New Insights into the Biological and Pharmaceutical Properties of Royal Jelly
Abstract
:1. Introduction
2. Bioactive Substances
2.1. Proteins and Peptides
2.2. Lipids and Fatty Acids
2.3. Other Constituents
3. Functional Properties of RJ
3.1. Biological Activity of RJ
3.1.1. Antimicrobial Activity
3.1.2. Antioxidant Activity
3.1.3. Wound Healing Activity
3.1.4. Immunomodulatory Activity
3.1.5. Anti-Aging Activity
3.2. Pharmaceutical Applications
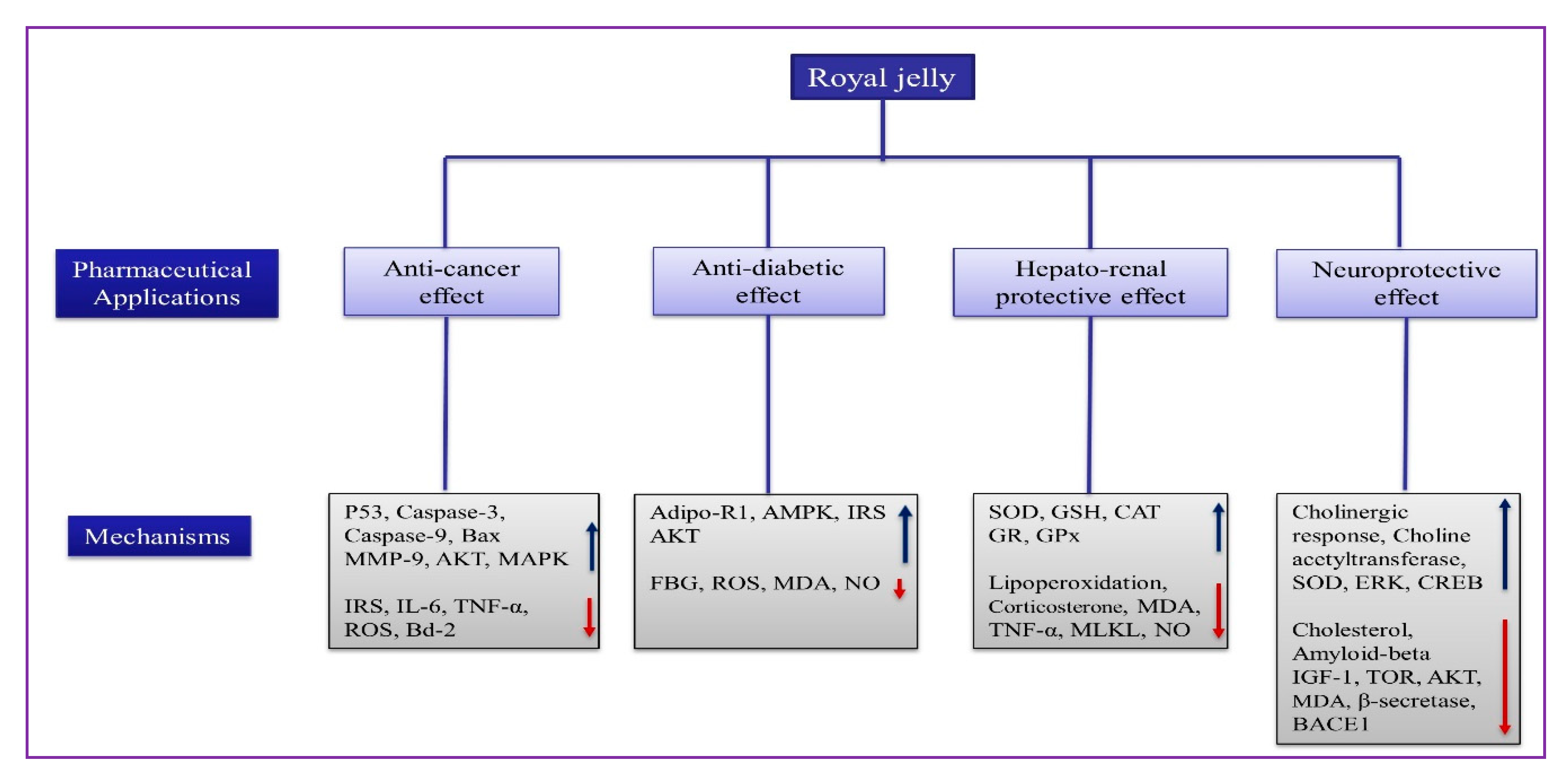
3.2.1. Anti-Cancer Effect
3.2.2. Anti-Diabetic Effect
3.2.3. Anti-Hypercholesterolemic Effect
3.2.4. Anti-Hypertension Effect
3.2.5. Anti-Inflammatory Effect
3.2.6. Organo-Protective Effect
Hepato-Renal Protective Effect
Neuroprotective Effect
Other Protective Effects
3.2.7. Effect on sexual Dysfunction and Fertility
Estrogenic Effect
Effect on Fertility
3.3. Side Effect of RJ Consumption
4. Conclusions and Future Research Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | Angiotensin 1-converting enzymes |
| AD | Alzheimer’s disease |
| AF | Aminofluorene |
| AMP | Adenosine monophosphate |
| ANO | Adenosine N1-oxide |
| ApoA-1 | Apolipoprotein A1 |
| BACE1 | β-site amyloid precursor protein cleaving enzymes |
| CAT | Catalase |
| CCl4 | Carbon tetrachloride |
| cGMP | Cyclic guanosine monophosphate |
| Con-A | Concanavalin A |
| DecDA | 1,10-decanedioic acid |
| DOX | Doxorubicin |
| EGF | Epidermal growth factor |
| EGFR | Anti-epidermal growth factor receptor |
| ERJ | Enzyme-treated RJ |
| FB | Fumonisin |
| FBG | Fasting blood glucose |
| FY4 | Food yellow 4 |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| 9-HDA | 9-hydroxy-2-decenoic acid |
| 10-HDA | 10-hydroxy-2-decenoic acid |
| 3-HHDA | 3-hydroxydecanoic acid |
| 10-HDDA | 10-hydroxydecenoic acid |
| 3,10-HDecDA | 3,10-dihydroxydecanedioic acid |
| 8-HOC | 8-hydroxy octanoic acid |
| HSV-1 | Herpes simplex virus type 1 |
| HuIFN-aN3 | Human interferon-alpha |
| IFN-ϒ | Interferon-gamma |
| IgE | Immunoglobulin E |
| IkBa | Inhibitor of kappa B |
| IkB-z | IkappaBzeta |
| IL | Interleukin |
| ILS | Insulin-like signaling |
| JNK-AP-1 | C-Jun N-terminal kinases-activating protein-1 |
| MDA | Malondialdehyde |
| 24-MET | 24-methylene cholesterol |
| MLKL | Mixed lineage kinase domain-like protein |
| MoDCs | Monocyte-derived dendritic cells |
| MRJPs | Major royal jelly proteins |
| NAT | N-acetyltransferase |
| NO | Nitric oxide |
| NPCs/NSs | Neural progenitors or neural stem cells |
| OXM | Oxymetholone |
| PC3 | Prostate cancer cell line |
| RJ | Royal jelly |
| RJBs | Royal jelly bees |
| SEA | Sebacic acid |
| SHR | Spontaneously hypertensive rats |
| SLE | Systemic lupus erythematosus |
| SOD | Superoxide dismutase |
| TKIs | Tyrosine kinase inhibitors |
| VLDL | Very low-density lipoprotein levels |
| VSMCs | Vascular smooth muscle cells |
References
- Knecht, D.; Kaatz, H. Patterns of larval food production by hypopharyngeal glands in adult worker honey bees. Apidologie 1990, 21, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Li, J.K.; Feng, M.; Begna, D.; Fang, Y.; Zheng, A.J. Proteome Comparison of Hypopharyngeal Gland Development between Italian and Royal Jelly-Producing Worker Honeybees (Apis mellifera L). J. Proteome Res. 2010, 9, 6578–6594. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and bioactivities of royal jelly. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 261–290. [Google Scholar]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; Almeida-Muradian, L. Quality and standardisation of royal jelly. J. ApiProd. ApiMed. Sci. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Bee Products-Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 181–190. [Google Scholar]
- Robinson, G.E.; Fahrbach, S.E.; Winston, M.L. Insect societies and the molecular biology of social behavior. Bioessays 1997, 19, 1099–1108. [Google Scholar] [CrossRef]
- Evans, J.D.; Wheeler, D.E. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proc. Natl. Acad. Sci. USA 1999, 96, 5575–5580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microb. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; McDonald, P. Australian Royal Jelly-Market Opportunity Assessment Based on Production That Uses New Labour Saving Technology; Rural Industries Research and Development Corporation: Wagga, NSW, Australia, 2017; Volume 4. [Google Scholar]
- Li, J.; Li, H.; Zhang, Z.; Pan, Y. Identification of the proteome complement of high royal jelly producing bees (Apis mellifera) during worker larval development. Apidologie 2007, 38, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Feng, M.; Fang, Y.; Han, B.; Xu, X.; Fan, P.; Hao, Y.; Qi, Y.; Hu, H.; Huo, X.; Meng, L. In-depth N-glycosylation reveals species-specific modifications and functions of the royal jelly protein from Western (Apis mellifera) and Eastern Honeybees (Apis cerana). J. Proteome Res. 2015, 14, 5327–5340. [Google Scholar] [CrossRef] [PubMed]
- Altaye, S.Z.; Meng, L.; Li, J. Molecular insights into the enhanced performance of royal jelly secretion by a stock of honeybee (Apis mellifera ligustica) selected for increasing royal jelly production. Apidologie 2019, 50, 436–453. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Aiping, W. Comprehensive technology for maximizing royal jelly production. Am. Bee J. 2005, 145, 661–664. [Google Scholar]
- Cao, L.-F.; Zheng, H.-Q.; Pirk, C.W.; Hu, F.-L.; Xu, Z.-W. High royal jelly-producing honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J. Econ. Entomol. 2016, 109, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Zhong, B.; Su, S. Genetic analysis for developmental behavior of honeybee colony’s royal jelly production traits in western honeybees. Yi Chuan Xue Bao Acta Genet. Sin. 2003, 30, 547–554. [Google Scholar]
