Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination
Abstract
1. Introduction
2. Results and Discussion
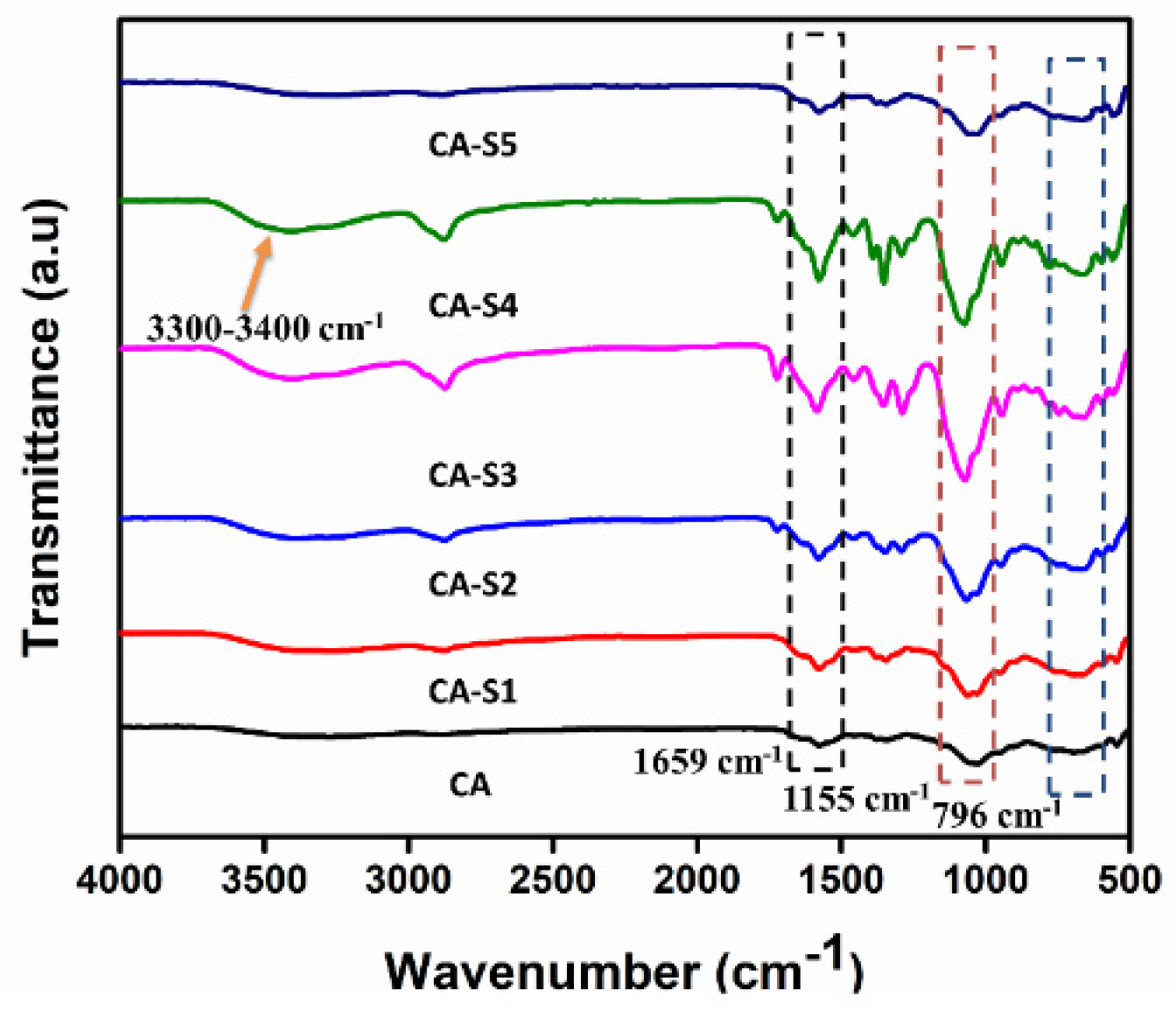
2.1. FTIR Analysis
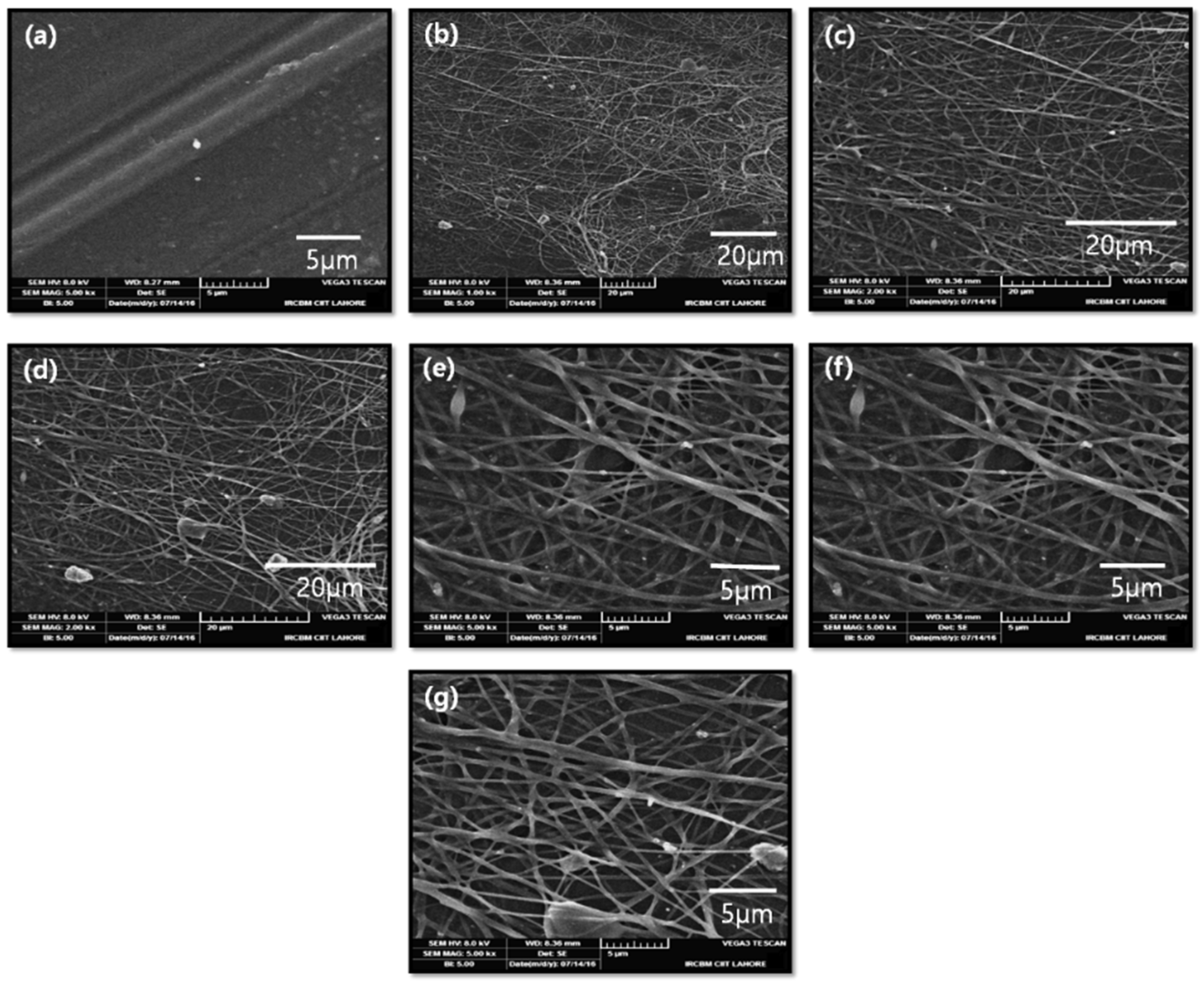
2.2. Scanning Electron Microscopy
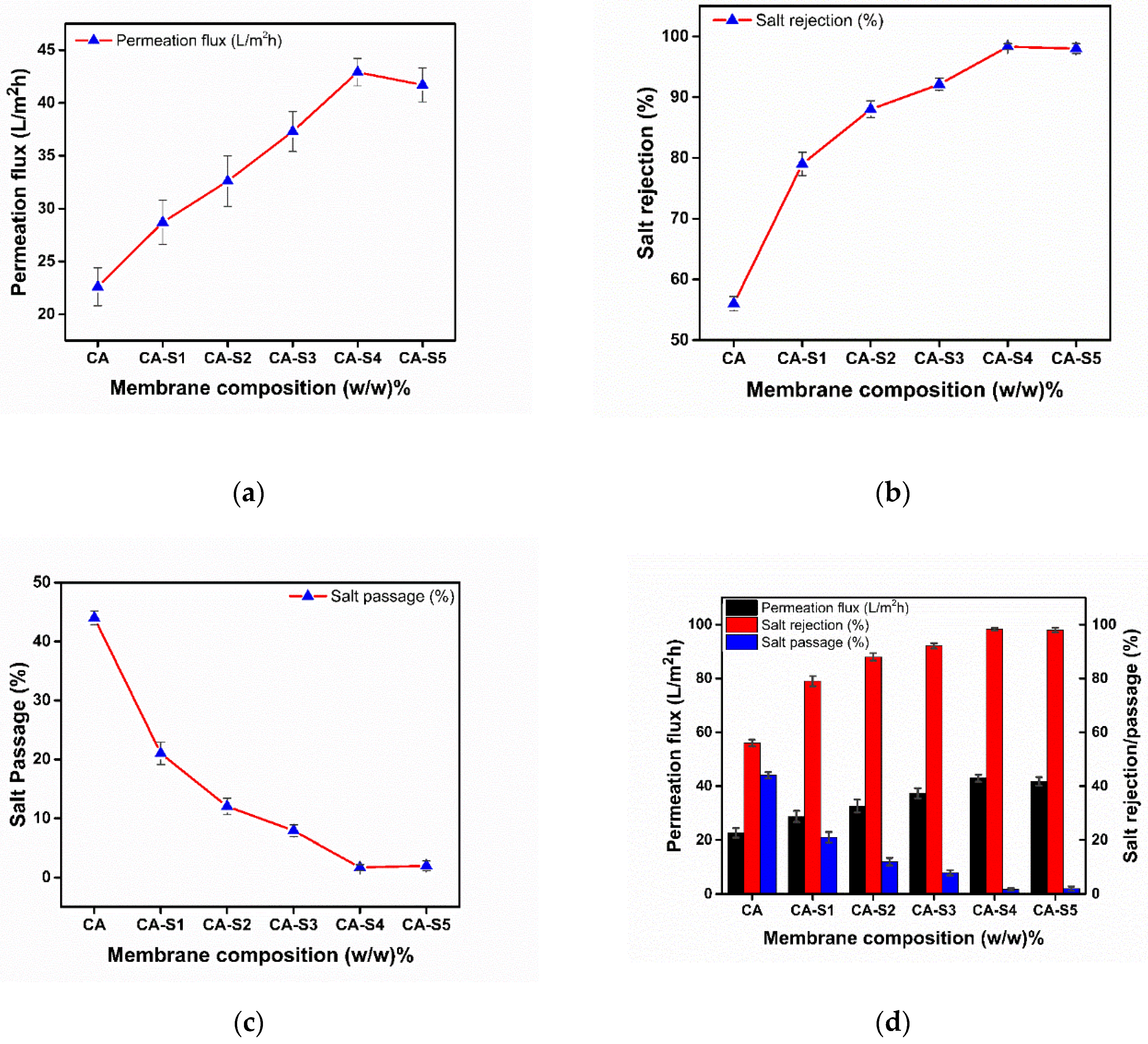
2.3. Desalination Performance of Membranes
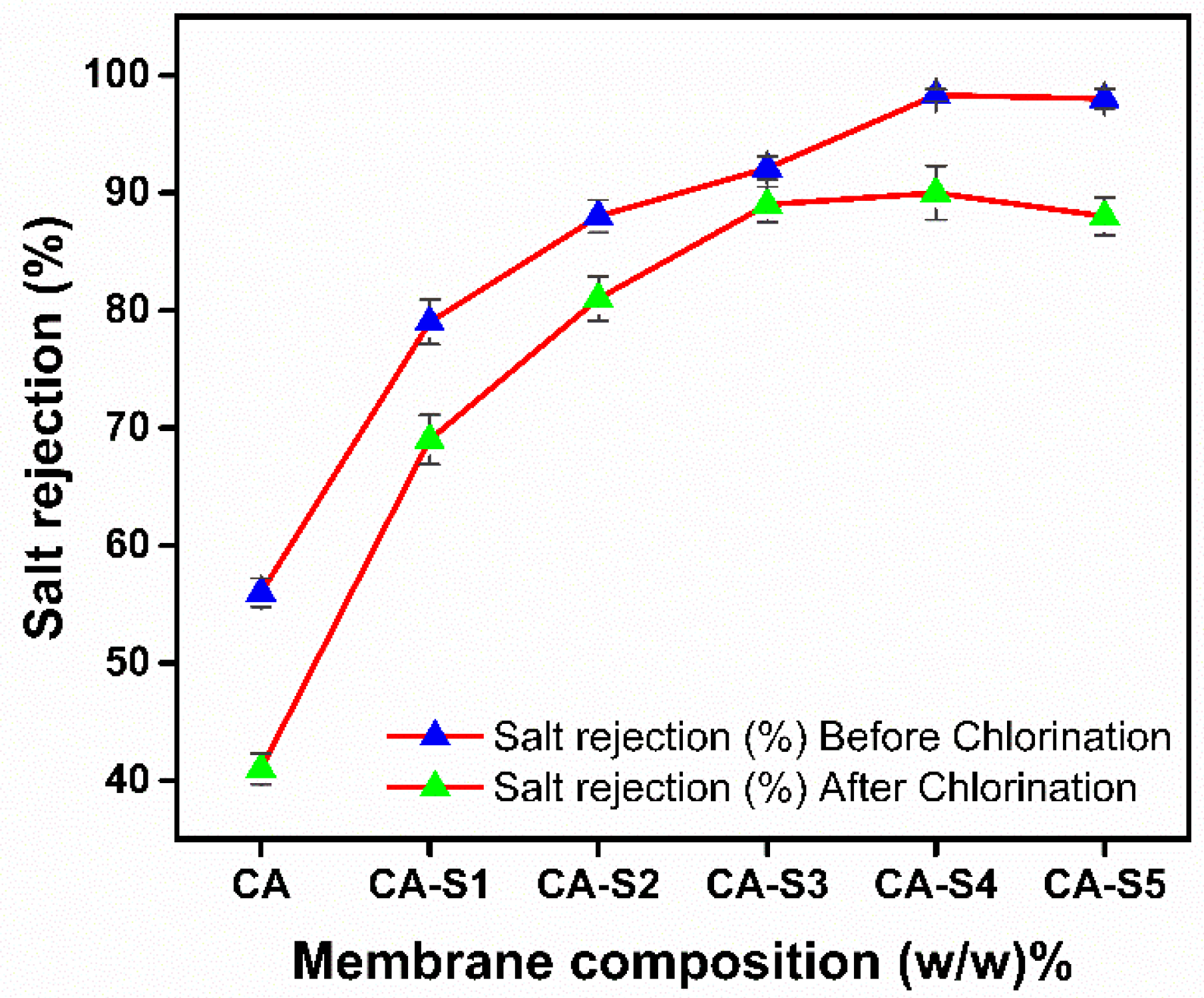
2.4. Membrane Chlorine Resistance
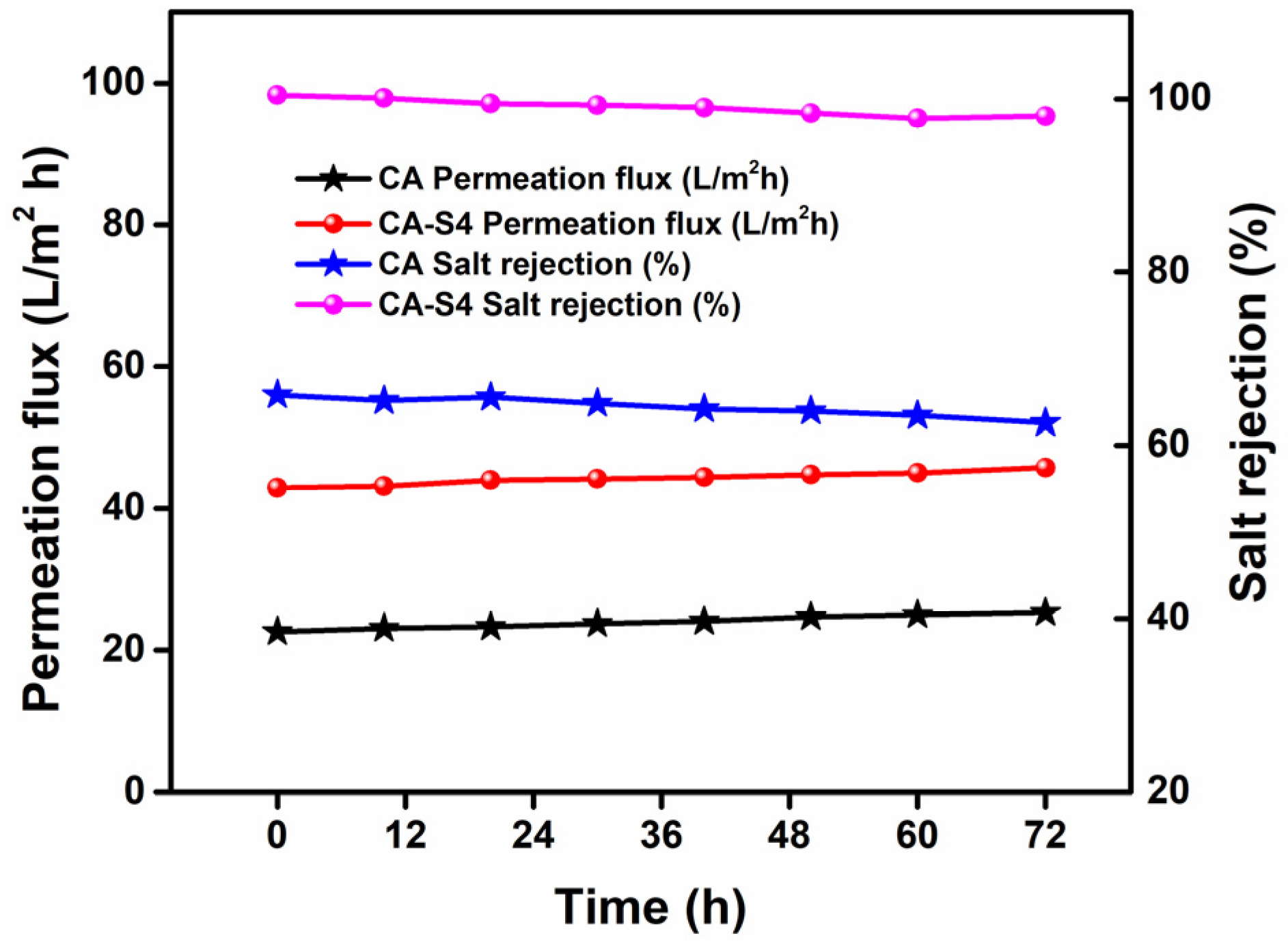
2.5. Stability Tests
2.6. Fouling Resistance
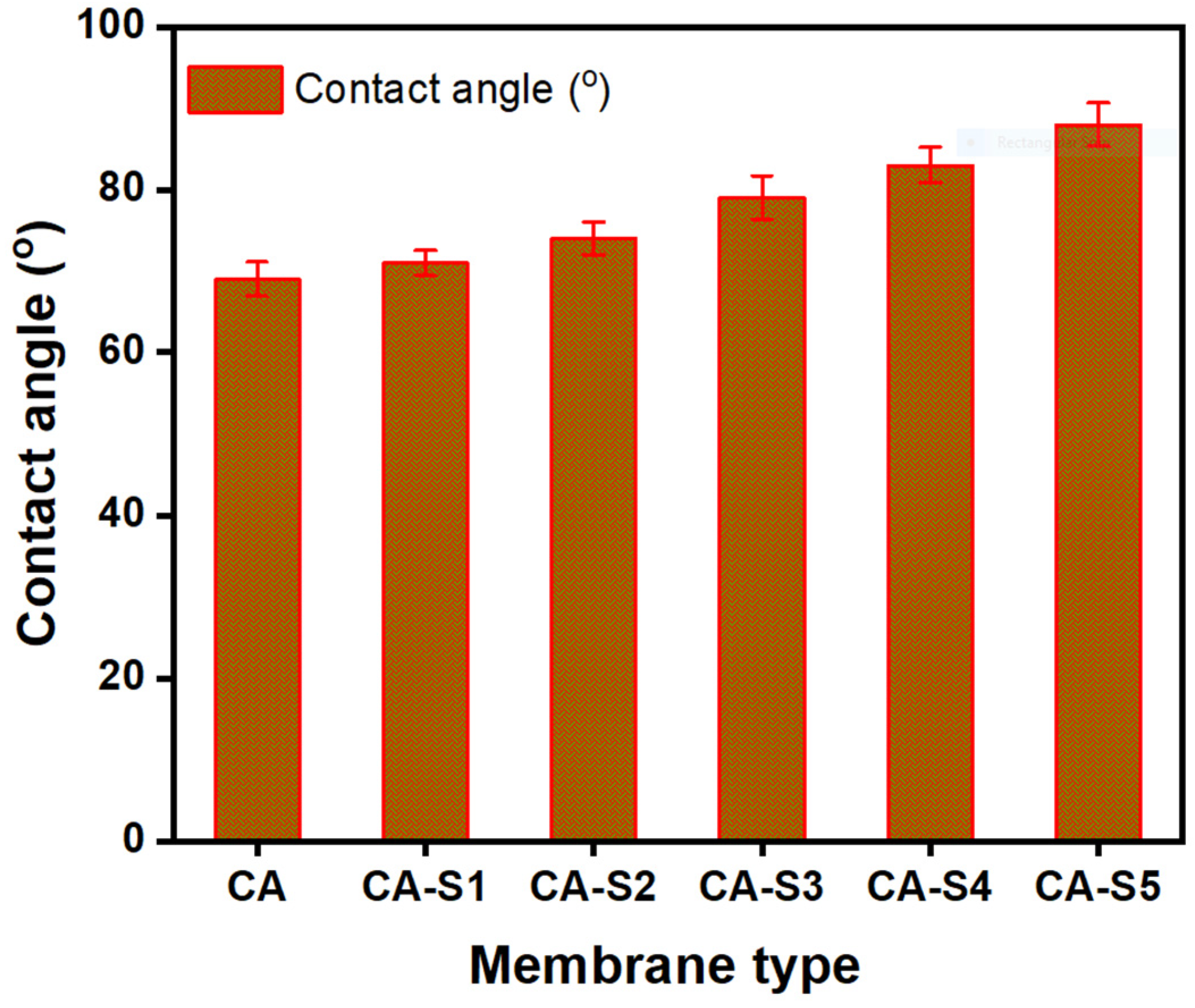
2.7. Contact Angle Determination
3. Materials and Methods
3.1. Materials
3.2. Preparation of NF-TFC Membrane
Preparation of CA (Cellulose Acetate) Substrate
3.3. Preparation of Electrospinning Solutions
3.3.1. CS/PVP Blends
3.3.2. Crosslinking of CS/PVP Blends with Maleic Acid
3.3.3. Electrospinning Process
3.4. FTIR Analysis
3.5. Scanning Electron Microscopy
3.6. Reverse Osmosis Performance Evaluation
3.7. Membrane Chlorine Resistance
3.8. Antifouling Studies
3.9. Contact Angle Determination
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marin, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sachit, D.E.; Veenstra, J.N. Analysis of reverse osmosis membrane performance during desalination of simulated brackish surface waters. J. Memb. Sci. 2014, 453, 136–154. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A.O. Alternative design to dual stage NF seawater desalination using high rejection brackish water membranes. Desalination 2011, 273, 391–397. [Google Scholar] [CrossRef]
