Prediction of Cyclosporin-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Model Characterizing Interplay of Drug Transporters and Enzymes
Abstract
:1. Introduction
2. Results
2.1. Collection of Drug Parameters Used for PBPK Prediction
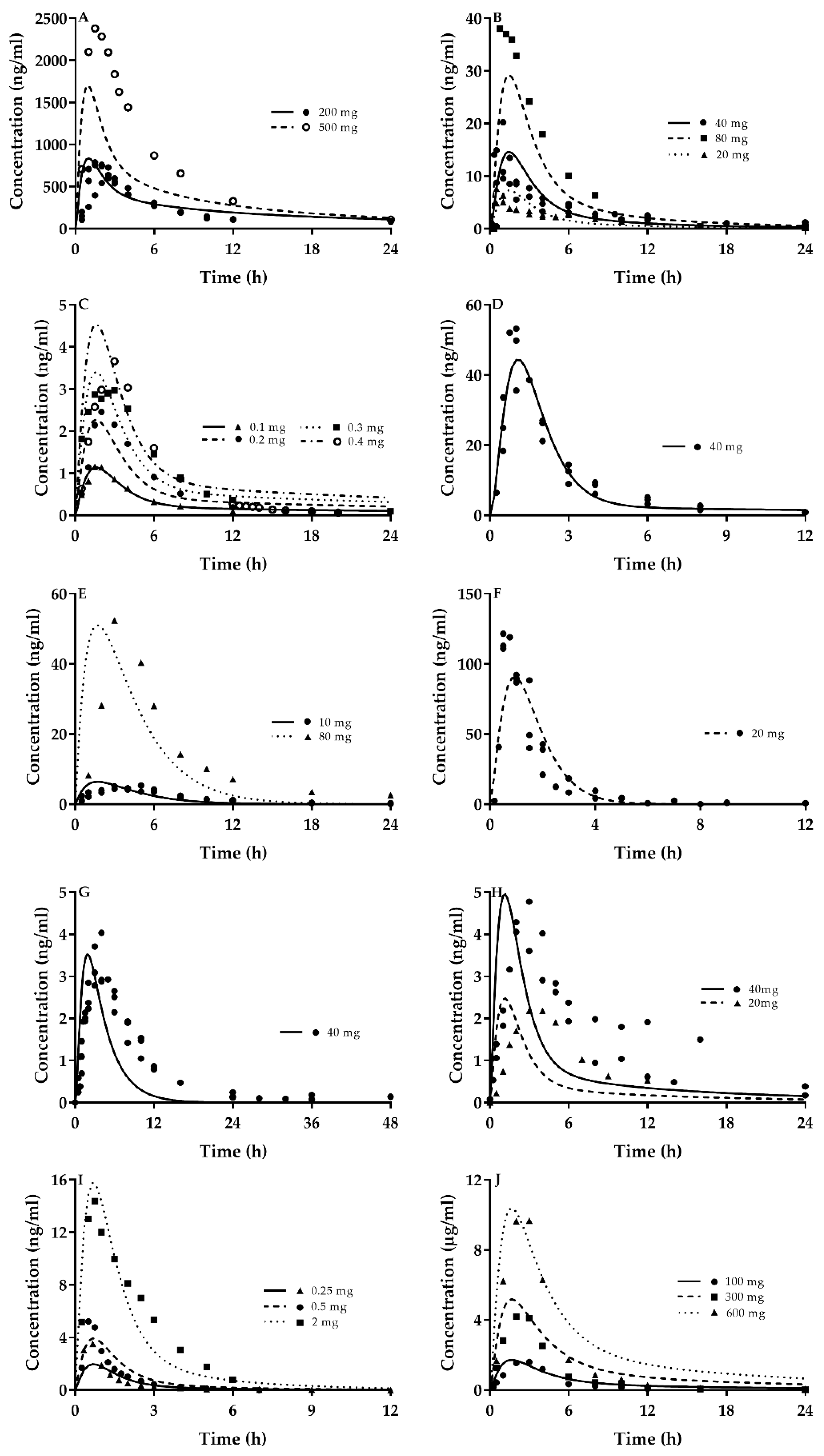
2.2. Quantitative Prediction of CsA and Victim Drugs Using the Developed PBPK Model
2.3. Quantitative DDIs of CsA with Victim Drugs
2.3.1. DDI of CsA with Atorvastatin
2.3.2. DDIs of CsA with Cerivastatin
2.3.3. DDIs of CsA with Pravastatin
2.3.4. DDI of CsA with Rosuvastatin
2.3.5. DDIs of CSA with Fluvastatin
2.3.6. DDI of CsA with Simvastatin
2.3.7. DDI of CsA with Lovastatin
2.3.8. DDI of CsA with Repaglinide
2.3.9. DDI of CsA with Bosentan
2.4. Sensitivity Analysis of Model Parameters
3. Discussion
4. Method
4.1. Collection of Data
4.2. Development of a Whole-Body PBPK Model Charactering Interplay of Enzymes and Transporters in Intestine, Liver and Kidney
4.3. Model Validation
4.4. Sensitivity Analysis of Model Parameters
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CYP450s | Cytochrome P450 enzymes |
| UGTs | UDP-glucuronosyltransferases |
| OATPs | Organic Anion transporting polypeptides |
| P-gp | P-glycoprotein |
| MRP | Multidrug resistance-associated protein |
| BCRP | Breast cancer resistance protein |
| CsA | Cyclosporin A |
| DDIs | Drug–Drug Interactions |
| PBPK | Physiologically based pharmacokinetic |
| fub | unbound fraction of drug in blood |
| Rb | blood/plasma ratio |
| Kt | Transition rate |
| SF | Empirical scaling factor |
| AUC | Area under the curve |
| Cmax | Peak concentration |
| AUCR | Ratio of AUC for victim drugs with CsA to without CsA |
| CmaxR | Ratio of Cmax for victim drugs with CsA to without CsA |
References
- Lu, C.; Di, L. In vitro and in vivo methods to assess pharmacokinetic drug- drug interactions in drug discovery and development. Biopharm. Drug Dispos. 2020, 41, 3–31. [Google Scholar] [CrossRef] [Green Version]
- Bteich, M.; Poulin, P.; Haddad, S. The potential protein-mediated hepatic uptake: Discussion on the molecular interactions between albumin and the hepatocyte cell surface and their implications for the in vitro-to-in vivo extrapolations of hepatic clearance of drugs. Expert Opin. Drug Metab. Toxicol. 2019, 15, 633–658. [Google Scholar] [CrossRef]
- Liu, X. SLC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 101–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X. Imbalance of Drug Transporter-CYP450s Interplay by Diabetes and Its Clinical Significance. Pharmaceutics 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Ann. Rev. Pharm. Toxicol. 1999, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. ABC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 13–100. [Google Scholar] [CrossRef] [PubMed]
- Lennernas, H. Clinical pharmacokinetics of atorvastatin. Clin. Pharm. 2003, 42, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Volpe, D.A.; Wang, Y.; Zhang, W.; Bode, C.; Owen, A.; Hidalgo, I.J. Use of transporter knockdown Caco-2 cells to investigate the in vitro efflux of statin drugs. Drug Metab. Dispos. 2011, 39, 1196–1202. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.C.; Hawksworth, G.M.; Weaver, R.J. ATP-dependent transport of statins by human and rat MRP2/Mrp2. Toxicol. Appl. Pharm. 2013, 269, 187–194. [Google Scholar] [CrossRef]
- Kalliokoski, A.; Niemi, M. Impact of OATP transporters on pharmacokinetics. Br. J. Pharm. 2009, 158, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Lechner, C.; Reichel, V.; Moenning, U.; Reichel, A.; Fricker, G. Development of a fluorescence-based assay for drug interactions with human Multidrug Resistance Related Protein (MRP2; ABCC2) in MDCKII-MRP2 membrane vesicles. Eur. J. Pharm. Biopharm. 2010, 75, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Shitara, Y. Clinical importance of OATP1B1 and OATP1B3 in drug-drug interactions. Drug Metab. Pharm. 2011, 26, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; O’Loughlin, K.L.; Fricke, S.M.; Williamson, N.A.; Greco, W.R.; Minderman, H.; Baer, M.R. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin. Cancer Res. 2005, 11, 2320–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, M.; Asberg, A.; Christensen, H.; Holdaas, H.; Hartmann, A.; Reubsaet, J.L. Substantially elevated levels of atorvastatin and metabolites in cyclosporine-treated renal transplant recipients. Clin. Pharm. 2004, 76, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Lemahieu, W.P.; Hermann, M.; Asberg, A.; Verbeke, K.; Holdaas, H.; Vanrenterghem, Y.; Maes, B.D. Combined therapy with atorvastatin and calcineurin inhibitors: No interactions with tacrolimus. Am. J. Transpl. 2005, 5, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Asberg, A.; Hartmann, A.; Fjeldsa, E.; Bergan, S.; Holdaas, H. Bilateral pharmacokinetic interaction between cyclosporine A and atorvastatin in renal transplant recipients. Am. J. Transpl. 2001, 1, 382–386. [Google Scholar] [CrossRef]
- Muck, W.; Mai, I.; Fritsche, L.; Ochmann, K.; Rohde, G.; Unger, S.; Johne, A.; Bauer, S.; Budde, K.; Roots, I.; et al. Increase in cerivastatin systemic exposure after single and multiple dosing in cyclosporine-treated kidney transplant recipients. Clin. Pharm. 1999, 65, 251–261. [Google Scholar] [CrossRef]
