Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models
Abstract
:1. Introduction
2. Results
2.1. HH Exhibited Anti-Inflammatory Effect
2.2. Identification of Atraric Acid
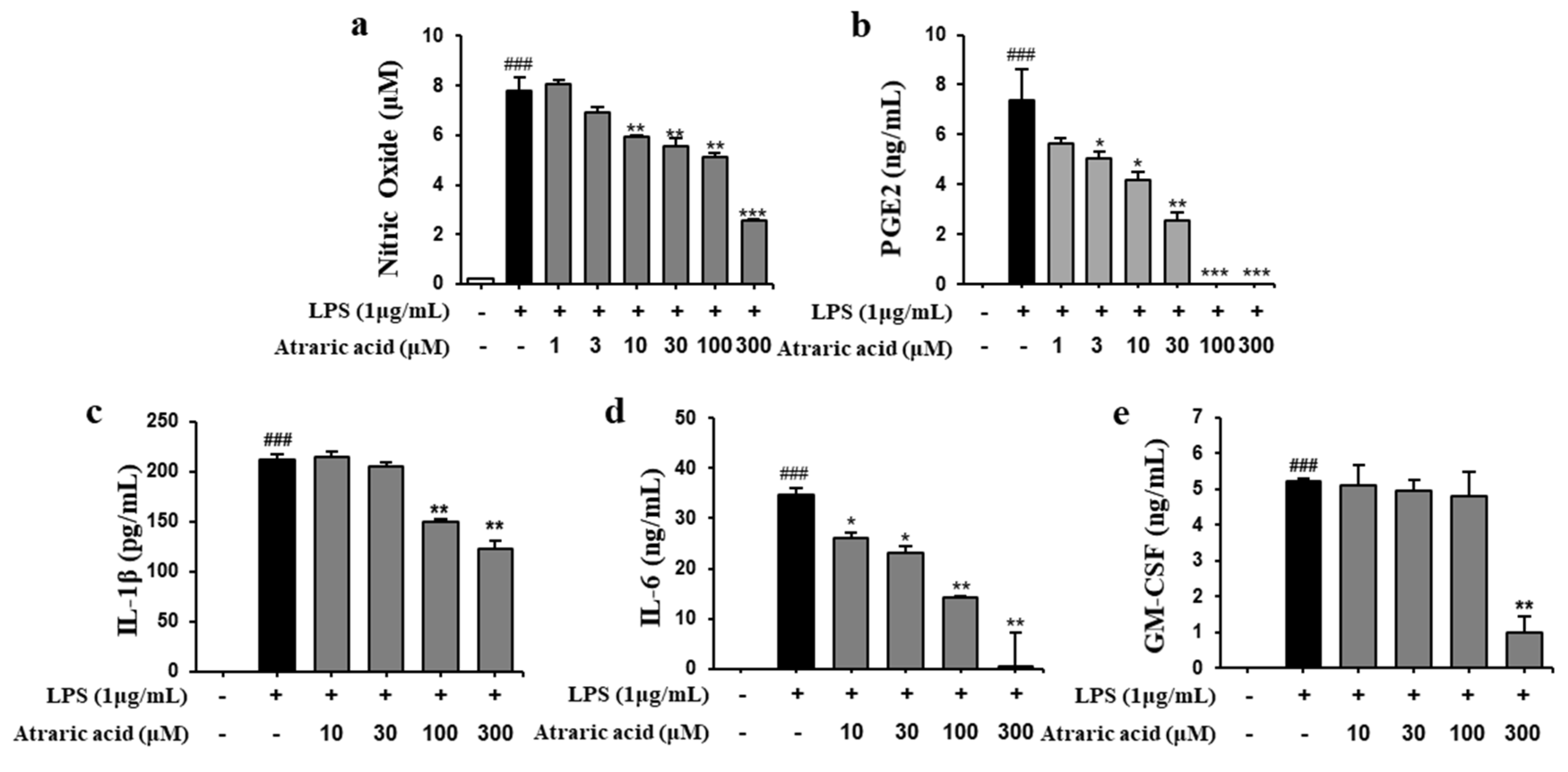
2.3. Atraric Acid Inhibited the Production of NO and Pro-Inflammatory Cytokines
2.4. Atraric Acid Inhibited LPS-Induced Expression of iNOS and COX-2
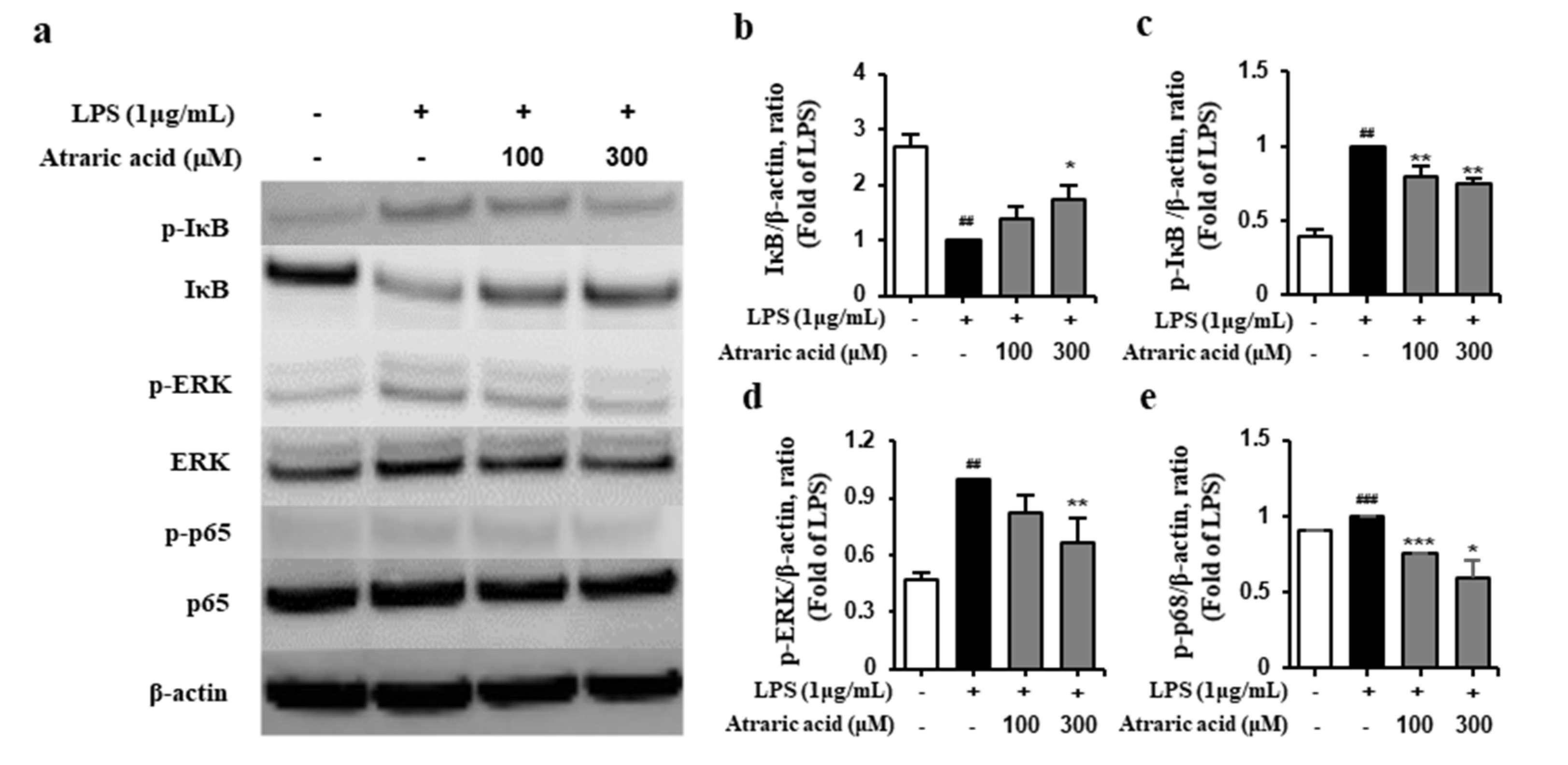
2.5. Atraric Acid Suppressed LPS-Stimulated Phosphorylation of the Nfκb Signaling Pathway
2.6. Atraric Acid Exhibited Anti-Inflammatory Effects on LPS-Induced Endotoxin Shock in Mice
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of the Lichen
4.2. Chemicals and Reagents
4.3. Isolation and Analysis
4.4. Instruments and Data Collection
4.5. Cell Culture
4.6. Cell Viability Assay
4.7. Measurement of NO and Cytokines
4.8. Western Blot Analysis
4.9. Animals and Experimental Design
4.10. Histopathological Examination
4.11. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| HH | Heterodermia hypoleuca |
| NFκB | nuclear factor kappa B |
| NO | nitric oxide |
| COX-2 | cyclooxygenase-2 |
| PGE2 | prostaglandin E2 |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| TLR-4 | toll-like receptor 4 |
| CCK-8 | cell counting kit-8 |
| i.p. | intraperitoneal |
References
- Lind, L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis 2003, 169, 203–214. [Google Scholar] [CrossRef]
- Bertolini, A.; Ottani, A.; Sandrini, M. Dual acting anti-inflammatory drugs: A reappraisal. Pharmacol. Res. 2001, 44, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Lee, S.J.; Kang, K.Y.; Hur, J.S.; Yee, S.T. Immunosuppressive effects of Bryoria sp. (Lichen-Forming Fungus) extracts via inhibition of CD8+ T-Cell proliferation and IL-2 production in CD4+ T Cells. J. Microbiol. Biotechnol. 2017, 27, 1189–1197. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.H.; So, Y.; Kang, S.Y.; Jeong, H.G.; Jin, C.H. Anti-inflammatory effect of Lupinalbin A isolated from Apios americana on lipopolysaccharide-treated RAW264.7 cells. Molecules 2018, 23, 583. [Google Scholar] [CrossRef] [Green Version]