- Hochstrat, R.; Wintgens, T.; Kazner, C.; Melin, T.; Gebel, J. Options for water scarcity and drought management—The role of desalination. Desalin. Water Treat. 2010, 18, 96–102. [Google Scholar] [CrossRef]
- Charcosset, C. A review of membrane processes and renewable energies for desalination. Desalination 2009, 245, 214–231. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef]
- Trache, D.; Thakur, V.K. Nanocellulose and Nanocarbons Based Hybrid Materials: Synthesis, Characterization and Applications. Nanomaterials 2020, 10, 1800. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Lee, J.S.; Heo, S.A.; Jo, H.J.; Min, B.R. Preparation and characteristics of cross-linked cellulose acetate ultrafiltration membranes with high chemical resistance and mechanical strength. React. Funct. Polym. 2016, 99, 114–121. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef]
- Bernd, G.K.S.; Lee, D.W. Modular Chitosan-Based Adsorbents for Tunable Uptake of Sulfate from Water. Int. J. Mol. Sci. 2020, 21, 7130. [Google Scholar] [CrossRef]
- Taha, A.A.; Wu, Y.N.; Wang, H.; Li, F. Preparation and application of functionalized cellulose acetate/silica composite nanofibrous membrane via electrospinning for Cr(VI) ion removal from aqueous solution. J. Environ. Manag. 2012, 112, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Liu, Q.L.; Xiong, Y.; Zhu, A.M.; Chen, Y.; Zhang, Q.G. Pervaporation dehydration of ethyl acetate/ethanol/water azeotrope using chitosan/poly (vinyl pyrrolidone) blend membranes. J. Memb. Sci. 2009, 327, 274–280. [Google Scholar] [CrossRef]