- Olbricht, C.; Wanner, C.; Eisenhauer, T.; Kliem, V.; Doll, R.; Boddaert, M.; O’Grady, P.; Krekler, M.; Mangold, B.; Christians, U. Accumulation of lovastatin, but not pravastatin, in the blood of cyclosporine-treated kidney graft patients after multiple doses. Clin. Pharm. 1997, 62, 311–321. [Google Scholar] [CrossRef]
- Park, J.W.; Siekmeier, R.; Merz, M.; Krell, B.; Harder, S.; Marz, W.; Seidel, D.; Schuler, S.; Gross, W. Pharmacokinetics of pravastatin in heart-transplant patients taking cyclosporin A. Int. J. Clin. Pharm. 2002, 40, 439–450. [Google Scholar] [CrossRef]
- Simonson, S.G.; Raza, A.; Martin, P.D.; Mitchell, P.D.; Jarcho, J.A.; Brown, C.D.; Windass, A.S.; Schneck, D.W. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin. Pharm. 2004, 76, 167–177. [Google Scholar] [CrossRef]
- Park, J.W.; Siekmeier, R.; Lattke, P.; Merz, M.; Mix, C.; Schuler, S.; Jaross, W. Pharmacokinetics and pharmacodynamics of fluvastatin in heart transplant recipients taking cyclosporine A. J. Cardiovasc. Pharm. 2001, 6, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, N.; Takahara, S.; Kokado, Y.; Wang, J.D.; Hatori, M.; Kameoka, H.; Inoue, T.; Okuyama, A. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis 2001, 158, 417–423. [Google Scholar] [CrossRef]
- Kajosaari, L.I.; Niemi, M.; Neuvonen, M.; Laitila, J.; Neuvonen, P.J.; Backman, J.T. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin. Pharm. 2005, 78, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Binet, I.; Wallnofer, A.; Weber, C.; Jones, R.; Thiel, G. Renal hemodynamics and pharmacokinetics of bosentan with and without cyclosporine A. Kidney Int. 2000, 57, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeheng, T.; Na-Bangchang, K.; Karbwang, J. Utility of physiologically based pharmacokinetic (PBPK) modeling in oncology drug development and its accuracy: A systematic review. Eur. J. Clin. Pharm. 2018, 74, 1365–1376. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Xu, J.; Zhao, K.; Chen, Y.; Liang, L.; Li, P.; Chen, N.; Geng, D.; Zhang, X.; et al. Prediction of Atorvastatin Pharmacokinetics in High-Fat Diet and Low-Dose Streptozotocin-Induced Diabetic Rats Using a Semiphysiologically Based Pharmacokinetic Model Involving Both Enzymes and Transporters. Drug Metab. Dispos. 2019, 47, 1066–1079. [Google Scholar] [CrossRef]
- Varma, M.V.; Bi, Y.A.; Kimoto, E.; Lin, J. Quantitative prediction of transporter- and enzyme-mediated clinical drug-drug interactions of organic anion-transporting polypeptide 1B1 substrates using a mechanistic net-effect model. J. Pharm. Exp. 2014, 351, 214–223. [Google Scholar] [CrossRef]
- Varma, M.V.; Lai, Y.; Feng, B.; Litchfield, J.; Goosen, T.C.; Bergman, A. Physiologically based modeling of pravastatin transporter-mediated hepatobiliary disposition and drug-drug interactions. Pharm. Res. 2012, 29, 2860–2873. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef]
- Fujino, H.; Saito, T.; Tsunenari, Y.; Kojima, J.; Sakaeda, T. Metabolic properties of the acid and lactone forms of HMG-CoA reductase inhibitors. Xenobiotica 2004, 34, 961–971. [Google Scholar] [CrossRef]
- Kunze, A.; Poller, B.; Huwyler, J.; Camenisch, G. Application of the extended clearance concept classification system (ECCCS) to predict the victim drug-drug interaction potential of statins. Drug Metab. Pers. 2015, 30, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Neuvonen, P.J.; Backman, J.T.; Niemi, M. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin. Pharm. 2008, 47, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Eliasson, E.; Ask, B.; Inotsume, N.; Rane, A. Roles of different CYP enzymes in the formation of specific fluvastatin metabolites by human liver microsomes. Basic Clin. Pharm. Toxicol. 2009, 105, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.W.; Kari, P.H.; Lu, A.Y.; Thomas, P.E.; Guengerich, F.P.; Vyas, K.P. Biotransformation of lovastatin. IV. Identification of cytochrome P450 3A proteins as the major enzymes responsible for the oxidative metabolism of lovastatin in rat and human liver microsomes. Arch. Biochem. Biophys. 1991, 290, 355–361. [Google Scholar] [CrossRef]
- Kudo, T.; Goda, H.; Yokosuka, Y.; Tanaka, R.; Komatsu, S.; Ito, K. Estimation of the Contribution of CYP2C8 and CYP3A4 in Repaglinide Metabolism by Human Liver Microsomes Under Various Buffer Conditions. J. Pharm. Sci. 2017, 106, 2847–2852. [Google Scholar] [CrossRef]
- Srinivas, N.R. Clinical drug-drug interactions of bosentan, a potent endothelial receptor antagonist, with various drugs: Physiological role of enzymes and transporters. Gen. Physiol. Biophys. 2016, 35, 243–258. [Google Scholar] [CrossRef]
- Fahrmayr, C.; Konig, J.; Auge, D.; Mieth, M.; Munch, K.; Segrestaa, J.; Pfeifer, T.; Treiber, A.; Fromm, M. Phase I and II metabolism and MRP2-mediated export of bosentan in a MDCKII-OATP1B1-CYP3A4-UGT1A1-MRP2 quadruple-transfected cell line. Br. J. Pharm. 2013, 169, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Davies, B.; Morris, T. Physiological parameters in laboratory animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef]
