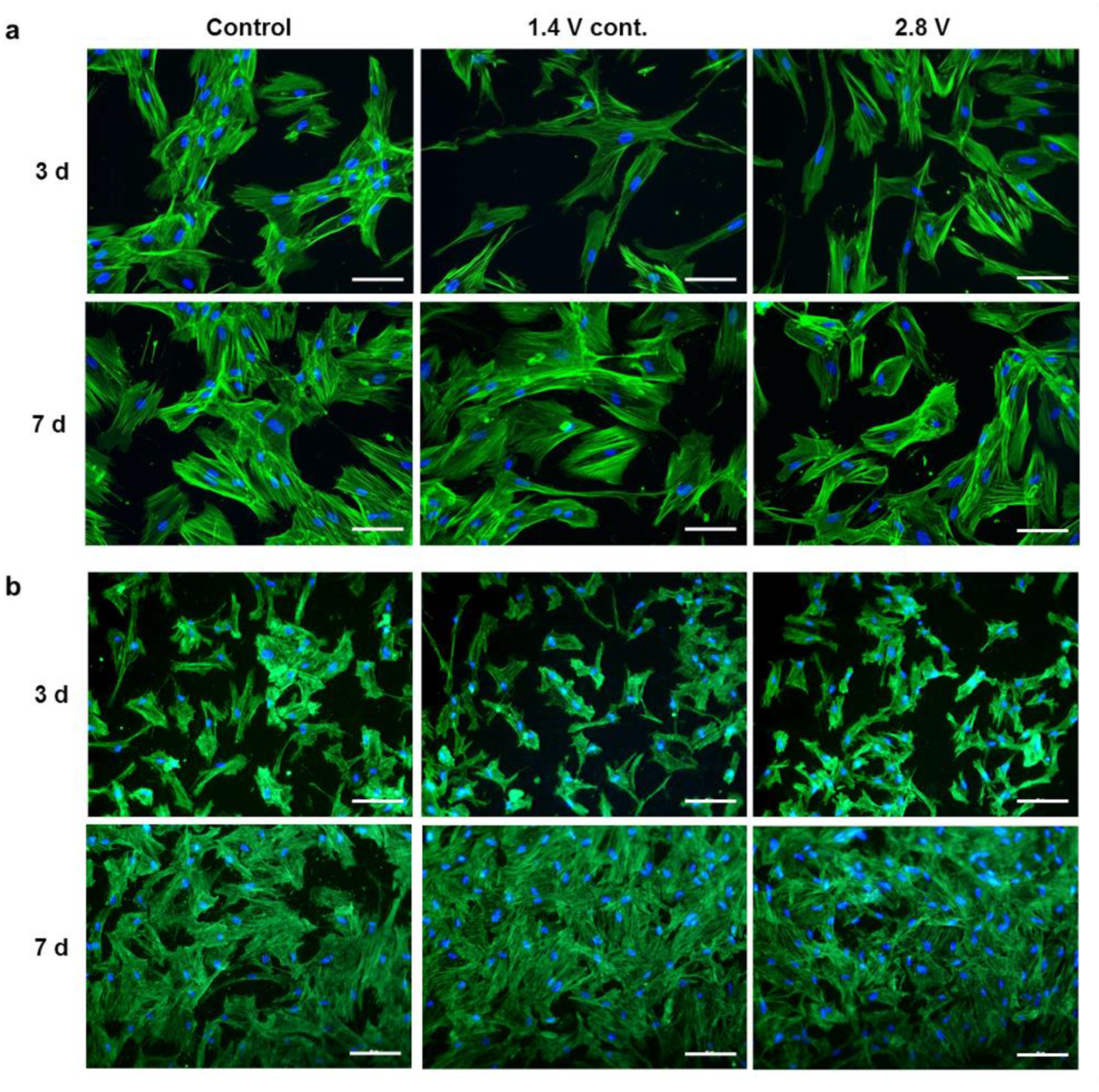
2.1. Influence of Alternating Electric Fields on Osteoblast Morphology
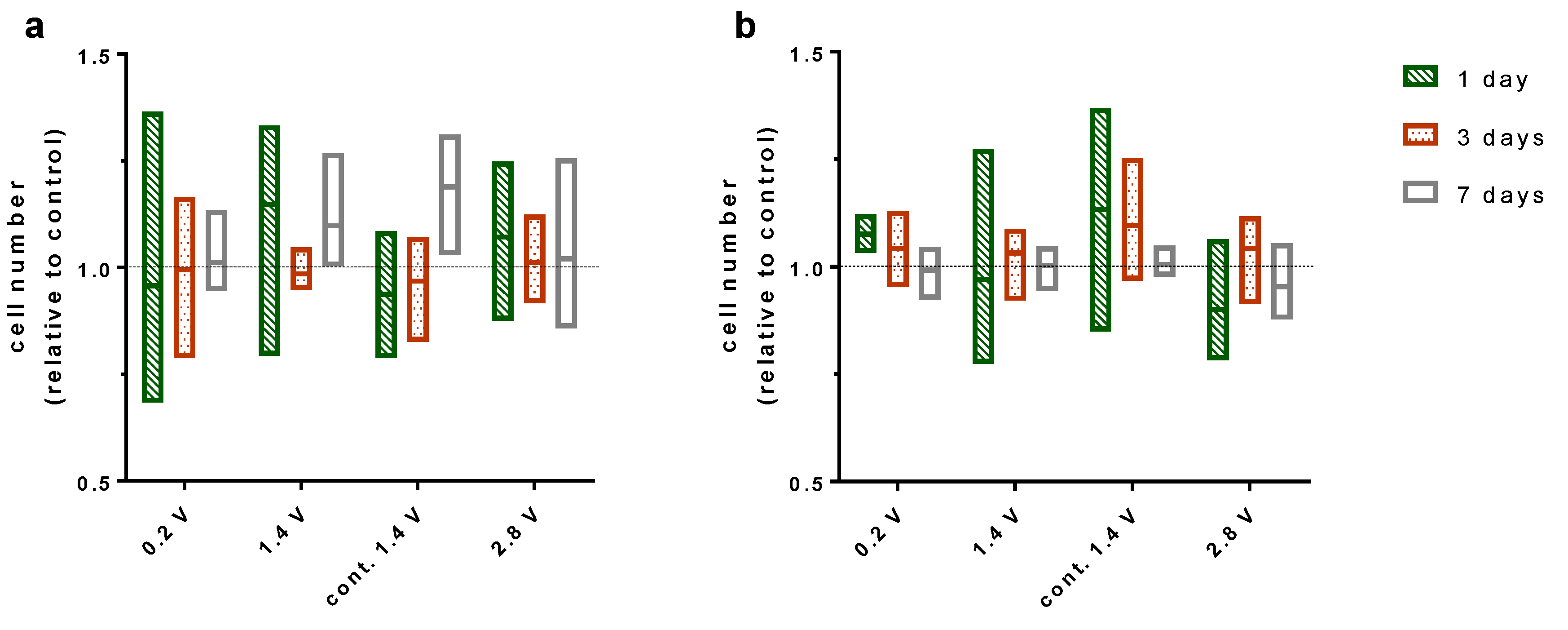
2.2. Effects of Alternating Electric Fields on Osteoblastic Proliferation
2.3. Effects of Alternating Electric Fields on Osteogenic Gene Expression
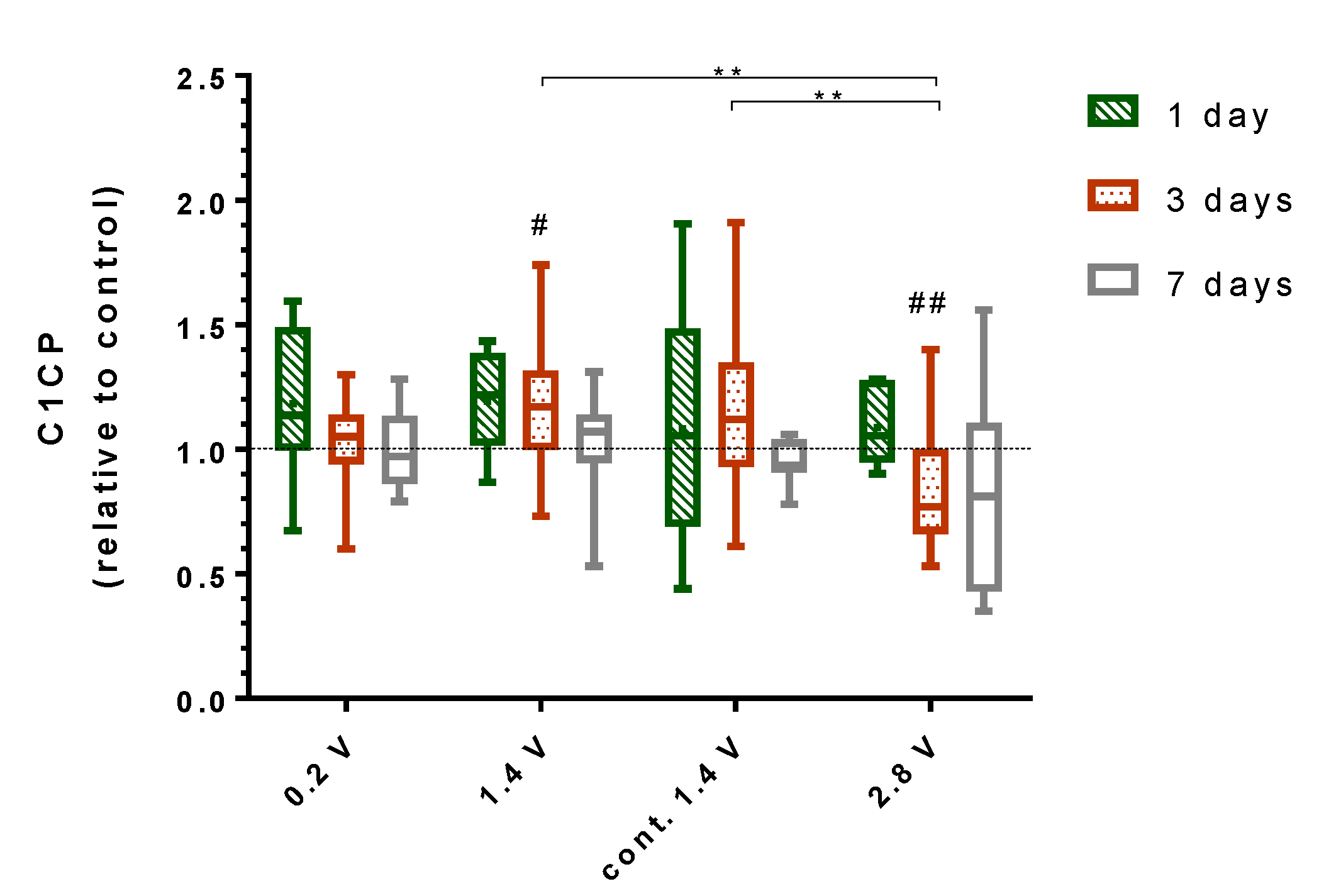
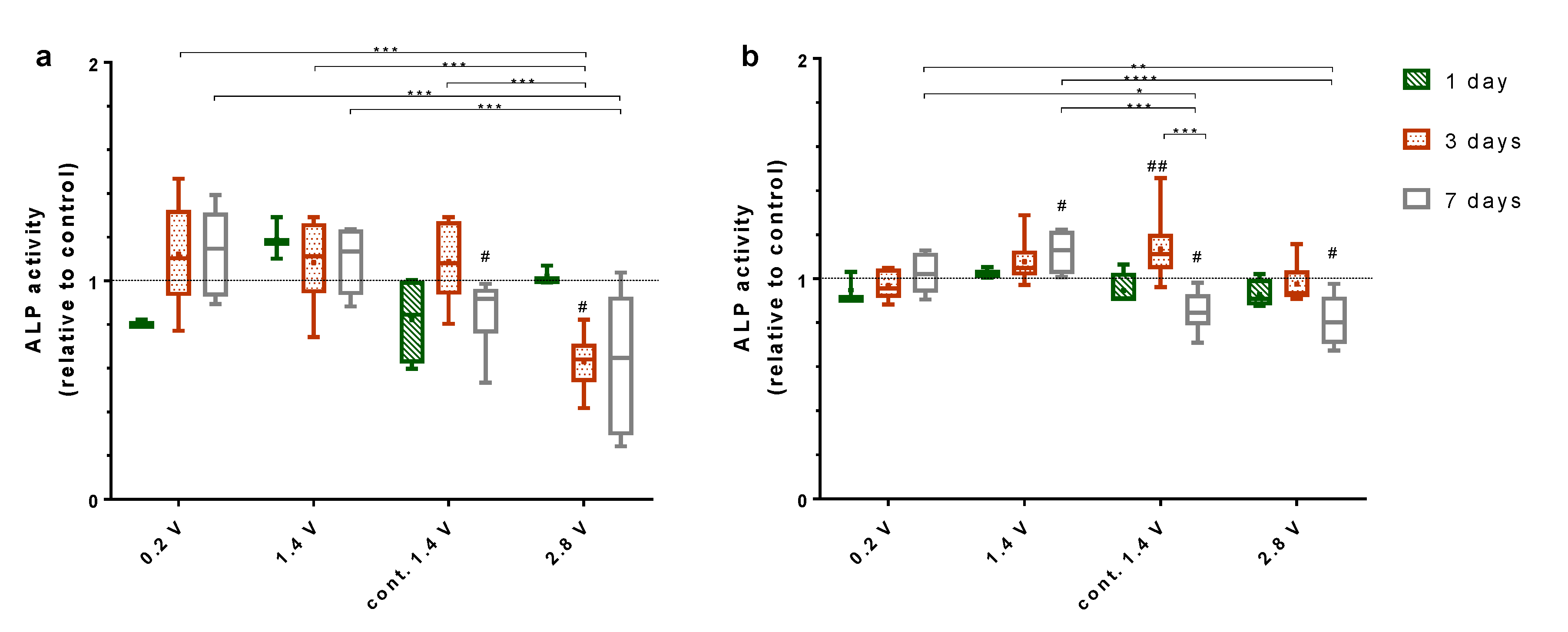
2.4. Effects of Alternating Electric Fields on Osteogenic Mediators
3. Discussion
4. Materials and Methods
4.1. Isolation and Cultivation of Human Osteoblasts
4.2. Electrical Stimulation Protocol
4.3. Actin Staining
4.4. Cell Proliferation
4.5. Gene Expression Analysis
4.6. Collagen Type I Synthesis
4.7. ALP Activity
4.8. Data Illustration and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Bordini, B.; Stea, S.; De Clerico, M.; Strazzari, S.; Sasdelli, A.; Toni, A. Factors affecting aseptic loosening of 4750 total hip arthroplasties: Multivariate survival analysis. BMC Musculoskelet. Disord. 2007, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Hailer, N.P.; Garellick, G.; Kärrholm, J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010, 81, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hooper, G.J.; Rothwell, A.G.; Stringer, M.; Frampton, C. Revision following cemented and uncemented primary total hip replacement. J. Bone Jt. Surg. Br. 2009, 91, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Corten, K.; Bourne, R.B.; Charron, K.D.; Au, K.; Rorabeck, C.H. What works best, a cemented or cementless primary total hip arthroplasty?: Minimum 17-year followup of a randomized controlled trial. Clin. Orthop. Relat. Res. 2011, 469, 209–217. [Google Scholar] [CrossRef]