- Sudha, K.M.K.; Harish, K.H.G.; Chandramani, R.; Radhakrishna, M.C. PVP Influence on PVA crystallinity and optical band gap. Arch. Phy. Res. 2015, 6, 18–21. [Google Scholar]
- Xu, D.; Hein, S.; Wang, K. Chitosan membrane in separation applications. Mater. Sci. Technol. 2008, 24, 1076–1087. [Google Scholar] [CrossRef]
- Shafiq, M.; Sabir, A.; Islam, A.; Khan, S.M.; Hussain, S.N.; Butt, M.T.Z.Z.; Jamil, T. Development and performance characteristics of silane crosslinked poly(vinyl alcohol)/chitosan membranes for reverse osmosis. J. Ind. Eng. Chem. 2017, 48, 99–107. [Google Scholar] [CrossRef]
- Ahmad, A.; Jamshed, F.; Riaz, T.; Sabad-E-Gul; Waheed, S.; Sabir, A.; Alanezi, A.A.; Adrees, M.; Jamil, T. Self-sterilized composite membranes of cellulose acetate/polyethylene glycol for water desalination. Carbohydr. Polym. 2016, 149, 207–216. [Google Scholar] [CrossRef]
- Sabir, A.; Shafiq, M.; Islam, A.; Jabeen, F.; Shafeeq, A.; Ahmad, A.; Zahid Butt, M.T.; Jacob, K.I.; Jamil, T. Conjugation of silica nanoparticles with cellulose acetate/polyethylene glycol 300 membrane for reverse osmosis using MgSO4 solution. Carbohydr. Polym. 2016, 136, 551–559. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T. Controlled delivery of drug from pH sensitive chitosan/poly (vinyl alcohol) blend. Carbohydr. Polym. 2012, 88, 1055–1060. [Google Scholar] [CrossRef]
- Waheed, S.; Ahmad, A.; Maqsood, S.; Sabad-e-Gul; Jamil, T.; Islam, A.; Hussain, T. Synthesis, characterization, permeation and antibacterial properties of cellulose acetate/polyethylene glycol membranes modified with chitosan. Desalination 2014, 351, 59–69. [Google Scholar] [CrossRef]