- DeSesso, J.M.; Jacobson, C.F. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem. Toxicol. 2001, 39, 209–228. [Google Scholar] [CrossRef]
- Perdaems, N.; Blasco, H.; Vinson, C.; Chenel, M.; Whalley, S.; Cazade, F.; Bouzom, F. Predictions of metabolic drug-drug interactions using physiologically based modelling: Two cytochrome P450 3A4 substrates coadministered with ketoconazole or verapamil. Clin. Pharm. 2010, 49, 239–258. [Google Scholar] [CrossRef]
- Mikkaichi, T.; Nakai, D.; Yoshigae, Y.; Imaoka, T.; Okudaira, N.; Izumi, T. Liver-selective distribution in rats supports the importance of active uptake into the liver via organic anion transporting polypeptides (OATPs) in humans. Drug Metab. Pharm. 2015, 30, 334–340. [Google Scholar] [CrossRef]
- Masuda, N.; Akasaka, I.; Ohtawa, M. Metabolic Fate of Fluvastatin, an Inhibitor of HMG-CoA Reductase (1): Absorption, Distribution and Excretion of [14C] Fluvastatin after Single Administration in Rats. Drug Metab. Pharmacokinet. 1995, 513–528. [Google Scholar]
- Liu, Y.; Zeng, B.H.; Shang, H.T.; Cen, Y.Y.; Wei, H. Bama miniature pigs (Sus scrofa domestica) as a model for drug evaluation for humans: Comparison of in vitro metabolism and in vivo pharmacokinetics of lovastatin. Comp. Med. 2008, 58, 580–587. [Google Scholar] [PubMed]
- Modry, D.L.; Stinson, E.B.; Oyer, P.E.; Jamieson, S.W.; Baldwin, J.C.; Shumway, N.E. Acute rejection and massive cyclosporine requirements in heart transplant recipients treated with rifampin. Transplantation 1985, 39, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Germershausen, J.I.; Hunt, V.M.; Bostedor, R.G.; Bailey, P.J.; Karkas, J.D.; Alberts, A.W. Tissue selectivity of the cholesterol-lowering agents lovastatin, simvastatin and pravastatin in rats in vivo. Biochem. Biophys. Res. Commun. 1989, 158, 667–675. [Google Scholar] [CrossRef]
- Camenisch, G.; Umehara, K. Predicting human hepatic clearance from in vitro drug metabolism and transport data: A scientific and pharmaceutical perspective for assessing drug-drug interactions. Biopharm. Drug Dispos. 2012, 33, 179–194. [Google Scholar] [CrossRef]
- Varma, M.V.; Lin, J.; Bi, Y.A.; Kimoto, E.; Rodrigues, A.D. Quantitative Rationalization of Gemfibrozil Drug Interactions: Consideration of Transporters-Enzyme Interplay and the Role of Circulating Metabolite Gemfibrozil 1-O-beta-Glucuronide. Drug Metab. Dispos. 2015, 43, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Shitara, Y.; Sato, H.; Yoshisue, K.; Hirano, M.; Ikeda, T.; Sugiyama, Y. The quantitative prediction of CYP-mediated drug interaction by physiologically based pharmacokinetic modeling. Pharm. Res. 2008, 25, 1891–1901. [Google Scholar] [CrossRef]
- Gertz, M.; Harrison, A.; Houston, J.B.; Galetin, A. Prediction of human intestinal first-pass metabolism of 25 CYP3A substrates from in vitro clearance and permeability data. Drug Metab. Dispos. 2010, 38, 1147–1158. [Google Scholar] [CrossRef]
- Kim, S.J.; Toshimoto, K.; Yao, Y.; Yoshikado, T.; Sugiyama, Y. Quantitative Analysis of Complex Drug-Drug Interactions Between Repaglinide and Cyclosporin A/Gemfibrozil Using Physiologically Based Pharmacokinetic Models With In Vitro Transporter/Enzyme Inhibition Data. J. Pharm. Sci. 2017, 106, 2715–2726. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Toshimoto, K.; Tomaru, A.; Yoshikado, T.; Tanaka, Y.; Hisaka, A.; Lee, W.; Sugiyama, Y. Physiologically Based Pharmacokinetic Modeling of Bosentan Identifies the Saturable Hepatic Uptake As a Major Contributor to Its Nonlinear Pharmacokinetics. Drug Metab. Dispos. 2018, 46, 740–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetkovic, Z.; Cvijic, S.; Vasiljevic, D. In vitro/in silico approach in the development of simvastatin-loaded self-microemulsifying drug delivery systems. Drug Dev. Ind. Pharm. 2018, 44, 849–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, M.; Houston, J.B.; Galetin, A. Physiologically based pharmacokinetic modeling of intestinal first-pass metabolism of CYP3A substrates with high intestinal extraction. Drug Metab. Dispos. 2011, 39, 1633–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, B.L.; Alberts, J.J.; Posada, M.M.; Rehmel, J.; Kolur, A.; Tham, L.S.; Loghin, C.; Hillgren, K.M.; Hall, S.D.; Dickinson, G.L. Physiologically-Based Pharmacokinetic Modeling of Atorvastatin Incorporating Delayed Gastric Emptying and Acid-to-Lactone Conversion. Cpt. Pharmacomet. Syst. Pharm. 2019, 8, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Zhao, P.; Zhang, L. Physiologically Based Pharmacokinetic (PBPK) Modeling of Pitavastatin and Atorvastatin to Predict Drug-Drug Interactions (DDIs). Eur. J. Drug Metab. Pharm. 2017, 42, 689–705. [Google Scholar] [CrossRef]
- Jones, H.M.; Barton, H.A.; Lai, Y.; Bi, Y.A.; Kimoto, E.; Kempshall, S.; Tate, S.C.; El-Kattan, A.; Houston, J.B.; Galetin, A.; et al. Mechanistic pharmacokinetic modeling for the prediction of transporter-mediated disposition in humans from sandwich culture human hepatocyte data. Drug Metab. Dispos. 2012, 40, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Nishimuta, H.; Sato, K.; Yabuki, M.; Komuro, S. Prediction of the intestinal first-pass metabolism of CYP3A and UGT substrates in humans from in vitro data. Drug Metab. Pharm. 2011, 26, 592–601. [Google Scholar] [CrossRef] [Green Version]