- Nie, H.; Liu, X.; Pan, J.; Li, W.; Li, Z.; Zhang, S.; Chen, S.; Miao, X.; Zheng, N.; Su, S. Identification of genes related to high royal jelly production in the honey bee (Apis mellifera) using microarray analysis. Genet. Mol. Biol. 2017, 40, 781–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hora, Z.A.; Altaye, S.Z.; Wubie, A.J.; Li, J. Proteomics Improves the New Understanding of Honeybee Biology. J. Agric. Food Chem. 2018, 66, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Altaye, S.Z.; Meng, L.F.; Lu, Y.; Li, J.K. The Emerging Proteomic Research Facilitates in-Depth Understanding of the Biology of Honeybees. Int. J. Mol. Sci. 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Health, Medicine. J. Bee Prod. Sci. 2011, 3, 8–19. [Google Scholar]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Miyata, Y.; Sakai, H. Anti-Cancer and Protective Effects of Royal Jelly for Therapy-Induced Toxicities in Malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [Green Version]
- Alu’datt, M.H.; Rababah, T.; Sakandar, H.A.; Imran, M.; Mustafa, N.; Alhamad, M.N.; Mhaidat, N.; Kubow, S.; Tranchant, C.; Al-Tawaha, A.R. Fermented food-derived bioactive compounds with anticarcinogenic properties: Fermented royal jelly as a novel source for compounds with health benefits. In Anticancer Plants: Properties and Application; Springer: Berlin/Heidelberg, Germany, 2018; pp. 141–165. [Google Scholar]
- Furusawa, T.; Rakwal, R.; Nam, H.W.; Shibato, J.; Agrawal, G.K.; Kim, Y.S.; Ogawa, Y.; Yoshida, Y.; Kouzuma, Y.; Masuo, Y. Comprehensive royal jelly (RJ) proteomics using one-and two-dimensional proteomics platforms reveals novel RJ proteins and potential phospho/glycoproteins. J. Proteome Res. 2008, 7, 3194–3229. [Google Scholar] [CrossRef]
- Zhang, L.; Han, B.; Li, R.; Lu, X.; Nie, A.; Guo, L.; Fang, Y.; Feng, M.; Li, J. Comprehensive identification of novel proteins and N-glycosylation sites in royal jelly. BMC Genome 2014, 15, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the Yellow/Major Royal Jelly Protein family and the emergence of social behavior in honey bees. Genome Res. 2006, 16, 1385–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, S.; Klaudiny, J. MRJP9, an ancient protein of the honeybee MRJP family with non-nutritional function. J. Apic. Res. 2007, 46, 99. [Google Scholar]
- Kimura, M.; Kimura, Y.; Tsumura, K.; Okihara, K.; Sugimoto, H.; Yamada, H.; Yonekura, M. 350-kDa royal jelly glycoprotein (apisin), which stimulates proliferation of human monocytes, bears the β1-3galactosylated N-glycan: Analysis of the N-glycosylation site. Biosci. Biotechnol. Biochem. 2003, 67, 2055–2058. [Google Scholar] [CrossRef] [Green Version]
- Furusawa, T.; Arai, Y.; Kato, K.; Ichihara, K. Quantitative analysis of Apisin, a major protein unique to royal jelly. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Qu, N.; Jiang, J.; Sun, L.; Lai, C.; Sun, L.; Wu, X. Proteomic characterization of royal jelly proteins in Chinese (Apis cerana cerana) and European (Apis mellifera) honeybees. Biochemistry 2008, 73, 676. [Google Scholar] [CrossRef] [Green Version]
- Sano, O.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Characterization of royal jelly proteins in both Africanized and European honeybees (Apis mellifera) by two-dimensional gel electrophoresis. J. Agric. Food Chem. 2004, 52, 15–20. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Li, R.; Feng, M.; Han, B.; Zhou, T.; Li, J. Towards posttranslational modification proteome of royal jelly. J. Proteom. 2012, 75, 5327–5341. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.S.; dos Santos, L.D.; Mendes, M.A.; de Souza, B.M.; Malaspina, O.; Palma, M.S. Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honeybees (Apis mellifera L.). Insect Biochem. Mol. Biol. 2005, 35, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.L.; Tian, L.Q.; Qin, Q.H.; Wu, X.B.; Yan, W.Y.; Zeng, Z.J. Transcriptome differences in the hypopharyngeal gland between Western Honeybees (Apis mellifera) and Eastern Honeybees (Apis cerana). BMC Genom. 2014, 15, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Li, C.; Zhang, L.; Fang, Y.; Feng, M.; Li, J. Novel royal jelly proteins identified by gel-based and gel-free proteomics. J. Agric. Food Chem. 2011, 59, 10346–10355. [Google Scholar] [CrossRef]
- Han, B.; Fang, Y.; Feng, M.; Lu, X.; Huo, X.; Meng, L.; Wu, B.; Li, J. In-depth phosphoproteomic analysis of royal jelly derived from western and eastern honeybee species. J. Proteome Res. 2014, 13, 5928–5943. [Google Scholar] [CrossRef]
- Silici, S.; Ekmekcioglu, O.; Eraslan, G.; Demirtas, A. Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology 2009, 74, 545–551. [Google Scholar] [CrossRef]
- Schmitzova, J.; Klaudiny, J.; Albert, Š.; Schröder, W.; Schreckengost, W.; Hanes, J.; Judova, J.; Šimúth, J. A family of major royal jelly proteins of the honeybee Apis mellifera L. Cell. Mol. Life Sci. CMLS 1998, 54, 1020–1030. [Google Scholar] [CrossRef]
- Scarselli, R.; Donadio, E.; Giuffrida, M.G.; Fortunato, D.; Conti, A.; Balestreri, E.; Felicioli, R.; Pinzauti, M.; Sabatini, A.G.; Felicioli, A. Towards royal jelly proteome. Proteomics 2005, 5, 769–776. [Google Scholar] [CrossRef]
- Fontana, R.; Mendes, M.A.; De Souza, B.M.; Konno, K.; César, L.l.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- Bulet, P.; Stocklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Li, X.a.; Huang, C.; Xue, Y. Contribution of lipids in honeybee (Apis mellifera) royal jelly to health. J. Med. Food 2013, 16, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Narukawa, M.; Watanabe, T. Specific hydroxy fatty acids in royal jelly activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.; Bakier, S.; Grzech, I. Gas chromatographic–mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B 2012, 885, 109–116. [Google Scholar] [CrossRef]