- Islam, A.; Imran, Z.; Yasin, T.; Gull, N.; Khan, S.M.; Shafiq, M.; Sabir, A.; Munawar, M.A.; Raza, M.H.; Jamil, T. An investigation of ac impedance and dielectric spectroscopic properties of conducting chitosan-silane crosslinked-poly (vinyl alcohol) blended films. Mater. Res. 2015, 18, 1256–1263. [Google Scholar] [CrossRef]
- Sokker, H.H.; Abdel Ghaffar, A.M.; Gad, Y.H.; Aly, A.S. Synthesis and characterization of hydrogels based on grafted chitosan for the controlled drug release. Carbohydr. Polym. 2009, 75, 222–229. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T.; Akhtar, M.J.; Imran, Z.; Sabir, A.; Sultan, M.; Khan, S.M.; Jamil, T. Impedance spectroscopy of chitosan/poly(vinyl alcohol) films. J. Solid State Electrochem. 2016, 20, 571–578. [Google Scholar] [CrossRef]
- Soundararajan, A.; Gomathi, T.; Sudha, P.N. Preparation and characterization of chitosan-povidone blend with glutaraldehyde as crossliking agent. World J. Pharm. Res. 2018, 7, 1926–1940. [Google Scholar] [CrossRef]
- Hasipoglu, H.N.; Yilmaz, E.; Yilmaz, O.; Caner, H. Preparation and characterization of maleic acid grafted chitosan. Int. J. Polym. Anal. Charact. 2005, 10, 313–327. [Google Scholar] [CrossRef]