- Varma, M.V.; Lai, Y.; Kimoto, E.; Goosen, T.C.; El-Kattan, A.F.; Kumar, V. Mechanistic modeling to predict the transporter- and enzyme-mediated drug-drug interactions of repaglinide. Pharm. Res. 2013, 30, 1188–1199. [Google Scholar] [CrossRef]
- Wu, X.; Whitfield, L.R.; Stewart, B.H. Atorvastatin transport in the Caco-2 cell model: Contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm. Res. 2000, 17, 209–215. [Google Scholar] [CrossRef]
- Kivisto, K.T.; Zukunft, J.; Hofmann, U.; Niemi, M.; Rekersbrink, S.; Schneider, S.; Luippold, G.; Schwab, M.; Eichelbaum, M.; Fromm, M.F. Characterisation of cerivastatin as a P-glycoprotein substrate: Studies in P-glycoprotein-expressing cell monolayers and mdr1a/b knock-out mice. Naunyn Schmiedebergs Arch. Pharm. 2004, 370, 124–130. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, W.; Huang, Y.; Hein, K.; Hidalgo, I.J. The role of a basolateral transporter in rosuvastatin transport and its interplay with apical breast cancer resistance protein in polarized cell monolayer systems. Drug Metab. Dispos. 2012, 40, 2102–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winiwarter, S.; Bonham, N.M.; Ax, F.; Hallberg, A.; Lennernas, H.; Karlen, A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 1998, 41, 4939–4949. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobian, M.; Haeri, A.; Bolourchian, N.; Shahhosseini, S.; Dadashzadeh, S. An Investigation into the Role of P-Glycoprotein in the Intestinal Absorption of Repaglinide: Assessed by Everted Gut Sac and Caco-2 Cell Line. Iran J. Pharm. Res. 2019, 18, 102–110. [Google Scholar] [PubMed]
- Guo, T.; Qin, H.-x.; Xia, D.-y. Pharmacokinetics and Bioequivalence of Three Preparations of Cyclosporine A in Chinese Healthy Volunteers. Chin. Pharmaceut. J. Beijing 2008, 43, 535. [Google Scholar]
- He, J.-C.; Feng, E.-F.; Zhang, Q.; Yin, Y.-Q.; He, H.-J.; Xu, G.-L. Study on bioequivalence of ciclosporin microemulsion oral solution in healthy volunteers. Pharm. Care Res. 2009, 9, 137–139. [Google Scholar]
- de la Pena, A.; Cui, X.; Geiser, J.; Loghin, C. No Dose Adjustment is Recommended for Digoxin, Warfarin, Atorvastatin or a Combination Oral Contraceptive When Coadministered with Dulaglutide. Clin. Pharm. 2017, 56, 1415–1427. [Google Scholar] [CrossRef]
- Guo, C.X.; Pei, Q.; Yin, J.Y.; Peng, X.D.; Zhou, B.T.; Zhao, Y.C.; Wu, L.X.; Meng, X.G.; Wang, G.; Li, Q.; et al. Effects of Ginkgo biloba extracts on pharmacokinetics and efficacy of atorvastatin based on plasma indices. Xenobiotica 2012, 42, 784–790. [Google Scholar] [CrossRef]
- Hausner, H.; Derving Karsbol, J.; Holst, A.G.; Jacobsen, J.B.; Wagner, F.D.; Golor, G.; Anderson, T.W. Effect of Semaglutide on the Pharmacokinetics of Metformin, Warfarin, Atorvastatin and Digoxin in Healthy Subjects. Clin. Pharm. 2017, 56, 1391–1401. [Google Scholar] [CrossRef] [Green Version]
- Hoch, M.; Hoever, P.; Theodor, R.; Dingemanse, J. Almorexant effects on CYP3A4 activity studied by its simultaneous and time-separated administration with simvastatin and atorvastatin. Eur J. Clin. Pharm. 2013, 69, 1235–1245. [Google Scholar] [CrossRef]
- Kubitza, D.; Becka, M.; Roth, A.; Mueck, W. Absence of clinically relevant interactions between rivaroxaban—An oral, direct Factor Xa inhibitor—And digoxin or atorvastatin in healthy subjects. J. Int Med Res. 2012, 40, 1688–1707. [Google Scholar] [CrossRef] [Green Version]
- DeGorter, M.K.; Urquhart, B.L.; Gradhand, U.; Tirona, R.G.; Kim, R.B. Disposition of atorvastatin, rosuvastatin, and simvastatin in oatp1b2-/- mice and intraindividual variability in human subjects. J. Clin. Pharm. 2012, 52, 1689–1697. [Google Scholar] [CrossRef]
- Blonk, M.; van Beek, M.; Colbers, A.; Schouwenberg, B.; Burger, D. Pharmacokinetic Drug-Drug Interaction Study Between Raltegravir and Atorvastatin 20 mg in Healthy Volunteers. J. Acquir. Immune Defic. Syndr. 2015, 69, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Khalilieh, S.; Yee, K.L.; Sanchez, R.I.; Triantafyllou, I.; Fan, L.; Maklad, N.; Jordan, H.; Martell, M.; Iwamoto, M. Results of a Doravirine-Atorvastatin Drug-Drug Interaction Study. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Ayalasomayajula, S.; Pan, W.; Han, Y.; Yang, F.; Langenickel, T.; Pal, P.; Zhou, W.; Yuan, Y.; Rajman, I.; Sunkara, G. Assessment of Drug-Drug Interaction Potential Between Atorvastatin and LCZ696, A Novel Angiotensin Receptor Neprilysin Inhibitor, in Healthy Chinese Male Subjects. Eur. J. Drug Metab. Pharm. 2017, 42, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.H.; Kim, T.E.; Kim, S.E.; Lee, M.G.; Song, I.S.; Yoon, S.H.; Cho, J.Y.; Jang, I.J.; Shin, S.G.; Yu, K.S. The effect of the newly developed angiotensin receptor II antagonist fimasartan on the pharmacokinetics of atorvastatin in relation to OATP1B1 in healthy male volunteers. J. Cardiovasc. Pharm. 2011, 58, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Muck, W. Clinical pharmacokinetics of cerivastatin. Clin. Pharm. 2000, 39, 99–116. [Google Scholar] [CrossRef]