- Springer, B.D.; Fehring, T.K.; Griffin, W.L.; Odum, S.M.; Masonis, J.L. Why revision total hip arthroplasty fails. Clin. Orthop. Relat. Res. 2009, 467, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Nilsdotter-Augustinsson, Å.; Briheim, G.; Herder, A.; Ljunghusen, O.; Wahlström, O.; Öhman, L. Inflammatory response in 85 patients with loosened hip prostheses: A prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007, 78, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Bori, G.; Soriano, A.; García, S.; Gallart, X.; Casanova, L.; Mallofre, C.; Almela, M.; Martínez, J.A.; Riba, J.; Mensa, J. Low sensitivity of histology to predict the presence of microorganisms in suspected aseptic loosening of a joint prosthesis. Mod. Pathol. 2006, 19, 874–877. [Google Scholar] [CrossRef]
- Wolff, J. Das Gesetz der Transformation der Knochen [The law of bone remodeling, translated by P. Maquet and R. Furlong]. A Hirshwald 1892, 1, 1–152. [Google Scholar]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Griffin, M.; Bayat, A. Electrical Stimulation in Bone Healing: Critical Analysis by Evaluating Levels of Evidence. Eplasty 2011, 11, e34. [Google Scholar] [PubMed]
- Bhavsar, M.B.; Han, Z.; DeCoster, T.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H. Electrical stimulation-based bone fracture treatment, if it works so well why do not more surgeons use it? Eur. J. Trauma Emerg. Surg. 2020, 46, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Ellenrieder, M.; Tischer, T.; Kreuz, P.C.; Fröhlich, S.; Fritsche, A.; Mittelmeier, W. Arthroskopisch gestützte Behandlung der aseptischen Hüftkopfnekrose [Arthroscopically assisted therapy of avascular necrosis of the femoral head]. Oper. Orthop. Traumatol. 2013, 25, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ebner, C.; Su, Y.; Zimmermann, U.; von Rienen, U.; Bader, R. A novel concept for active electrical stimulation of the osseointegration of an uncemented total hip stem. Biomed. Eng. Tech. 2014, 59, S1162. [Google Scholar]