- Marsano, E.; Vicini, S.; Skopińska, J.; Wisniewski, M.; Sionkowska, A. Chitosan and poly(vinyl pyrrolidone): Compatibility and miscibility of blends. Macromol. Symp. 2004, 218, 251–260. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. Polyvinyl alcohol as the barrier layer in thin film composite nanofiltration membranes: Preparation, characterization, and performance evaluation. J. Colloid Interface Sci. 2009, 338, 121–127. [Google Scholar] [CrossRef]
- Lonsdale, H.K.; Merten, U.; Riley, R.L.; Jay, J. Transport Properties of Cellulose Acetate Osmotic Membranes. J. Appl. Polym. Sci. 1965, 9, 1341–1362. [Google Scholar] [CrossRef]
- Burghoff, H.; Lee, K.L.; Pusch, W. Characterization of transport across cellulose-acetate membranes in the presence of strong solute-membrane interactions. J. Appl. Ploym. Sci. 1980, 25, 323. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Malaisamy, R.; Mahendran, R.; Mohan, D.; Rajendran, M.; Mohan, V. Cellulose acetate and sulfonated Polysulfone blend ultrafiltration membranes—I. Preparation and characterization. J. Appl. Polym. Sci. 2002, 86, 1749–1761. [Google Scholar] [CrossRef]
- Raza, M.A.; Islam, A.; Sabir, A.; Ali, I.; Mehmood, R.; Bae, J.; Hassan, G.; Khan, M.U. PVA/TEOS crosslinked membranes incorporating zinc oxide nanoparticles and sodium alginate to improve reverse osmosis performance for desalination. J. Appl. Polym. Sci. 2019, 47559, 1–10. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Highly improved reverse osmosis performance of novel PVA/DGEBA cross-linked membranes by incorporation of Pluronic F-127 and MWCNTs for water desalination. Desalination 2016, 397, 53–66. [Google Scholar] [CrossRef]