- Backman, J.T.; Kyrklund, C.; Neuvonen, M.; Neuvonen, P.J. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin. Pharm. 2002, 72, 685–691. [Google Scholar] [CrossRef]
- Bauer, S.; Mwinyi, J.; Stoeckle, A.; Gerloff, T.; Roots, I. Quantification of pravastatin in human plasma and urine after solid phase extraction using high performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 818, 257–262. [Google Scholar] [CrossRef]
- Neuvonen, P.J.; Kantola, T.; Kivisto, K.T. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin. Pharm. 1998, 63, 332–341. [Google Scholar] [CrossRef]
- Kantola, T.; Backman, J.T.; Niemi, M.; Kivisto, K.T.; Neuvonen, P.J. Effect of fluconazole on plasma fluvastatin and pravastatin concentrations. Eur. J. Clin. Pharm. 2000, 56, 225–229. [Google Scholar] [CrossRef]
- Cooper, K.J.; Martin, P.D.; Dane, A.L.; Warwick, M.J.; Schneck, D.W.; Cantarini, M.V. Effect of itraconazole on the pharmacokinetics of rosuvastatin. Clin. Pharm. 2003, 73, 322–329. [Google Scholar] [CrossRef]
- Keskitalo, J.E.; Kurkinen, K.J.; Neuvonen, M.; Backman, J.T.; Neuvonen, P.J.; Niemi, M. No significant effect of ABCB1 haplotypes on the pharmacokinetics of fluvastatin, pravastatin, lovastatin, and rosuvastatin. Br. J. Clin. Pharm. 2009, 68, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Kalafsky, G.; Smith, H.T.; Choc, M.G. High-performance liquid chromatographic method for the determination of fluvastatin in human plasma. J. Chromatogr. 1993, 614, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ayalasomayajula, S.; Han, Y.; Langenickel, T.; Malcolm, K.; Zhou, W.; Hanna, I.; Alexander, N.; Natrillo, A.; Goswami, B.; Hinder, M.; et al. In vitro and clinical evaluation of OATP-mediated drug interaction potential of sacubitril/valsartan (LCZ696). J. Clin. Pharm. 2016, 41, 424–431. [Google Scholar] [CrossRef]
- Harvey, R.D.; Aransay, N.R.; Isambert, N.; Lee, J.S.; Arkenau, T.; Vansteenkiste, J.; Dickinson, P.A.; Bui, K.; Weilert, D.; So, K.; et al. Effect of multiple-dose osimertinib on the pharmacokinetics of simvastatin and rosuvastatin. Br. J. Clin. Pharm. 2018, 84, 2877–2888. [Google Scholar] [CrossRef] [Green Version]
- Chu, N.N.; Chen, W.L.; Xu, H.R.; Li, X.N. Pharmacokinetics and safety of ezetimibe/simvastatin combination tablet: An open-label, single-dose study in healthy Chinese subjects. Clin. Drug Investig. 2012, 32, 791–798. [Google Scholar] [CrossRef]
- Yin, O.Q.; Mak, V.W.; Hu, M.; Fok, B.S.; Chow, M.S.; Tomlinson, B. Impact of CYP2D6 polymorphisms on the pharmacokinetics of lovastatin in Chinese subjects. Eur. J. Clin. Pharm. 2012, 68, 943–949. [Google Scholar] [CrossRef]
- Tornio, A.; Vakkilainen, J.; Neuvonen, M.; Backman, J.T.; Neuvonen, P.J.; Niemi, M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of lovastatin acid. Pharm. Genom. 2015, 25, 382–387. [Google Scholar] [CrossRef]
- Jia, M.M.; Zhou, Y.; He, X.M.; Wu, Y.L.; Li, H.Q.; Chen, H.; Li, W.Y. Development of a liquid chromatography-tandem mass spectrometry method for determination of butoconazole nitrate in human plasma and its application to a pharmacokinetic study. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 431–436. [Google Scholar] [CrossRef]
- Weber, C.; Schmitt, R.; Birnboeck, H.; Hopfgartner, G.; van Marle, S.P.; Peeters, P.A.; Jonkman, J.H.; Jones, C.R. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin. Pharm. 1996, 60, 124–137. [Google Scholar] [CrossRef]
- Li, M.; de Graaf, I.A.; de Jager, M.H.; Groothuis, G.M. P-gp activity and inhibition in the different regions of human intestine ex vivo. Biopharm. Drug Dispos. 2017, 38, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.; Roth, D. Evaluation of fluvastatin in the treatment of hypercholesterolemia in renal transplant recipients taking cyclosporine. Transplantation 1996, 62, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Arnadottir, M.; Eriksson, L.O.; Thysell, H.; Karkas, J.D. Plasma concentration profiles of simvastatin 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitory activity in kidney transplant recipients with and without ciclosporin. Nephron 1993, 65, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Campana, C.; Iacona, I.; Regazzi, M.B.; Gavazzi, A.; Perani, G.; Raddato, V.; Montemartini, C.; Vigano, M. Efficacy and pharmacokinetics of simvastatin in heart transplant recipients. Ann. Pharm. 1995, 29, 235–239. [Google Scholar] [CrossRef]
- Gullestad, L.; Nordal, K.P.; Berg, K.J.; Cheng, H.; Schwartz, M.S.; Simonsen, S. Interaction between lovastatin and cyclosporine A after heart and kidney transplantation. Transpl. Proc. 1999, 31, 2163–2165. [Google Scholar] [CrossRef]
- Gullestad, L.; Nordal, K.P.; Forfang, K.; Ihlen, H.; Hostmark, A.; Berg, K.J.; Cheng, H.; Schwartz, M.S.; Geiran, O.; Simonsen, S. Post-transplant hyperlipidaemia: Low-dose lovastatin lowers atherogenic lipids without plasma accumulation of lovastatin. J. Intern Med. 1997, 242, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Izumi, S.; Nozaki, Y.; Komori, T.; Maeda, K.; Takenaka, O.; Kusano, K.; Yoshimura, T.; Kusuhara, H.; Sugiyama, Y. Substrate-dependent inhibition of organic anion transporting polypeptide 1B1: Comparative analysis with prototypical probe substrates estradiol-17beta-glucuronide, estrone-3-sulfate, and sulfobromophthalein. Drug Metab. Dispos. 2013, 41, 1859–1866. [Google Scholar] [CrossRef] [Green Version]