- Schmidt, C.; Zimmermann, U.; van Rienen, U. Modeling of an optimized electrostimulative hip revision system under consideration of uncertainty in the conductivity of bone tissue. IEEE J. Biomed. Health Inform. 2015, 19, 1321–1330. [Google Scholar] [CrossRef]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. Biotechniques 2016, 60, 95–98. [Google Scholar] [CrossRef]
- Hu, W.-W.; Hsu, Y.-T.; Cheng, Y.-C.; Li, C.; Ruaan, R.-C.; Chien, C.-C.; Chung, C.-A.; Tsao, C.-W. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 28–36. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, Z.; Rouabhia, M. Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. J. Bone Miner. Metab. 2011, 29, 535–544. [Google Scholar] [CrossRef]
- Zhai, M.; Jing, D.; Tong, S.; Wu, Y.; Wang, P.; Zeng, Z.; Shen, G.; Wang, X.; Xu, Q.; Luo, E. Pulsed electromagnetic fields promote in vitro osteoblastogenesis through a Wnt/β-catenin signaling-associated mechanism. Bioelectromagnetics 2016, 37, 152–162. [Google Scholar] [CrossRef]
- Fassina, L.; Visai, L.; Benazzo, F.; Benedetti, L.; Calligaro, A.; De Angelis, M.G.C.; Farina, A.; Maliardi, V.; Magenes, G. Effects of Electromagnetic Stimulation on Calcified Matrix Production by SAOS-2 Cells over a Polyurethane Porous Scaffold. Tissue Eng. 2006, 12, 1985–1999. [Google Scholar] [CrossRef]