- Marletta, M.A. Nitric oxide synthase structure and mechanism. J. Biol. Chem. 1993, 268, 12231–12234. [Google Scholar] [PubMed]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine-production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.M.; Amin, A.R.; Abramson, S.B. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998, 41, 1141–1151. [Google Scholar] [CrossRef]
- Jo, A.; Yoo, H.J.; Lee, M. Robustaflavone isolated from Nandina domestica using bioactivity-guided fractionation downregulates inflammatory mediators. Molecules 2019, 24. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.O.; So, Y.; Jin, C.H.; Nam, B.M.; Yee, S.T.; Jeong, I.Y. 3-deoxysilybin exerts anti-inflammatory effects by suppressing NF-κB activation in lipopolysaccharide-stimulated RAW264.7 macrophages. Biosci. Biotechnol. Biochem. 2001, 78, 2051–2058. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Pang, H.; Chen, Y.; Huang, L.; Liu, H.; Zheng, Y.; Sun, C.; Zhang, G.; Wang, G. Anti-inflammatory effect of a polyphenol-enriched fraction from Acalypha wilkesiana on lipopolysaccharide-stimulated RAW 264.7 macrophages and acetaminophen-induced liver injury in Mice. Oxid. Med. Cell Longev. 2018, 7858094. [Google Scholar] [CrossRef] [Green Version]
- Abarca-Vargas, R.; Petricevich, V.L. Extract from Bougainvillea xbuttiana (Variety Orange) inhibits production of LPS-induced inflammatory mediators in macrophages and exerts a protective effect in vivo. Biomed. Res. Int. 2019, 2034247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Kang, K.Y.; Hwang, Y.H.; Jeong, M.H.; Hur, J.S.; Yee, S.T. Induction of apoptosis in HL-60 cells treated with the extract of lichen forming fungus, Hemithecium oryzaeforme (Fée) Staiger. IJARNP 2017, 10, 25–31. [Google Scholar]
- Huneck, S. The significance of lichens and their metabolites. Naturwissenschaften 1999, 86, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Huneck, S.; Yoshimura, I. Identification of lichen substances. Identif. Lichen Subst. 1999, 11–123. [Google Scholar]
- Huneck, S. New results on the chemistry of lichen substances. Fortschr. Chem. Org. Naturst. 2001, 81, 1–276. [Google Scholar]
- Hessenkemper, W.; Roediger, J.; Bartsch, S.; Houtsmuller, A.B.; Van-royen, M.E.; Petersen, I.; Grimm, M.O.; Baniahmad, A. A natural androgen receptor antagonist induces cellular senescence in prostate cancer cells. Mol. Endocrinol. 2014, 28, 1831–1840. [Google Scholar] [CrossRef] [Green Version]