- Ahmad, A.; Jamshaid, F.; Adrees, M.; Sagar, S.; Sabir, A.; Riaz, T.; Zaheer, H.; Islam, A.; Jamil, T. Novel Polyurethane/Polyvinyl chloride-co-vinyl acetate crosslinked membrane for reverse osmosis (RO). Desalination 2017, 420, 136–144. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef]
- Chae, H.; Lee, J.; Lee, C.; Kim, I.; Park, P. Graphene oxide-embedded thin-film composite reverse osmosis membrane with High flux, anti-biofouling, and chlorine resistance Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling and chlorine resistance. J. Memb. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Shafiq, M.; Sabir, A.; Islam, A.; Khan, S.M.; Gull, N.; Hussain, S.N.; Butt, M.T.Z. Cellulose acetate based thin film nanocomposite reverse osmosis membrane incorporated with TiO2 nanoparticles for improved performance. Carbohydr. Polym. 2018, 186, 367–376. [Google Scholar] [CrossRef]
- Sabir, A.; Islam, A.; Shafiq, M.; Shafeeq, A.; Butt, M.T.Z.; Ahmad, N.M.; Sanaullah, K.; Jamil, T. Novel polymer matrix composite membrane doped with fumed silica particles for reverse osmosis desalination. Desalination 2015, 368, 159–170. [Google Scholar] [CrossRef]
- Ayyavoo, J.; Nguyen, T.P.N.; Jun, B.M.; Kim, I.C.; Kwon, Y.N. Protection of polymeric membranes with antifouling surfacing via surface modifications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 190–201. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewska, S.J.; Planecka, A.; Kozlowska, J. The influence of UV irradiation on the properties of chitosan films containing keratin. Polym. Degrad Stab. 2010, 95, 2486–2491. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer (Guildf.) 