- Makino, J.; Ogasawara, R.; Kamiya, T.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Itoh, A.; Adachi, T. Royal jelly constituents increase the expression of extracellular superoxide dismutase through histone acetylation in monocytic THP-1 cells. J. Nat. Prod. 2016, 79, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Nabas, Z.; Haddadin, M.S.; Haddadin, J.; Nazer, I.K. Chemical composition of royal jelly and effects of synbiotic with two different locally isolated probiotic strains on antioxidant activities. Pol. J. Food Nutr. Sci. 2014, 64, 171–180. [Google Scholar] [CrossRef]
- Kanbur, M.; Eraslan, G.; Beyaz, L.; Silici, S.; Liman, B.C.; Altınordulu, Ş.; Atasever, A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp. Toxicol. Pathol. 2009, 61, 123–132. [Google Scholar] [CrossRef] [PubMed]
- De Paula, R.; Rabalski, I.; Messia, M.C.; Abdel-Aal, E.-S.M.; Marconi, E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res. Int. 2017, 102, 136–143. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; del Mar Aguilera-Luiz, M.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Fast analysis of polyphenols in royal jelly products using automated TurboFlow™-liquid chromatography–Orbitrap high resolution mass spectrometry. J. Chromatogr. B 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. AMP N1-oxide, a unique compound of royal jelly, induces neurite outgrowth from pc12 vells via signaling by protein kinase A independent of that by mitogen-activated protein kinase. Evid.-Based Complement. Altern. Med. 2010, 7, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Hattori, N.; Nomoto, H.; Mishima, S.; Inagaki, S.; Goto, M.; Sako, M.; Furukawa, S. Identification of AMP N1-oxide in royal jelly as a component neurotrophic toward cultured rat pheochromocytoma PC12 cells. Biosci. Biotechnol. Biochem. 2006, 70, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.F.; Zhou, J.H.; Wu, L.M.; Fu, L.H.; Zhao, J. HPLC determination of adenosine in royal jelly. Food Chem. 2009, 115, 715–719. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly-induced neurite outgrowth from rat pheochromocytoma PC12 cells requires integrin signal independent of activation of extracellular signalregulated kinases. Biomed. Res. 2007, 28, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. AMP N1-oxide potentiates astrogenesis by cultured neural stem/progenitor cells through STAT3 activation. Biomed. Res. 2007, 28, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Wei, M.; Kang, X.; Deng, H.; Lu, Z. A novel method developed for acetylcholine detection in royal jelly by using capillary electrophoresis coupled with electrogenerated chemiluminescence based on a simple reaction. Electrophoresis 2009, 30, 1949–1952. [Google Scholar] [CrossRef]
- Vittek, J.; Slomiany, B. Testosterone in royal jelly. Experientia 1984, 40, 104–106. [Google Scholar] [CrossRef]
- Carvalho, V.D.C.; Silveira, V.Á.S.; do Prado, R.F.; Carvalho, Y.R. Effect of estrogen therapy, soy isoflavones, and the combination therapy on the submandibular gland of ovariectomized rats. Pathol.-Res. Pract. 2011, 207, 300–305. [Google Scholar] [CrossRef]
- Takaki-Doi, S.; Hashimoto, K.; Yamamura, M.; Kamei, C. Antihypertensive activities of royal jelly protein hydrolysate and its fractions in spontaneously hypertensive rats. Acta Med. Okayama 2009, 63, 57–64. [Google Scholar]
- Heyl, H.L. An Observation Suggesting the Presence of a Gonadotropic Hormone in Royal Jelly. Science 1939, 89, 540–541. [Google Scholar] [CrossRef]
- Gardner, T.S. The use of Drosophila melanogaster as a screening agent for longevity factors; pantothenic acid as a longevity factor in royal jelly. J. Gerontol. 1948, 3, 1–8. [Google Scholar] [CrossRef]
- Bărnuţiu, L.I.; Mărghitaş, L.A.; Dezmirean, D.S.; Mihai, C.M.; Bobiş, O. Chemical composition and antimicrobial activity of Royal Jelly-REVIEW. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 67–72. [Google Scholar]
- Kim, B.Y.; Lee, K.S.; Jung, B.; Choi, Y.S.; Kim, H.K.; Yoon, H.J.; Gui, Z.-Z.; Lee, J.; Jin, B.R. Honeybee (Apis cerana) major royal jelly protein 4 exhibits antimicrobial activity. J. Asia-Pac. Entomol. 2019, 22, 175–182. [Google Scholar] [CrossRef]
- Coutinho, D.; Karibasappa, S.N.; Mehta, D.S. Royal Jelly Antimicrobial Activity against Periodontopathic Bacteria. J. Interdiscip. Dent. 2018, 8, 18. [Google Scholar]
- Park, M.J.; Kim, B.Y.; Park, H.G.; Deng, Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly. J. Asia-Pac. Entomol. 2019, 22, 684–689. [Google Scholar] [CrossRef]
- Bíliková, K.; Wu, G.; Šimúth, J. Isolation of a peptide fraction from honeybee royal jelly as a potential antifoulbrood factor. Apidologie 2001, 32, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Bílikova, K.; Huang, S.-C.; Lin, I.-P.; Šimuth, J.; Peng, C.-C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and bioactivity of royal jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef] [PubMed]
- Moselhy, W.; Fawzy, A.; Kamel, A. An evaluation of the potent antimicrobial effects and unsaponifiable matter analysis of the royal jelly. Life Sci. J. 2013, 2, 290–296. [Google Scholar]
- Sauerwald, N. Combined antibacterial and antifungal properties of water-soluble fraction of royal jelly. Adv. Food Sci. 1998, 20, 46–52. [Google Scholar]
- Cihan, Y. Protective Role of Royal Jelly Against Radiation-Induced Oxidative Stress in Rats. Int. J. Hematol. Oncol. 2013, 28, 79–87. [Google Scholar] [CrossRef]