- Luo, F.; Hou, T.; Zhang, Z.; Xie, Z.; Wu, X.; Xu, J. Effects of Pulsed Electromagnetic Field Frequencies on the Osteogenic Differentiation of Human Mesenchymal Stem Cells. Orthopedics 2012, 35, e526–e531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; An, Y.; Li, F.; Li, D.; Jing, D.; Guo, T.; Luo, E.; Ma, C. The effects of pulsed electromagnetic field on the functions of osteoblasts on implant surfaces with different topographies. Acta Biomater. 2014, 10, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Eischen-Loges, M.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, e4959. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Neville, A.; Dowson, D. Biotribocorrosion—An appraisal of the time dependence of wear and corrosion interactions: I. The role of corrosion. J. Phys. D Appl. Phys. 2006, 39, 3200–3205. [Google Scholar] [CrossRef]
- Dauben, T.J.; Ziebart, J.; Bender, T.; Zaatreh, S.; Kreikemeyer, B.; Bader, R. A Novel In Vitro System for Comparative Analyses of Bone Cells and Bacteria under Electrical Stimulation. Biomed Res. Int. 2016, 2016, 5178640. [Google Scholar] [CrossRef]
- Su, C.-Y.; Fang, T.; Fang, H.-W. Effects of Electrostatic Field on Osteoblast Cells for Bone Regeneration Applications. Biomed Res. Int. 2017, 2017, 7124817. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Hronik-Tupaj, M.; Rice, W.L.; Cronin-Golomb, M.; Kaplan, D.L.; Georgakoudi, I. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed. Eng. Online 2011, 10, 9. [Google Scholar] [CrossRef]
- McCullen, S.D.; McQuilling, J.P.; Grossfeld, R.M.; Lubischer, J.L.; Clarke, L.I.; Loboa, E.G. Application of low-frequency alternating current electric fields via interdigitated electrodes: Effects on cellular viability, cytoplasmic calcium, and osteogenic differentiation of human adipose-derived stem cells. Tissue Eng. Part C Methods 2010, 16, 1377–1386. [Google Scholar] [CrossRef]