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Cheng, L.; Zhang, L.; Chen, H. Preparation of thin film composite nanofiltration membrane by interfacial polymerization with 3,5-diaminobenzoylpiperazine and trimesoyl chloride. Chin. J. Chem. Eng. 2011, 19, 262–266. [Google Scholar] [CrossRef]
- Jahangiri, F.; Mousavi, S.A.; Farhadi, F.; Vatanpour, V.; Sabzi, B.; Chenari, Z. Effect of CO2-laser irradiation on properties and performance of thin-film composite polyamide reverse osmosis membrane. Korean J. Chem. Eng. 2016, 33, 1028–1036. [Google Scholar] [CrossRef]
- Sabir, A.; Falath, W.; Jacob, K.I.; Shafiq, M.; Munawar, M.A.; Islam, A.; Gull, N.; Butt, M.T.Z.; Sanaullah, K.; Jamil, T. Hyperbranched polyethyleneimine induced polycationic membranes for improved fouling resistance and high RO performance. Eur. Polym. J. 2016, 85, 266–278. [Google Scholar] [CrossRef]


| Articles | Permeation Flux (L/m2h) | Salt Rejection (%) | Reference |
|---|---|---|---|
| PVA/NaAlg/ZnO–NPs | 34.00 | 97.00 | [33] |
| PVA/DGEBA/MWCNT | 84.00 | 92.00 | [34] |
| PU/PVC–co-VA | 3.58 ± 0.38 | 96.00 ± 0.59 | [35] |
| Modified PVA/Gum Arabic conjugate | 94.00 | 98.00 | [36] |
| GO | 9.18 | 99.30 | [37] |
| CA/TiO2(NPs) | 0.42 | 76.10 | [38] |
| CS/PVP | 42.90 | 98.30 | Present work |
| CA/PEG/FSP | 1.312 | 78.00 | [39] |
| Sample | Salt Rejection (%) Before Chlorine Stability Test | Salt Rejection (%) After Chlorine Stability Test |
|---|---|---|
| CA | 56.0 ± 1.2 | 41.0 ± 1.3 |
| CA-S1 | 79.0 ± 1.9 | 69.0 ± 2.1 |