- Kimura, Y. Antitumor and antimetastatic actions of various natural products. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; Volume 34, pp. 35–76. [Google Scholar]
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 253–255. [Google Scholar] [CrossRef]
- Premratanachai, P.; Chanchao, C. Review of the anticancer activities of bee products. Asian Pac. J. Trop. Biomed. 2014, 4, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipic, B.; Gradisnik, L.; Rihar, K.; Soos, E.; Pereyra, A.; Potokar, J. The influence of royal jelly and human interferon-alpha (HuIFN-alpha N3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arh. Hig. Rada Toksiko. 2015, 66, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmoradian, S.; Mahdavi, R.; Mobasseri, M.; Faramarzi, E.; Mobasseri, M. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: A randomized clinical trial. Chin. J. Integr. Med. 2014, 20, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Münstedt, K.; Bargello, M.; Hauenschild, A. Royal jelly reduces the serum glucose levels in healthy subjects. J. Med. Food 2009, 12, 1170–1172. [Google Scholar] [CrossRef]
- Ghanbari, E.; Nejati, V.; Azadbakht, M. Protective effect of royal jelly against renal damage in streptozotocin induced diabetic rats. Iran. J. Toxicol. 2015, 9, 1258–1263. [Google Scholar]
- Ghanbari, E.; Nejati, V.; Khazaei, M. Improvement in serum biochemical alterations and oxidative stress of liver and pancreas following use of royal jelly in streptozotocin-induced diabetic rats. Cell J. (Yakhteh) 2016, 18, 362. [Google Scholar]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Dzopalic, T.; Vucevic, D.; Tomic, S.; Djokic, J.; Chinou, I.; Colic, M. 3,10-Dihydroxy-decanoic acid, isolated from royal jelly, stimulates Th1 polarising capability of human monocyte-derived dendritic cells. Food Chem. 2011, 126, 1211–1217. [Google Scholar] [CrossRef]
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu, F. Royal jelly supplementation improves lipoprotein metabolism in humans. J. Nutr. Sci. Vitaminol. 2007, 53, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vittek, J. Effect of royal jelly on serum lipids in experimental animals and humans with atherosclerosis. Experientia 1995, 51, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016, 32, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.-F.; Chen, B.-K.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm. Biol. 2017, 55, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.-H.; Yoshida, C.; Suzuki, K.-M.; Maruyama, H.; Futamura, Y.; Araki, Y.; Mishima, S. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol. Pharm. Bull. 2004, 27, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.; Han, B.; Feng, M.; Fang, Y.; Zhang, L.; Hu, H.; Hao, Y.; Qi, Y.; Zhang, X.; Li, J. Functional and proteomic investigations reveal major royal jelly protein 1 associated with anti-hypertension activity in mouse vascular smooth muscle cells. Sci. Rep. 2016, 6, 30230. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Rong, Y.; You, M.; Ma, Q.; Chen, M.; Hu, F. Royal jelly causes hypotension and vasodilation induced by increasing nitric oxide production. Food Sci. Nutr. 2019, 7, 1361–1370. [Google Scholar] [CrossRef] [Green Version]
- Aslan, Z.; Aksoy, L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz. J. Urol. 2015, 41, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, S.; Karadeniz, A.; Karakoc, A.; Yildirim, A.; Kalkan, Y.; Şimşek, N. Effects of royal jelly on liver paraoxonase activity in rats treated with cisplatin. Turk. J. Med. Sci. 2012, 42, 367–375. [Google Scholar]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Abu-Serie, M.M.; Habashy, N.H. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: Major royal jelly protein 2 and its novel isoform X1. Int. J. Biol. Macromol. 2019, 128, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.R.; Jalili, C.; Roshankhah, S. Can royal jelly protect against renal ischemia/reperfusion injury in rats? Chin. J. Physiol. 2019, 62, 131–137. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 2007, 28, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yu, Y.; Sun, P.; Fan, Z.; Zhang, W.; Feng, C. Royal jelly peptides: Potential inhibitors of beta-secretase in N2a/APP695swe cells. Sci. Rep. 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Xu, J.; Jin, P.; Yang, Q.; Zhu, K.; You, M.; Chen, M.; Hu, F. Royal Jelly Ameliorates Behavioral Deficits, Cholinergic System Deficiency, and Autonomic Nervous Dysfunction in Ovariectomized Cholesterol-Fed Rabbits. Molecules 2019, 24, 1149. [Google Scholar] [CrossRef] [Green Version]
- You, M.; Pan, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Si, J.; Wang, K.; Hu, F. Royal jelly alleviates cognitive deficits and β-amyloid accumulation in APP/PS1 mouse model via activation of the cAMP/PKA/CREB/BDNF pathway and inhibition of neuronal apoptosis. Front. Aging Neurosci. 2018, 10, 428. [Google Scholar] [CrossRef] [Green Version]
- Jenkhetkan, W.; Thitiorul, S.; Jansom, C.; Ratanavalachai, T. Genoprotective effects of thai royal jelly against doxorubicin in human lymphocytes in vitro. Nat. Prod. Commun. 2018, 13, 1934578X1801300124. [Google Scholar] [CrossRef] [Green Version]
- Park, H.M.; Hwang, E.; Lee, K.G.; Han, S.-M.; Cho, Y.; Kim, S.Y. Royal Jelly Protects Against Ultraviolet B–Induced Photoaging in Human Skin Fibroblasts via Enhancing Collagen Production. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Al-Sanafi, A.E.; Mohssin, S.A.; Abdulla, S.M. Effect of royal jelly on male infertility. Thi-Qar Med. J. 2007, 1, 1–12. [Google Scholar]