- Grunert, P.C.; Jonitz-Heincke, A.; Su, Y.; Souffrant, R.; Hansmann, D.; Ewald, H.; Krüger, A.; Mittelmeier, W.; Bader, R. Establishment of a Novel In Vitro Test Setup for Electric and Magnetic Stimulation of Human Osteoblasts. Cell Biochem. Biophys. 2014, 70, 805–817. [Google Scholar] [CrossRef]
- Wieland, D.C.F.; Krywka, C.; Mick, E.; Willumeit-Romer, R.; Bader, R.; Kluess, D. Investigation of the inverse piezoelectric effect of trabecular bone on a micrometer length scale using synchrotron radiation. Acta Biomater. 2015, 25, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Hiemer, B.; Ziebart, J.; Jonitz-Heincke, A.; Grunert, P.C.; Su, Y.; Hansmann, D.; Bader, R. Magnetically induced electrostimulation of human osteoblasts results in enhanced cell viability and osteogenic differentiation. Int. J. Mol. Med. 2016, 38, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Kim, G. The effect of sinusoidal AC electric stimulation of 3D PCL/CNT and PCL/β-TCP based bio-composites on cellular activities for bone tissue regeneration. J. Mater. Chem. B 2013, 1, 1439. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Kang, E.-T.; Neoh, K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.-Y.; Fang, H.-Y.; Lin, Y.-H.; Lee, A.K.-X.; Yu, J.; Chen, Y.-W. Application of piezoelectric cells printing on three-dimensional porous bioceramic scaffold for bone regeneration. Int. J. Bioprinting 2019, 5, 210. [Google Scholar] [CrossRef]
- Polley, C.; Distler, T.; Detsch, R.; Lund, H.; Springer, A.; Boccaccini, A.R.; Seitz, H. 3D Printing of Piezoelectric Barium Titanate-Hydroxyapatite Scaffolds with Interconnected Porosity for Bone Tissue Engineering. Materials 2020, 13, 1773. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors-A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Boccaccini, A.R. Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials 2014, 35, 9068–9086. [Google Scholar] [CrossRef]