| CA-S2 | 88.0 ± 1.4 | 81.0 ± 1.9 |
| CA-S3 | 92.1 ± 1.0 | 89.0 ± 1.5 |
| CA-S4 | 98.3 ± 0.5 | 90.0 ± 2.3 |
| CA-S5 | 98.0 ± 0.8 | 88.0 ± 1.6 |
| Membranes | CS | PVP | Maleic Acid |
|---|---|---|---|
| (g) | (g) | (g) | |
| CA | 1 | 4 | - |
| CA-S1 | 1 | 4 | 0.05 |
| CA-S2 | 1 | 4 | 0.1 |
| CA-S3 | 1 | 4 | 0.15 |
| CA-S4 | 1 | 4 | 0.2 |
| CA-S5 | 1 | 4 | 0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Raza, M.A.; Mehmood, R.; Islam, A.; Sabir, A.; Gull, N.; Haider, B.; Park, S.H.; Khan, R.U. Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination. Int. J. Mol. Sci. 2020, 21, 7338. https://doi.org/10.3390/ijms21197338
Ali I, Raza MA, Mehmood R, Islam A, Sabir A, Gull N, Haider B, Park SH, Khan RU. Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination. International Journal of Molecular Sciences. 2020; 21(19):7338. https://doi.org/10.3390/ijms21197338
Chicago/Turabian StyleAli, Israr, Muhammad Asim Raza, Rashid Mehmood, Atif Islam, Aneela Sabir, Nafisa Gull, Bilal Haider, Sang Hyun Park, and Rafi Ullah Khan. 2020. "Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination" International Journal of Molecular Sciences 21, no. 19: 7338. https://doi.org/10.3390/ijms21197338
APA StyleAli, I., Raza, M. A., Mehmood, R., Islam, A., Sabir, A., Gull, N., Haider, B., Park, S. H., & Khan, R. U. (2020). Novel Maleic Acid, Crosslinked, Nanofibrous Chitosan/Poly (Vinylpyrrolidone) Membranes for Reverse Osmosis Desalination. International Journal of Molecular Sciences, 21(19), 7338. https://doi.org/10.3390/ijms21197338