- Elewa, Y.H.; Mohamed, A.A.-R.; Galal, A.A.; El-naseery, N.I.; Ichii, O.; Kon, Y. Food Yellow4 reprotoxicity in relation to localization of DMC1 and apoptosis in rat testes: Roles of royal jelly and cod liver oil. Ecotoxicol. Environ. Saf. 2019, 169, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Kohguchi, M.; Inoue, S.-I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Effect of royal jelly diet on the testicular function of hamsters. Food Sci. Technol. Res. 2007, 10, 420–423. [Google Scholar] [CrossRef]
- Moriyama, T.; Yanagihara, M.; Yano, E.; Kimura, G.; Seishima, M.; Tani, H.; Kanno, T.; Nakamura-Hirota, T.; Hashimoto, K.; Tatefuji, T. Hypoallergenicity and immunological characterization of enzyme-treated royal jelly from Apis mellifera. Biosci. Biotechnol. Biochem. 2013, 77, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Al-Kushi, A.G.; Header, E.A.; ElSawy, N.A.; Moustafa, R.A.; Alfky, N.A.A. Antioxidant effect of royal jelly on immune status of hyperglycemic rats. Pharmacogn. Mag. 2018, 14, 528. [Google Scholar] [CrossRef]
- Malekinejad, H.; Ahsan, S.; Delkhosh-Kasmaie, F.; Cheraghi, H.; Rezaei-Golmisheh, A.; Janbaz-Acyabar, H. Cardioprotective effect of royal jelly on paclitaxel-induced cardio-toxicity in rats. Iran. J. Basic Med. Sci. 2016, 19, 221–227. [Google Scholar]
- Shi, J.-L.; Liao, C.-H.; Wang, Z.-L.; Wu, X.-B. Effect of royal jelly on longevity and memory-related traits of Apis mellifera workers. J. Asia-Pac. Entomol. 2018, 21, 1430–1433. [Google Scholar] [CrossRef]
- Šedivá, M.; Laho, M.; Kohútová, L.; Mojžišová, A.; Majtán, J.; Klaudiny, J. 10-HDA, A Major Fatty Acid of Royal Jelly, Exhibits pH Dependent Growth-Inhibitory Activity Against Different Strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef] [Green Version]
- Park, H.G.; Kim, B.Y.; Park, M.J.; Deng, Y.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antibacterial activity of major royal jelly proteins of the honeybee (Apis mellifera) royal jelly. J. Asia-Pac. Entomol. 2019, 22, 737–741. [Google Scholar] [CrossRef]
- Splith, K.; Neundorf, I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur. Biophys. J. 2011, 40, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.S.; Novak, A.F.; Taber, S. 10-hydroxy-Δ2-decenoic acid, an antibiotic found in royal jelly. Science 1959, 130, 452–453. [Google Scholar] [CrossRef]
- Gunalp, R.; Ulusoy, M.; Celebier, I.; Keskin, N. Antifungal effect of royal jelly on Candida albicans. J. Biotechnol. 2018, 280, S70–S71. [Google Scholar] [CrossRef]
- Stocker, A. Isolation and Characterisation of Substances from Royal Jelly; Technische Universität München: München, Germany, 2003. [Google Scholar]
- Hashemipour, M.A.; Tavakolineghad, Z.; Arabzadeh, S.A.M.; Iranmanesh, Z.; Nassab, S.A.H.G. Antiviral Activities of Honey, Royal Jelly, and Acyclovir Against HSV-1. Wounds 2014, 26, 47–54. [Google Scholar] [PubMed]
- El-Nekeety, A.A.; El-Kholy, W.; Abbas, N.F.; Ebaid, A.; Amra, H.A.; Abdel-Wahhab, M.A. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 2007, 50, 256–269. [Google Scholar] [CrossRef]
- Çavuşoğlu, K.; Yapar, K.; Yalçin, E. Royal jelly (honey bee) is a potential antioxidant against cadmium-induced genotoxicity and oxidative stress in albino mice. J. Med. Food 2009, 12, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid. Med. Cell. Longev. 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Song, I.-B.; Han, H.-J.; Lee, N.-Y.; Cha, J.-Y.; Son, Y.-K.; Kwon, J. Antioxidant Activity of Royal Jelly Hydrolysates Obtained by Enzymatic Treatment. Korean J. Food Sci. Anim. Resour. 2018, 38, 135. [Google Scholar] [PubMed]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Yun, H.; Park, H.; Kim, S.Y.; Lee, K.G.; Han, S.M.; Cho, Y. Royal jelly enhances migration of human dermal fibroblasts and alters the levels of cholesterol and sphinganine in an in vitro wound healing model. Nutr. Res. Pract. 2010, 4, 362–368. [Google Scholar] [CrossRef] [Green Version]
- El-Gayar, M.H.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Antivirulence and wound healing effects of royal jelly and garlic extract for the control of MRSA skin infections. Wound Med. 2016, 13, 18–27. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Kohno, K.; Inoue, S.; Koya-Miyata, S.; Okamoto, I.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 2003, 3, 1313–1324. [Google Scholar] [CrossRef]
- Suemaru, K.; Cui, R.; Li, B.; Watanabe, S.; Okihara, K.; Hashimoto, K.; Yamada, H.; Araki, H. Topical application of royal jelly has a healing effect for 5-fluorouracil-induced experimental oral mucositis in hamsters. Methods Find. Exp. Clin. Pharmacol. 2008, 30, 103–106. [Google Scholar] [CrossRef]
- Siavash, M.; Shokri, S.; Haghighi, S.; Mohammadi, M.; Shahtalebi, M.A.; Farajzadehgan, Z. The efficacy of topical Royal Jelly on diabetic foot ulcers healing: A case series. J. Res. Med. Sci. 2011, 16, 904. [Google Scholar]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef] [Green Version]
- Labro, M.-T. Immunomodulatory effects of antimicrobial agents. Part I: Antibacterial and antiviral agents. Exp. Rev. Anti-Infect. Ther. 2012, 10, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Elsayh, K.I.; Saad, K.; Eloseily, E.M.; Osman, N.S.; Alblihed, M.A.; Badr, G.; Mahmoud, M.H. Effects of royal jelly supplementation on regulatory T cells in children with SLE. Food Nutr. Res. 2016, 60, 32963. [Google Scholar] [CrossRef] [Green Version]
- Gasic, S.; Vucevic, D.; Vasilijic, S.; Antunovic, M.; Chinou, I.; Colic, M. Evaluation of the immunomodulatory activities of royal jelly components in vitro. Immunopharmacol. Immunotoxicol. 2007, 29, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Vucevic, D.; Melliou, E.; Vasilijic, S.; Gasic, S.; Ivanovski, P.; Chinou, I.; Colic, M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. [Google Scholar] [CrossRef]