- Harwood, M.D.; Neuhoff, S.; Carlson, G.L.; Warhurst, G.; Rostami-Hodjegan, A. Absolute abundance and function of intestinal drug transporters: A prerequisite for fully mechanistic in vitro-in vivo extrapolation of oral drug absorption. Biopharm. Drug Dispos. 2013, 34, 2–28. [Google Scholar] [CrossRef]
- Maeda, K.; Ikeda, Y.; Fujita, T.; Yoshida, K.; Azuma, Y.; Haruyama, Y.; Yamane, N.; Kumagai, Y.; Sugiyama, Y. Identification of the rate-determining process in the hepatic clearance of atorvastatin in a clinical cassette microdosing study. Clin. Pharm. 2011, 90, 575–581. [Google Scholar] [CrossRef]
- Tuegel, C.; Bansal, N. Heart failure in patients with kidney disease. Heart 2017, 103, 1848–1853. [Google Scholar] [CrossRef]
- Lee, H.H.; Ho, R.H. Interindividual and interethnic variability in drug disposition: Polymorphisms in organic anion transporting polypeptide 1B1 (OATP1B1; SLCO1B1). Br. J. Clin. Pharm. 2017, 83, 1176–1184. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Liu, C.; Li, J.; Zhang, M.; Hu, M.; Xu, P.; Liu, L.; Liu, X. A mechanistic physiologically based pharmacokinetic-enzyme turnover model involving both intestine and liver to predict CYP3A induction-mediated drug-drug interactions. J. Pharm. Sci. 2013, 102, 2819–2836. [Google Scholar] [CrossRef] [PubMed]
- Englund, G.; Rorsman, F.; Ronnblom, A.; Karlbom, U.; Lazorova, L.; Grasjo, J.; Kindmark, A.; Artursson, P. Regional levels of drug transporters along the human intestinal tract: Co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur. J. Pharm. Sci. 2006, 29, 269–277. [Google Scholar] [CrossRef]
- Peters, S.A.; Jones, C.R.; Ungell, A.L.; Hatley, O.J. Predicting Drug Extraction in the Human Gut Wall: Assessing Contributions from Drug Metabolizing Enzymes and Transporter Proteins using Preclinical Models. Clin. Pharm. 2016, 55, 673–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paine, M.F.; Khalighi, M.; Fisher, J.M.; Shen, D.D.; Kunze, K.L.; Marsh, C.L.; Perkins, J.D.; Thummel, K.E. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J. Pharm. Exp. 1997, 283, 1552–1562. [Google Scholar]
- Mathialagan, S.; Piotrowski, M.A.; Tess, D.A.; Feng, B.; Litchfield, J.; Varma, M.V. Quantitative Prediction of Human Renal Clearance and Drug-Drug Interactions of Organic Anion Transporter Substrates Using In Vitro Transport Data: A Relative Activity Factor Approach. Drug Metab. Dispos. 2017, 45, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, M.; Cartwright, C.M.; Hobbs, M.J.; Kenworthy, K.E.; Rowland, M.; Houston, J.B.; Galetin, A. Cyclosporine inhibition of hepatic and intestinal CYP3A4, uptake and efflux transporters: Application of PBPK modeling in the assessment of drug-drug interaction potential. Pharm. Res. 2013, 30, 761–780. [Google Scholar] [CrossRef]



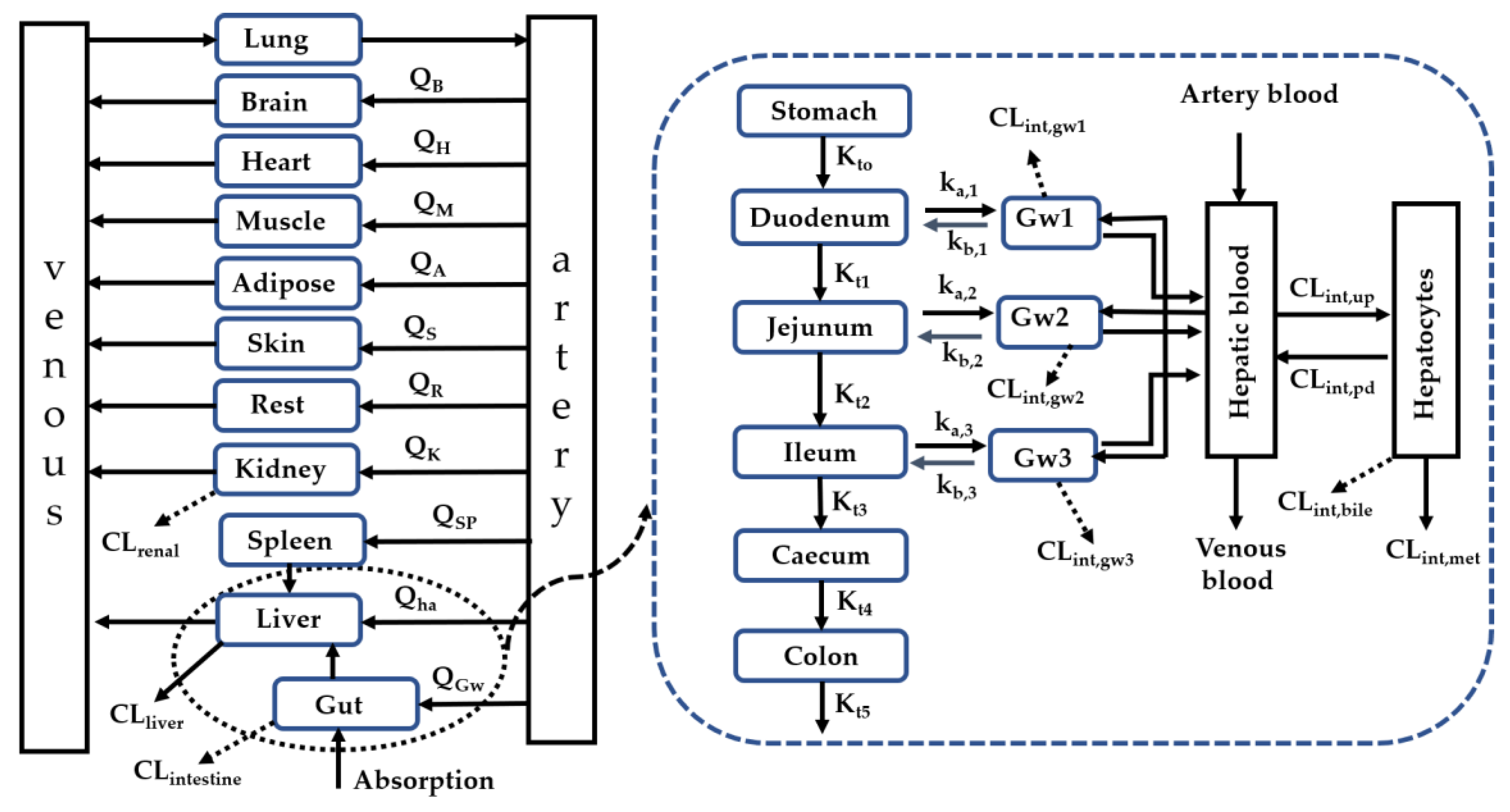
| Tissue | V (mL) [38,39] | Q (mL/min) [38] | Transit Rate Constant (min−1) [40] |
|---|---|---|---|
| Lung | 1170 | 5600 | / |
| Kidney | 280 | 1240 | / |
| Heart | 310 | 240 | / |
| Liver | 1690 | 1518 | / |
| Muscle | 35,000 | 750 | / |
| Skin | 7800 | 300 | / |
| Brain | 1450 | 700 | / |
| Adipose | 10,000 | 260 | / |
| Rob | 5100 | 592 | / |
| Spleen | 190 | 80 | / |
| Artery | 1730 | 5600 | / |
| Venous | 3470 | 5600 | / |
| Stomach | 160 | 38 | 0.0462 |
| Duodenum | 70 | 118 | 0.0462 |
| Jejunum | 209 | 413 | 0.012 |
| Ileum | 139 | 244 | 0.0058 |
| Cecum | 116 | 44 | 0.004 |
| Colon | 1116 | 281 | 0.0013 |
| Tissue | CsA a | Ato a | Cer [41] | Pra a | Ros [41] | Flu [42] | Sim [41] | Lov [43] | Rep [41] | Bos [41] |
|---|---|---|---|---|---|---|---|---|---|---|
| Adipose | 253.35 | 181 | 0.43 a | 0.14 | 0.82 | 0.339 | 0.39 | 298.06 | 0.2 | 0.52 |
| Liver | 4.13 [44] | 4.57 | 51 | 56 [45] | 10.60 | 34.13 | 22.7 | 25.63 | 15.8 | 51 |
| Muscle | 3.67 | 2.64 | 0.2 | 0.21 | 0.20 | 0.147 | 0.219 | 4.69 | 0.302 | 0.23 |
| Lung | 7.75 | 5.6 | 1.13 | 0.36 | 0.40 | 0.407 | 0.426 | 9.06 | 0.604 | 1.01 |
| Kidney | 5.79 | 4.18 | 2.93 | 48.5 [45] | 1.12 | 1.59 | 2.87 | 4.25 | 1.48 | 1.14 |
| Brain | 11.77 | 8.44 | 0.2 | 0.22 | 0.01 | 0.094 | 0.207 | 0.88 | 0.078 | 0.35 |
| Heart | 5.22 | 3.78 | 2 | 0.32 | 0.40 | 0.82 | 0.612 | 11.63 | 0.702 | 0.64 |
| Intestine | 5.99 [44] | 8.97 | 0.2 a | 0.21 | 0.18 a | 8.2 | 20.2 a | 36.2 | 0.07 a | 0.47 a |
| Skin | 18.28 | 13.16 | 0.533 | 0.38 | 0.38 | 0.313 | 0.39 | 21.46 | 0.209 | 0.48 |
| Spleen | 3.36 | 2.43 | 0.733 | 0.26 | 0.23 | 0.23 | 0.292 | 0.31 | 0.346 | 0.52 |
| Stomach | 3.67 | 2.64 | 0.09 | 0.21 | 0.20 a | 1.22 | 20.2 a | 62.69 | 0.07 a | 0.16 a |
| Rest | 5.24 | 1 | 0.01 | 0.11 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| fub b | 0.05 [27] | 0.084 [46] | 0.017 [47] | 0.56 [28] | 0.174 [27] | 0.014 [27] | 0.107 [48] | 0.03 [49] | 0.011 [50] | 0.02 [51] |
| Rb b | 1.36 [27] | 0.61 [27] | 0.76 [47] | 0.839 [28] | 0.69 [27] | 0.57 [27] | 0.56 [52] | 0.57 [53] | 0.62 [50] | 0.6 [51] |
| Drug | CLint met | CLint Uptake | SF | CLint, pd | CLint, bile | |||
|---|---|---|---|---|---|---|---|---|
| CYP3A4 | Other | P-gp | BCRP | MRP2 | ||||