- Güvenç, A.; Küpeli Akkol, E.; Süntar, I.; Keleş, H.; Yıldız, S.; Calış, I. Biological activities of Pseudevernia furfuracea (L.) Zopf extracts and isolation of the active compounds. J. Ethnopharmacol. 2012, 144, 726–734. [Google Scholar] [CrossRef]
- Ahad, A.M.; Goto, Y.; Kiuchi, F.; Tsuda, Y.; Kondo, K.; Sato, T. Nematocidal principles in ‘‘oakmoss absolute’’ and nematocidal activity of 2,4-dihydroxybenzoates. Chem. Pharm. Bull. 1991, 39, 1043–1046. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Chang, S.M.; Chen, C.H. Chemical constituents from Alseodaphne andersonii. Nat. Prod. 2001, 64, 1548–1551. [Google Scholar] [CrossRef]
- Gormann, R.; Kaloga, M.; Li, X.C.; Ferreira, D.; Bergenthal, D.; Kolodziej, H. Furanonaphthoquinones, atraric acid and a benzofuran from the stem barks of Newbouldia laevis. Phytochemistry 2003, 64, 583–587. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Choi, H.; Jo, A.; Kang, H.; Yun, H.; Im, S.; Choi, C. Anti-inflammatory effects of a Stauntonia hexaphylla Fruit extract in lipopolysaccharide-activated RAW264.7 macrophages and Rats by Carrageenan-induced hind paw swelling. Nutrients 2018, 10. [Google Scholar]
- Park, J.; Kwak, C.H.; Ha, S.H.; Kwon, K.M.; Abekura, F.; Cho, S.H.; Chang, Y.C.; Lee, Y.C.; Ha, K.T.; Chung, T.W.; et al. Ganglioside GM3 suppresses lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophage cells through NF-κB, AP-1, and MAPKs signaling. J. Cell Biochem. 2018, 119, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Ro, M.; Kim, J.H. Mediatory roles of leukotriene B4 receptors in LPS-induced endotoxic shock. Sci. Rep. 2019, 9, 5936. [Google Scholar] [CrossRef]
- Fu, S.; Lu, W.; Yu, W.; Hu, J. Protective effect of Cordyceps sinensis extract on lipopolysaccharide-induced acute lung injury in mice. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive compounds in Moringa oleifera Lam. leaves inhibit the pro-inflammatory mediators in lipopolysaccharide-induced human monocyte-derived macrophages. Molecules 2020, 25. [Google Scholar] [CrossRef] [Green Version]