- Mihajlovic, D.; Rajkovic, I.; Chinou, I.; Colic, M. Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J. Funct. Foods 2013, 5, 838–846. [Google Scholar] [CrossRef]
- Pyrzanowska, J.; Piechal, A.; Blecharz-Klin, K.; Joniec-Maciejak, I.; Graikou, K.; Chinou, I.; Widy-Tyszkiewicz, E. Long-term administration of Greek Royal Jelly improves spatial memory and influences the concentration of brain neurotransmitters in naturally aged Wistar male rats. J. Ethnopharmacol. 2014, 155, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Cook, L.F.; Grasso, L.M.; Cao, M.; Dong, Y.Q. Royal Jelly-Mediated Prolongevity and Stress Resistance in Caenorhabditis elegans Is Possibly Modulated by the Interplays of DAF-16, SIR-2.1, HCF-1, and 14-3-3 Proteins. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 70, 827–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Detienne, G.; De Haes, W.; Ernst, U.R.; Schoofs, L.; Temmerman, L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 2014, 60, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.X.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.Q.; Shen, L.R. Supplementation with Major Royal-Jelly Proteins Increases Lifespan, Feeding, and Fecundity in Drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Pajovic, B.; Radojevic, N.; Dimitrovski, A.; Tomovic, S.; Vukovic, M. The therapeutic potential of royal jelly in benign prostatic hyperplasia. Comparison with contemporary literature. Aging Male 2016, 19, 192–196. [Google Scholar] [CrossRef]
- Abandansari, R.M.; Parsian, H.; Kazerouni, F.; Porbagher, R.; Zabihi, E.; Rahimipour, A. Effect of Simultaneous Treatment with Royal Jelly and Doxorubicin on the Survival of the Prostate Cancer Cell Line (PC3): An In Vitro Study. Int. J. Cancer Manag. 2018, 11, e13780. [Google Scholar] [CrossRef]
- Alsharif, F.H.; Mazanec, S.R. The use of complementary and alternative medicine among women with breast cancer in Saudi Arabia. Appl. Nurs. Res. 2019, 48, 75–80. [Google Scholar] [CrossRef]
- Gismondi, A.; Trionfera, E.; Canuti, L.; Di Marco, G.; Canini, A. Royal jelly lipophilic fraction induces antiproliferative effects on SH-SY5Y human neuroblastoma cells. Oncol. Rep. 2017, 38, 1833–1844. [Google Scholar] [CrossRef] [Green Version]
- Shidfar, F.; Jazayeri, S.; Mousavi, S.N.; Malek, M.; fateme HOSSEINI, A.; Khoshpey, B. Does supplementation with royal jelly improve oxidative stress and insulin resistance in type 2 diabetic patients? Iran. J. Public Health 2015, 44, 797. [Google Scholar] [PubMed]
- Khoshpey, B.; Djazayeri, S.; Amiri, F.; Malek, M.; Hosseini, A.F.; Hosseini, S.; Shidfar, S.; Shidfar, F. Effect of royal jelly intake on serum glucose, Apolipoprotein AI (ApoA-I), Apolipoprotein B (ApoB) and ApoB/ApoA-I ratios in patients with type 2 Diabetes: A randomized, double-blind clinical trial study. Can. J. Diabetes 2016, 40, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2016, 16-0458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.; Jazayeri, S.; Khoshpay, B.; Malek, M.; Hosseini, A.; Hosseini, S.; Shidfar, F. Royal jelly decreases blood pressure, serum glucose, and interleukin-6 in patients with type 2 diabetes on an iso-caloric diet. J. Nutr. Food Secur. 2017, 2, 300–307. [Google Scholar]
- Cheraghi, O.; Abdollahpourasl, M.; Rezabakhsh, A.; Rahbarghazi, R. Distinct effects of royal jelly on human endothelial cells under high glucose condition. Iran. J. Pharm. Res. IJPR 2018, 17, 1361. [Google Scholar] [PubMed]
- Zamami, Y.; Takatori, S.; Goda, M.; Koyama, T.; Iwatani, Y.; Jin, X.; Takai-Doi, S.; Kawasaki, H. Royal Jelly Ameliorates Insulin Resistance in Fructose-Drinking Rats. Biol. Pharm. Bull. 2008, 31, 2103–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: A meta-analysis. J. Funct. Foods 2018, 41, 202–209. [Google Scholar] [CrossRef]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Karaca, T.; Simsek, N.; Uslu, S.; Kalkan, Y.; Can, I.; Kara, A.; Yoruk, M. The effect of royal jelly on CD3(+), CD5(+), CD45(+) T-cell and CD68(+) cell distribution in the colon of rats with acetic acid-induced colitis. Allergol. Immunopathol. 2012, 40, 357–361. [Google Scholar] [CrossRef]
- Ibrahim, S.E.M.; Kosba, A.A. Royal jelly supplementation reduces skeletal muscle lipotoxicity and insulin resistance in aged obese rats. Pathophysiology 2018, 25, 307–315. [Google Scholar] [CrossRef] [PubMed]
- You, M.-M.; Chen, Y.-F.; Pan, Y.-M.; Liu, Y.-C.; Tu, J.; Wang, K.; Hu, F.-L. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Song, I.-B.; Han, H.-J.; Lee, N.-Y.; Cha, J.-Y.; Son, Y.-K.; Kwon, J. Anti-inflammatory and immune-enhancing effects of enzyme-treated royal jelly. Appl. Biol. Chem. 2018, 61, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Yang, D.S.; Wei, Z.; Wang, J.M.; Li, C.Y.; Hui, Y.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; et al. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef]
- Sugiyama, T.; Takahashi, K.; Tokoro, S.; Gotou, T.; Neri, P.; Mori, H. Inhibitory effect of 10-hydroxy-trans-2-decenoic acid on LPS-induced IL-6 production via reducing IkappaB-zeta expression. Innate Immun. 2012, 18, 429–437. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef] [Green Version]
- Kohno, K.; Ohashi, E.; Sano, O.; Kusano, H.; Kunikata, T.; Arai, N.; Hanaya, T.; Kawata, T.; Nishimoto, T.; Fukuda, S. Anti-inflammatory effects of adenosine N1-oxide. J. Inflamm. 2015, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Nejati, V.; Zahmatkesh, E.; Babaei, M. Protective effects of royal jelly on oxymetholone-induced liver injury in mice. Iran. Biomed. J. 2016, 20, 229. [Google Scholar]