| mL/min | mL/min | mL/min | mL/min | |||||
| CsA | 5432 [46] | / | 10,857 [46] | 0.1 a | 2933 [46] | 637 [46] | / | / |
| Ato | 3469.5 [27] | 612.3 [27] | 6374.7 [54] | 32.6 [27] | 3916.79 [46] | / | 302.4 [55] | / |
| Cer | 979.8 [27] | 1197.6 [27] | 1942.8 [56] | 12.5 [27] | 3541.5 [27] | 40.5 [27] | / | / |
| Pra | / | / | 283.3 [27] | 19.4 [27] | 80.9 [27] | / | / | 80.9 [27] |
| Ros | / | / | 1841.6 [27] | 9.2 [27] | 242.8 [27] | / | 566.6 [27] | / |
| Flu | 2386.3 [56] | 2801.3 [56] | 9106.6 [56] | 21 a | 4047.4 [56] | / | 3440 [56] | / |
| Sim | 231,391 [57] | / | 9234.1 [31] | 25 a | 23,695.5 [31] | 101.2 [31] | / | / |
| Lov | 6893.96 [43] | / | 13,129.8 [31] | 4 a | 11,569.5 [31] | / | / | / |
| Rep | 2503.7 [27] | 6438 [27] | 7184 [27] | 16.9 [58] | 4452.14 [27] | / | 20.24 [27] | / |
| Bos | 1365.14 [27] | / | 7184 [27] | 1.1 [27] | 2023.7 [27] | 1472.6 [51] | / | / |
| Drug | ka | Peff, A-B | Peff, B-A | CLint, gut [53] | ||||
|---|---|---|---|---|---|---|---|---|
| min −1 | cm/min | cm/min | mL/min | |||||
| P-gp | BCRP | MRP2 | Duodenum | Jejunum | Ileum | |||
| CsA | 0.025 [28] | / | 0.01 [28] | / | / | 11.25 | 44.6 | 25.98 |
| Ato | / | 0.0094 [59] | 0.0185 [8] | 0.0118 [8] | 0.011 [8] | 12.2 | 48.273 | 28.22 |
| Cer | / | 0.0133 [47] | 0.0099 [60] | 0.0065 [60] | 0.0118 [60] | / | / | / |
| Pra | 0.021 [28] | / | / | / | 43.7 a | / | / | / |
| Ros | 0.0022 [61] | / | 0.012 [8] | 0.013 [8] | 0.011 [8] | / | / | / |
| Flu | / | 0.038 [62] | 0.035 [8] | 0.026 [8] | 0.029 [8] | / | / | / |
| Sim | / | 0.01 [49] | 0.0093 [49] | / | / | 755.2 | 2991.3 | 1745 |
| Lov | / | 0.016 [49] | / | 0.014 [49] | 871 | 89.8 | 2011 | |
| Rep | 0.04 [58] | 0.0148 [63] | / | / | / | |||
| Bos | / | 0.014 [51] | 5.84 b [51] | / | / | / | ||
| Drug | Ref. | Dose | Cmax | Pred/Obs | AUC | Pred/Obs | AAFE | ||
|---|---|---|---|---|---|---|---|---|---|
| mg | ng/mL | ng·h/mL | |||||||
| Pred | Obs | Pred | Obs | ||||||
| CsA | [64] | 200 | 681.17 | 947.16 | 0.72 | 5456.12 | 7113.47 | 0.77 | 1.5 |
| [64] | 200 | 681.17 | 758.86 | 0.90 | 5456.12 | 6771.87 | 0.81 | 1.5 | |
| [64] | 200 | 681.17 | 938.71 | 0.73 | 5456.12 | 7253.95 | 0.75 | 1.6 | |
| [65] | 500 | 1702.93 | 2480 | 0.69 | 11,984.7 | 16,100 | 0.74 | 1.7 | |
| Ato | [66] | 40 | 14.59 | 19.5 | 0.75 | 71.69 | 82.8 | 0.87 | 2.3 |
| [67] | 40 | 14.59 | 20.22 | 0.72 | 71.77 | 87.48 | 0.82 | 1.8 | |
| [68] | 40 | 13.5 | 9.45 | 1.43 | 73.18 | 89.66 | 0.82 | 2.1 | |
| [69] | 40 | 14.59 | 11.6 | 1.26 | 66.70 | 69.4 | 0.96 | 1.6 | |
| [70] | 20 | 6.75 | 4.7 | 1.44 | 35.78 | 47.4 | 0.75 | 2.5 | |
| [71] | 20 | 7.29 | 8.9 | 0.82 | 32.65 | 34.5 | 0.95 | 1.7 | |
| [72] | 20 | 7.29 | 9.28 | 0.79 | 34.22 | 43.7 | 0.78 | 1.7 | |
| [73] | 20 | 6.75 | 7.87 | 0.86 | 37.25 | 39.9 | 0.93 | 1.7 | |
| [74] | 80 | 29.18 | 38.07 | 0.77 | 136.39 | 182.96 | 0.75 | 1.5 | |
| [75] | 80 | 29.18 | 88.9 | 0.33 | 143.5 | 302.5 | 0.47 | 1.9 | |
| Cer | [76] | 0.1 | 1.13 | 1.28 | 0.88 | 6.06 | 6.49 | 0.93 | 1.3 |
| [76] | 0.2 | 2.26 | 2.61 | 0.87 | 13.13 | 13.1 | 1.00 | 1.2 | |
| [77] | 0.3 | 3.4 | 3.2 | 1.06 | 21.07 | 20.9 | 1.01 | 1.7 | |
| [76] | 0.4 | 4.53 | 3.66 | 1.24 | 26.25 | 24.6 | 1.07 | 2.3 | |
| Pra | [78] | 40 | 50.07 | 53.23 | 0.95 | 129.86 | 133.63 | 0.97 | 1.3 |
| [79] | 40 | 50.07 | 36.9 | 1.37 | 127.98 | 93.69 | 1.37 | 1.4 | |
| [80] | 40 | 50.07 | 49.5 | 1.02 | 118.62 | 109 | 1.09 | 1.2 | |
| Ros | [20] | 10 | 6.3 | 4.58 | 1.38 | 34.04 | 40.1 | 0.85 | 2.1 |
| [81] | 10 | 6.3 | 5.8 | 1.09 | 34.04 | 45.9 | 0.74 | 2.6 | |
| [81] | 80 | 50.42 | 53.5 | 0.94 | 272.75 | 397 | 0.69 | 2.0 | |
| Flu | [82] | 20 | 91.03 | 121.6 | 0.75 | 264.62 | 199.5 | 1.33 | 2.7 |
| [82] | 20 | 91.03 | 112.9 | 0.81 | 264.62 | 192.5 | 1.37 | 2.1 | |
| [83] | 20 | 91.03 | 119.06 | 0.76 | 247.18 | 144.62 | 1.71 | 1.9 | |
| Sim | [84] | 40 | 3.87 | 11.5 | 0.34 | 16.48 | 26.5 | 0.62 | 4.2 |
| [85] | 40 | 3.87 | 4.3 | 0.90 | 16.48 | 29.3 | 0.56 | 7.7 | |
| [86] | 40 | 3.87 | 3.37 | 1.15 | 16.48 | 32.50 | 0.51 | 4.1 | |
| Lov | [87] | 40 | 4.91 | 5.00 | 0.98 | 20.20 | 29.2 | 0.69 | 2.7 |
| [82] | 20 | 2.46 | 2.2 | 1.12 | 10.44 | 15.5 | 0.67 | 1.9 | |
| [88] | 40 | 4.91 | 4.06 | 1.21 | 22.40 | 34.1 | 0.66 | 2.6 | |
| Rep | [89] | 2 | 15.7 | 15.28 | 1.03 | 31.19 | 34.77 | 0.90 | 1.4 |
| [23] | 0.25 | 1.97 | 3.99 | 0.49 | 4.15 | 4.44 | 0.93 | 1.4 | |
| [10] | 0.5 | 3.92 | 5.22 | 0.75 | 7.96 | 6.36 | 1.25 | 1.5 | |
| Bos | [90] | 100 | 1689.97 | 1786 | 0.95 | 11,365.74 | 8180 | 1.39 | 1.9 |
| [90] | 300 | 5074.94 | 5000 | 1.01 | 33,714.30 | 18,450 | 1.83 | 2.8 | |
| [90] | 600 | 10,163.50 | 9987 | 1.02 | 67,476.63 | 41,480 | 1.63 | 2.6 | |
| Drug. | Dose | CsA Dose | Cmax-with | Cmax-without | CmaxR | AUC-with | AUC-without | AUCR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg | mg | ng/mL | ng/mL | ng·h/mL | ng·h/mL | |||||||||
| Pred | Obs | Pred | Obs | Pred | Obs | Pred | Obs | Pred | Obs | Pred | Obs | |||
| Ato | 10 [14,16] | 886 BID 28-day | 28.5 | 37.3 | 3.31 | 3.5 | 8.61 | 10.66 | 123.22 | 226 | 17.21 | 26 | 7.16 | 8.69 |
| 40 [15] | 175 | 74.3 | 362 | 14.6 | 26.5 | 5.10 | 13.66 | 204.78 | 1026 | 49.84 | 67 | 4.11 | 15.31 | |