- Yuan, R.; Huang, L.; Du, L.J.; Feng, J.F.; Li, J.; Luo, Y.Y.; Xu, Q.M.; Yang, S.L.; Gao, H.; Feng, Y.L. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacol. Res. 2019, 142, 102–114. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, H.Y.; Liu, G.M.; Ni, W.H.; Wang, F.; Tai, G.X. Escherichia coli Maltose-Binding protein induces M1 polarity of RAW264.7 macrophage cells via a TLR2- and TLR4-dependent manner. Int. J. Mol. Sci. 2015, 16, 9896–9909. [Google Scholar] [CrossRef]
- Jeong, J.B.; Shin, Y.K.; Lee, S.H. Anti-inflammatory activity of patchouli alcohol in RAW264.7 and HT-29 cells. Food Chem. Toxicol. 2013, 55, 229–233. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Zhou, Y.; Li, M.; Yang, H.; Mu, L.; Qian, Q.; Wu, J.; Xu, W. Anti-inflammatory activities of Aedes aegypti cecropins and their protection against murine endotoxin shock. Parasit. Vectors 2018, 11, 470. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Hong, S.G.; Mun, S.K.; Kim, S.J.; Lee, S.J.; Kim, J.J.; Kang, K.Y.; Yee, S.T. The protective effects of astaxanthin on the OVA-induced asthma mice model. Molecules 2017, 22. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, S.-K.; Kang, K.-Y.; Jang, H.-Y.; Hwang, Y.-H.; Hong, S.-G.; Kim, S.-J.; Cho, H.-W.; Chang, D.-J.; Hur, J.-S.; Yee, S.-T. Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models. Int. J. Mol. Sci. 2020, 21, 7070. https://doi.org/10.3390/ijms21197070
Mun S-K, Kang K-Y, Jang H-Y, Hwang Y-H, Hong S-G, Kim S-J, Cho H-W, Chang D-J, Hur J-S, Yee S-T. Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models. International Journal of Molecular Sciences. 2020; 21(19):7070. https://doi.org/10.3390/ijms21197070
Chicago/Turabian StyleMun, Seul-Ki, Kyung-Yun Kang, Ho-Yeol Jang, Yun-Ho Hwang, Seong-Gyeol Hong, Su-Jin Kim, Hyun-Wook Cho, Dong-Jo Chang, Jae-Seoun Hur, and Sung-Tae Yee. 2020. "Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models" International Journal of Molecular Sciences 21, no. 19: 7070. https://doi.org/10.3390/ijms21197070
APA StyleMun, S. -K., Kang, K. -Y., Jang, H. -Y., Hwang, Y. -H., Hong, S. -G., Kim, S. -J., Cho, H. -W., Chang, D. -J., Hur, J. -S., & Yee, S. -T. (2020). Atraric Acid Exhibits Anti-Inflammatory Effect in Lipopolysaccharide-Stimulated RAW264.7 Cells and Mouse Models. International Journal of Molecular Sciences, 21(19), 7070. https://doi.org/10.3390/ijms21197070