- Ahmed, W.M.S.; Khalaf, A.A.; Moselhy, W.A.; Safwat, G.M. Royal jelly attenuates azathioprine induced toxicity in rats. Environ. Toxicol. Pharmacol. 2014, 37, 431–437. [Google Scholar] [CrossRef]
- Caixeta, D.C.; Teixeira, R.R.; Peixoto, L.G.; Machado, H.L.; Baptista, N.B.; de Souza, A.V.; Vilela, D.D.; Franci, C.R.; Espindola, F.S. Adaptogenic potential of royal jelly in liver of rats exposed to chronic stress. PLoS ONE 2018, 13, e0191889. [Google Scholar] [CrossRef] [Green Version]
- Cihan, Y.B.; Arsav, V.; Gocen, E. Royal Jelly in the Prevention of Radiation-Induced Brain Damages. J. Neurol. Sci. (Turk.) 2011, 28, 475–486. [Google Scholar]
- Morita, H.; Ikeda, T.; Kajita, K.; Fujioka, K.; Mori, I.; Okada, H.; Uno, Y.; Ishizuka, T. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, A.; Cemek, M.; Buyukokuroglu, M.E.; Altunbas, K.; Bas, O.; Yurumez, Y. Royal jelly can diminish secondary neuronal damage after experimental spinal cord injury in rabbits. Food Chem. Toxicol. 2012, 50, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.-R.; Galal, A.A.; Elewa, Y.H. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem. 2015, 117, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Minami, A.; Matsushita, H.; Leno, D.; Matsuda, Y.; Horii, Y.; Ishii, A.; Takahashi, T.; Kanazawa, H.; Wakatsuki, A.; Suzuki, T. Improvement of neurological disorders in postmenopausal model rats by administration of royal jelly. Climacteric 2016, 19, 568–573. [Google Scholar] [CrossRef]
- Rafat, N.; Monfared, A.S.; Shahidi, M.; Pourfallah, T.A. The modulating effect of royal jelly consumption against radiation-induced apoptosis in human peripheral blood leukocytes. J. Med. Phys. Assoc. Med. Phys. India 2016, 41, 52. [Google Scholar]
- Araki, K.; Miyata, Y.; Ohba, K.; Nakamura, Y.; Matsuo, T.; Mochizuki, Y.; Sakai, H. Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial. Medicines 2018, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef] [Green Version]
- Mishima, S.; Suzuki, K.-M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef]
- Husein, M.Q.; Kridli, R.T. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Anim. Reprod. Sci. 2002, 74, 45–53. [Google Scholar] [CrossRef]
- Shahzad, Q.; Mehmood, M.U.; Khan, H.; ul Husna, A.; Qadeer, S.; Azam, A.; Naseer, Z.; Ahmad, E.; Safdar, M.; Ahmad, M. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim. Reprod. Sci. 2016, 167, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Seyyedi, F.; Rafiean-Kopaei, M.; Miraj, S. Comparison of the effects of vaginal royal jelly and vaginal estrogen on quality of life, sexual and urinary function in postmenopausal women. J. Clin. Diagn. Res. JCDR 2016, 10, QC01. [Google Scholar] [CrossRef] [PubMed]
- Taavoni, S.; Barkhordari, F.; Goushegir, A.; Haghani, H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: A randomized, triple-blind, placebo-controlled study. Complement. Ther. Med. 2014, 22, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Eshtiyaghi, M.; Deldar, H.; Pirsaraei, Z.A.; Shohreh, B. Royal jelly may improve the metabolism of glucose and redox state of ovine oocytes matured in vitro and embryonic development following in vitro fertilization. Theriogenology 2016, 86, 2210–2221. [Google Scholar] [CrossRef]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal jelly promotes ovarian follicles growth and increases steroid hormones in immature rats. Int. J. Fertil. Steril. 2018, 11, 263. [Google Scholar]
- Suzuki, K.-M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid.-Based Complement. Altern. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.Y.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A.; et al. Fatty Acids Derived from Royal Jelly Are Modulators of Estrogen Receptor Functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef] [Green Version]
- Abdelhafiz, A.T.; Muhamad, J.A. Midcycle pericoital intravaginal bee honey and royal jelly for male factor infertility. Int. J. Gynecol. Obstet. 2008, 101, 146–149. [Google Scholar] [CrossRef]
- Campos, P.K.A.; Barbosa, L.P.; Neves, M.M.; Melo, B.E.S.; Morais, A.C.T.; Morais, D.B. Seminal quality and testicular morphometry of rabbits (Oryctolagus cuniculus) supplemented with royal jelly. Arq. Bras. Med. Vet. Zootec. 2012, 64, 1563–1568. [Google Scholar] [CrossRef] [Green Version]
- Moradi, A.R.; Malekinejad, H.; Farrokhi-Ardabili, F.; Bernousi, I. Royal Jelly improves the sperm parameters of ram semen during liquid storage and serves as an antioxidant source. Small Rumin. Res. 2013, 113, 346–352. [Google Scholar] [CrossRef]
- El-Hanoun, A.M.; Elkomy, A.E.; Fares, W.A.; Shahien, E.H. Impact of Royal Jelly to Improve Reproductive Performance of Male Rabbits under Hot Summer Conditions. World Rabbit Sci. 2014, 22, 241–248. [Google Scholar] [CrossRef]
- Temamoğulları, F.; Aral, F.; Yılmaz, R. Royal jelly protection on flunixin meglumine-induced spermiotoxicity and testicular degeneration in mice. Pol. J. Vet. Sci. 2018, 21, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Mahdivand, N.; Najafi, G.; Nejati, V.; Shalizar-Jalali, A.; Rahmani, F. Royal jelly protects male rats from heat stress-induced reproductive failure. Andrologia 2019, 51, e13213. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Aoki, M.; Kawana, S. Case of anaphylaxis caused by ingestion of royal jelly. J. Dermatol. 2008, 35, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Nagasawa, C.; Shibuya, Y.; Seishima, M. A case of royal jelly-induced anaphylaxis. J. Am. Acad. Dermatol. 2007, 56, Ab79. [Google Scholar]
- Rosmilah, M.; Shahnaz, M.; Patel, G.; Lock, J.; Rahman, D.; Masita, A.; Noormalin, A. Characterization of major allergens of royal jelly Apis mellifera. Trop. Biomed. 2008, 25, 243–251. [Google Scholar]