| Cer | 0.2 [17] | 225 BID 1 day | 4.02 | 7.8 | 2.26 | 1.56 | 1.78 | 5.00 | 27.4 | 36.2 | 9.5 | 9.53 | 2.88 | 3.80 |
| 225 BID 7-day | 5.11 | 7.82 | 2.26 | 1.56 | 2.26 | 5.01 | 49.27 | 45.3 | 9.5 | 9.53 | 5.19 | 4.75 | ||
| Pra | 20 [18] | 420 BID 1 day | 91.2 | 84 | 25.35 | 23.27 | 3.60 | 3.61 | 211.33 | 249 | 62.74 | 66.9 | 3.37 | 4.44 |
| 420 BID 28-day | 95.44 | 80 | 25.35 | 23.27 | 3.76 | 3.44 | 243.02 | 241 | 62.74 | 66.9 | 3.87 | 4.30 | ||
| 10 [19] | 400 BID 1 day | 53.96 | 96 | 12.68 | 13.7 | 4.26 | 7.00 | 126.39 | 307.05 | 31.37 | 26.25 | 4.03 | 11.70 | |
| 400 BID 8-day | 55.82 | 98 | 12.68 | 13.7 | 4.40 | 7.15 | 141.86 | 303.52 | 31.37 | 26.25 | 4.52 | 11.56 | ||
| 400 BID 29-day | 56.3 | 115.1 | 12.68 | 13.7 | 4.44 | 8.40 | 143.95 | 345.9 | 31.37 | 26.25 | 4.59 | 13.18 | ||
| Ros | 10 [20] | 200 BID 1 day | 15.86 | 39.8 | 6.3 | 4.58 | 2.52 | 8.69 | 62.56 | 197 | 34 | 40.1 | 1.84 | 4.91 |
| 200 BID 10-day | 18.47 | 48.7 | 6.3 | 4.58 | 2.93 | 10.63 | 81.9 | 284 | 34 | 40.1 | 2.41 | 7.06 | ||
| 20 [20] | 200 BID 1 day | 31.7 | 66.5 | \ | \ | \ | \ | 125.11 | 308 | \ | \ | \ | \ | |
| 200 BID 10-day | 36.94 | 83.0 | \ | \ | \ | \ | 163.81 | 424 | \ | \ | \ | \ | ||
| Flu | 40 [21] | 200 BID 1 day | 622.96 | 869.4 | 182.06 | 211.9 | 3.42 | 4.10 | 1667.4 | 1948.8 | 494.4 | 549.4 | 3.37 | 3.55 |
| 200 BID 28-day | 646.87 | 1530 | 190.14 | 254.7 | 3.40 | 6.01 | 2062.23 | 2615.3 | 640 | 841.8 | 3.22 | 3.11 | ||
| 20 [92] | 200 BID 28-day | 314 | 155 | \ | \ | \ | \ | 1056 | 373 | \ | \ | \ | \ | |
| Sim | 20 [93] | 266 BID | 13.91 | 20.6 | 1.9 | 9.9 | 7.32 | 2.08 | 28.84 | 101 | 8.08 | 39.6 | 3.57 | 2.55 |
| 10 [94] | 1h 200 BID a | 18.77 | 6.8 | 1.65 | 2 b | 11.38 | 3.40 | |||||||
| 2 h 200 BID a | 11.56 | 8.4 | 1.83 | 2 b | 6.32 | 4.20 | ||||||||
| 3 h 200 BID a | 4.56 | 12.1 | 1.44 | 2 b | 3.17 | 6.05 | ||||||||
| Lov | 20 [18] | 420 BID 1 day | 30.58 | 46 | 2.46 | 2.2 | 12.43 | 20.91 | 113.98 | 243 | 10.44 | 15.5 | 10.92 | 15.68 |
| 420 BID 28-day | 33.12 | 75 | 2.46 | 2.2 | 13.46 | 34.09 | 164.89 | 459 | 10.44 | 15.5 | 15.79 | 29.61 | ||
| 10 [95] | 273 BID 10-day c | 31.33 | 175 | 3.89 | 26 | 8.05 | 6.73 | |||||||
| 230 BID 10-day c | 27.87 | 110 | 3.89 | 26 | 7.16 | 4.23 | ||||||||
| 290 BID 10-day c | 32.64 | 110 | 3.89 | 26 | 8.39 | 4.23 | ||||||||
| 5 [96] | 300 d | 5.09 | 4.05 | 1 | 4.6 | 5.09 | 0.88 | |||||||
| 5 | 300 d | 5.93 | 11.47 | 1 | 4.6 | 5.93 | 2.49 | |||||||
| 10 | 300 d | 12.27 | 21.08 | 1 | 4.6 | 12.27 | 4.58 | |||||||
| 15 | 300 d | 18.91 | 25.25 | 1 | 4.6 | 18.91 | 5.49 | |||||||
| 20 | 300 d | 24.77 | 19.79 | 1 | 4.6 | 24.77 | 4.30 | |||||||
| 20 | 300 d | 25.7 | 19.05 | 1 | 4.6 | 25.70 | 4.14 | |||||||
| Rep | 0.25 [23] | 100 | 4.64 | 6.7 | 1.97 | 3.9 | 2.36 | 1.72 | 10.38 | 10.82 | 4.15 | 4.44 | 2.50 | 2.44 |
| Bos e | 500 [24] | 300 BID | 18.55 | 7.92 | 8.47 | 4.74 | 2.19 | 1.67 | 189.49 | 48.9 | 50.44 | 24.78 | 3.76 | 1.97 |
| Factors | Tmax | Cmax | AUC |
|---|---|---|---|
| h | ng/mL | ng·h/mL | |
| DDI Part | |||
| Whole | 1.17 | 75.99 | 264.82 |
| Ki (2-fold) | 1.17 | 53.65 | 185.75 |
| Ki (0.5-fold) | 1.17 | 103.21 | 378.30 |
| CLint,up (2-fold) | 1.17 | 48.13 | 154.69 |
| CLint,up (0.5-fold) | 1.33 | 107.23 | 419.24 |
| CsA dosage (100 mg) | 1.17 | 51.48 | 178.84 |
| CsA dosage (400 mg) | 1.33 | 106.77 | 392.31 |
| Kt (2-fold) | 0.83 | 82.94 | 228.64 |
| Kt (0.5-fold) | 1.67 | 60.31 | 266.78 |
| SF (2-fold) | 1.17 | 48.13 | 154.69 |
| SF (0.5-fold) | 1.33 | 107.23 | 419.24 |
| non-CYP3A4 | 1.17 | 68.91 | 240.63 |
| non-liver | 1.33 | 19.23 | 77.52 |
| non-gut | 1.17 | 55.96 | 214.06 |
| Alone Part | |||
| Whole | 1.50 | 14.59 | 66.88 |
| non-liver CYP3A4 | 2.33 | 25.23 | 172.17 |
| non-liver BCRP | 1.50 | 14.96 | 69.11 |
| non-gut CYP3A4 | 1.50 | 15.91 | 74.17 |
| non-gut P-gp | 1.50 | 15.76 | 69.35 |
| non-gut-BCRP | 1.50 | 15.48 | 68.67 |
| non-gut-MRP2 | 1.33 | 15.63 | 68.80 |
| non-gut | 1.33 | 20.36 | 82.16 |
| non-liver-OATP | 1.67 | 136.13 | 1077.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, P.; Zhang, Z.; Wang, Z.; Liu, L.; Liu, X. Prediction of Cyclosporin-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Model Characterizing Interplay of Drug Transporters and Enzymes. Int. J. Mol. Sci. 2020, 21, 7023. https://doi.org/10.3390/ijms21197023
Yang Y, Li P, Zhang Z, Wang Z, Liu L, Liu X. Prediction of Cyclosporin-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Model Characterizing Interplay of Drug Transporters and Enzymes. International Journal of Molecular Sciences. 2020; 21(19):7023. https://doi.org/10.3390/ijms21197023
Chicago/Turabian StyleYang, Yiting, Ping Li, Zexin Zhang, Zhongjian Wang, Li Liu, and Xiaodong Liu. 2020. "Prediction of Cyclosporin-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Model Characterizing Interplay of Drug Transporters and Enzymes" International Journal of Molecular Sciences 21, no. 19: 7023. https://doi.org/10.3390/ijms21197023
APA StyleYang, Y., Li, P., Zhang, Z., Wang, Z., Liu, L., & Liu, X. (2020). Prediction of Cyclosporin-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Model Characterizing Interplay of Drug Transporters and Enzymes. International Journal of Molecular Sciences, 21(19), 7023. https://doi.org/10.3390/ijms21197023