- Lochner, K.; Fritsche, A.; Jonitz, A.; Hansmann, D.; Mueller, P.; Mueller-hilke, B.; Bader, R. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med. 2011, 1055–1063. [Google Scholar] [CrossRef]
- Su, Y.; Souffrant, R.; Kluess, D.; Ellenrieder, M.; Mittelmeier, W.; van Rienen, U.; Bader, R. Evaluation of electric field distribution in electromagnetic stimulation of human femoral head. Bioelectromagnetics 2014, 35, 547–558. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−ΔΔCT) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahm, F.; Ziebart, J.; Jonitz-Heincke, A.; Hansmann, D.; Dauben, T.; Bader, R. Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes. Int. J. Mol. Sci. 2020, 21, 6944. https://doi.org/10.3390/ijms21186944
Sahm F, Ziebart J, Jonitz-Heincke A, Hansmann D, Dauben T, Bader R. Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes. International Journal of Molecular Sciences. 2020; 21(18):6944. https://doi.org/10.3390/ijms21186944
Chicago/Turabian StyleSahm, Franziska, Josefin Ziebart, Anika Jonitz-Heincke, Doris Hansmann, Thomas Dauben, and Rainer Bader. 2020. "Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes" International Journal of Molecular Sciences 21, no. 18: 6944. https://doi.org/10.3390/ijms21186944
APA StyleSahm, F., Ziebart, J., Jonitz-Heincke, A., Hansmann, D., Dauben, T., & Bader, R. (2020). Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes. International Journal of Molecular Sciences, 21(18), 6944. https://doi.org/10.3390/ijms21186944