- Takahashi, M.; Matsuo, I.; Ohkido, M. Contact dermatitis due to honeybee royal jelly. Contact Dermat. 1983, 9, 452–455. [Google Scholar] [CrossRef]
- Peacock, S.; Murray, V.; Turton, C. Respiratory distress and royal jelly. BMJ 1995, 311, 1472. [Google Scholar]
- Laporte, J.R.; Ibanez, L.; Vendrell, L.; Ballarin, E. Bronchospasm induced by royal jelly. Allergy 1996, 51, 440. [Google Scholar] [CrossRef]
- Yonei, Y.; Shibagaki, K.; Tsukada, N.; Nagasu, N.; Inagaki, Y.; Miyamoto, K.; Suzuki, O.; Kiryu, Y. Case report: Haemorrhagic colitis associated with royal jelly intake. J. Gastroenterol. Hepatol. 1997, 12, 495–499. [Google Scholar] [CrossRef]
- Gómez, T.E.; Méndez, D.Y.; Borja, S.J.; Feo, B.J.; Alfaya, A.T.; Galindo, B.P.; Ledesma, F.A.; García, R.R. Occupational allergic respiratory disease due to royal jelly. Ann. Allergy Asthma Immunol. 2016, 117, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Guan, K. Occupational Asthma Caused by Inhalable Royal Jelly and Its Cross-reactivity with Honeybee Venom. Chin. Med. J.-Peking 2016, 129, 2888–2889. [Google Scholar] [CrossRef] [PubMed]
- Thien, F.; Leung, R.; Baldo, B.; Weinbr, J.; Plomley, R.; Czarny, D. Asthma and anaphylaxis induced by royal jelly. Clin. Exp. Allergy 1996, 26, 216–222. [Google Scholar] [CrossRef] [PubMed]
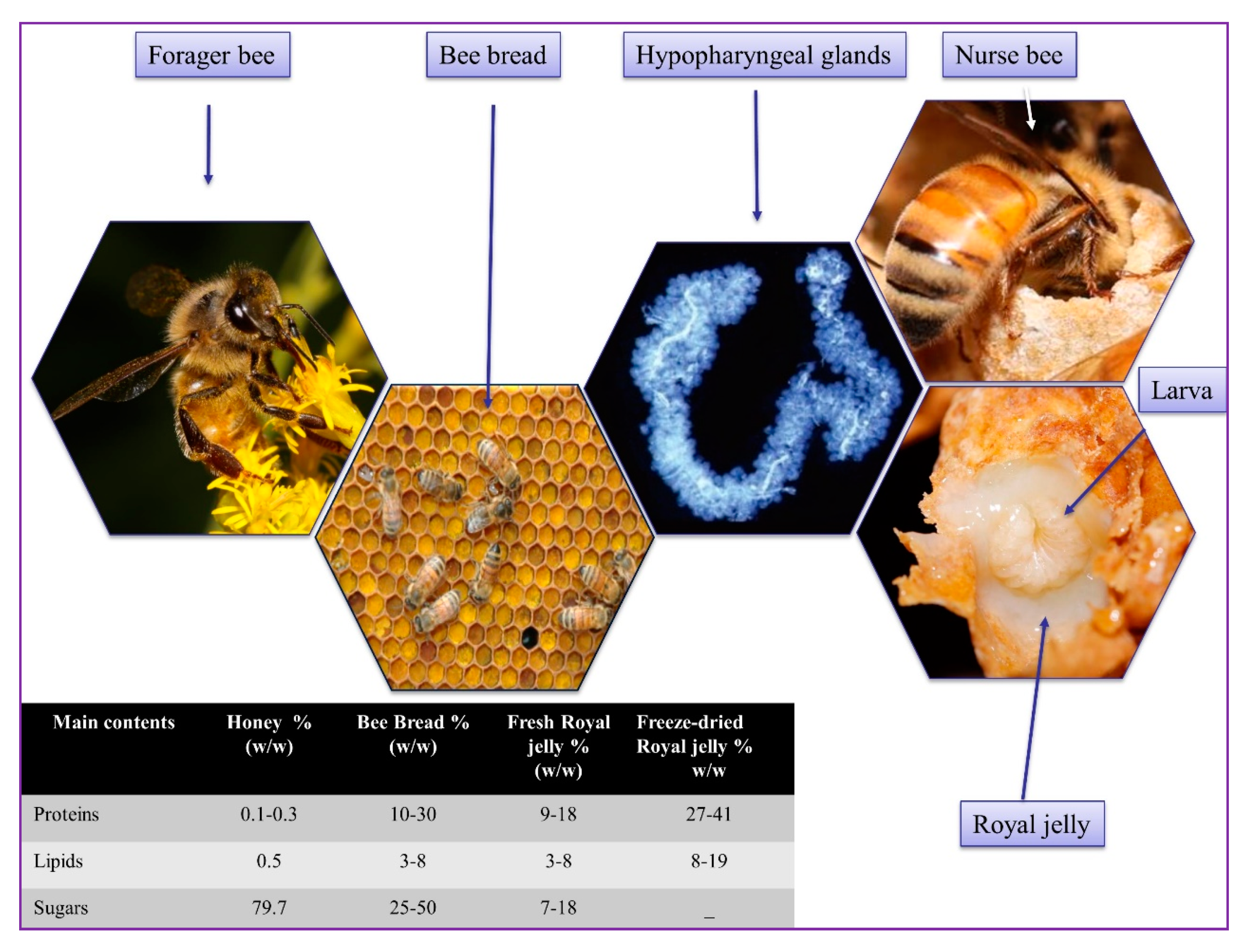



| Bioactive Compounds/Experimental Models | Effects | Sources |
|---|---|---|
| RJ, MRJP-2, and MRJP-4 (Micro-organisms) | Antibacterial, antifungal, anti-yeast Induce damage and dysfunction in microbial cell wall and membrane | [66,67,68] |
| Royalisin and 10-HDA (Micro-organisms) | Antibacterial (Gram+, Gram−), antifungal Inhibit growth | [9,69,70] |
| Jelleine I-III, jelleine-II (pS), and jelleine-II (pT) (Micro-organisms) | Antibacterial (Gram+, Gram−) Cell degranulation, hemolysis, and increase immune defense | [39,43] |
| RJ, 10H∆2DA, 3,10-HDA, 11S, 10-HDA, 10-acetooxy-2-DEA, and Native jelleine-11 (Micro-organisms) | Antifungal and anti-yeast Strongly inhibit growth | [39,43,71,72,73] |
| Pre and post administration of RJ (Animals) | Antioxidant activity Decrease oxidative stress (MDA) and increase antioxidant properties (CAT, GPx, and SOD) | [74] |
| RJ (Humans) | Anti-cancer effect Inhibit the tumor-induced angiogenesis, activate immune system, metabolism of 2-AF metabolites, and stop the damage of bisphenol A | [75,76,77] |
| Intravenously application of 10-HDA and the HuIFN-aN3 (Animals) | Anti-cancer effect Decrease the level of glutathione and enhance the level of lipid peroxidation via MDA | [78] |
| RJ (Animals and humans) | Anti-diabetic effect Improve the serum level of triglycerides, lipoprotein, and cholesterol Decrease glucose level and increase insulin concentration | [79,80,81,82,83] |
| MRJP-3 (Animals) | Immunomodulatory effect Decrease antigenicity and inhibit IL-4, IL-2, and IFN-ϒ production | [84] |
| 3,10-DDA (Humans) | Immunomodulatory effect Increase the production of IL-12, IL-18, and stimulate the production of IFN-ϒ | [85] |
| RJ (Animals and humans) | Hypocholesterolemic effect Reduced the level of triglyceride, insulin, total lipids, and cholesterol level by decreasing very low-density lipoprotein levels | [86,87,88] |
| RJ and MRJP-1 (Humans) | Hypocholesterolemic effect Decreased the total cholesterol and LDL-c level by improving the (HDL-c) level | [89,90] |
| RJ, ERJ, And MRJP-1 (Animals) | Anti- hypertension effect Reduce systolic blood pressure, diastolic blood pressure, and increase NO level | [62,91,92,93] |
| RJ (Animals) | Anti-inflammatory effect RJ inhibit the TNF-α, IL-1β, and, IL-18 levels in the blood due to its antiradicals and antioxidative effect | [94,95] |
| RJ and MRJP-2 (Animals) | Hepato-renal protective effect Reduce blood urea, MDA level, leukocyte infiltration, creatinine, adhesion molecule-1 expression, glomerular diameter, and TNF-a Increased SOD and GPx | [50,96,97,98,99] |
| RJ and 10-HDA Animals | Neurotrophic effects Inhibited production of oligodendrocytes, astrocytes, and stimulate neuron differentiation | [57,100] |
| RJ and RJPs (Animals and humans) | Neuroprotective Decrease cholesterol and amyloid-beta deposition by down-regulation of β-secretase Increase cholinergic response, estrogen level, and antioxidant capacities Improved blood-brain barrier, and autonomic nervous systems | [101,102,103] |
| RJ (Humans) | Genoprotective effect Increase of BCL2/BAX ratio for cell survival Enhance in hTERT/BAX for increasing age Increase in NRF2/BAX for antioxidative response | [104] |
| RJ and 10-HDA (Humans) | Protective effect Protect it from photo-aging by improving collagen production via up-regulation of TGF-β1 expression | [105] |
| RJ (Animals and humans) | Effect on fertility Increase sperm motility, luteinizing hormones, and testosterone levels | [81,106,107,108] |
| ERJ (Humans) | Anti-allergic Significantly reducing IgE-binding capacity of blood | [109] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. https://doi.org/10.3390/ijms21020382
Ahmad S, Campos MG, Fratini F, Altaye SZ, Li J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. International Journal of Molecular Sciences. 2020; 21(2):382. https://doi.org/10.3390/ijms21020382
Chicago/Turabian StyleAhmad, Saboor, Maria Graça Campos, Filippo Fratini, Solomon Zewdu Altaye, and Jianke Li. 2020. "New Insights into the Biological and Pharmaceutical Properties of Royal Jelly" International Journal of Molecular Sciences 21, no. 2: 382. https://doi.org/10.3390/ijms21020382
APA StyleAhmad, S., Campos, M. G., Fratini, F., Altaye, S. Z., & Li, J. (2020). New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. International Journal of Molecular Sciences, 21(2), 382. https://doi.org/10.3390/ijms21020382







