Docosahexaenoic Acid Attenuates Mitochondrial Alterations and Oxidative Stress Leading to Cell Death Induced by Very Long-Chain Fatty Acids in a Mouse Oligodendrocyte Model
Abstract
1. Introduction
2. Results
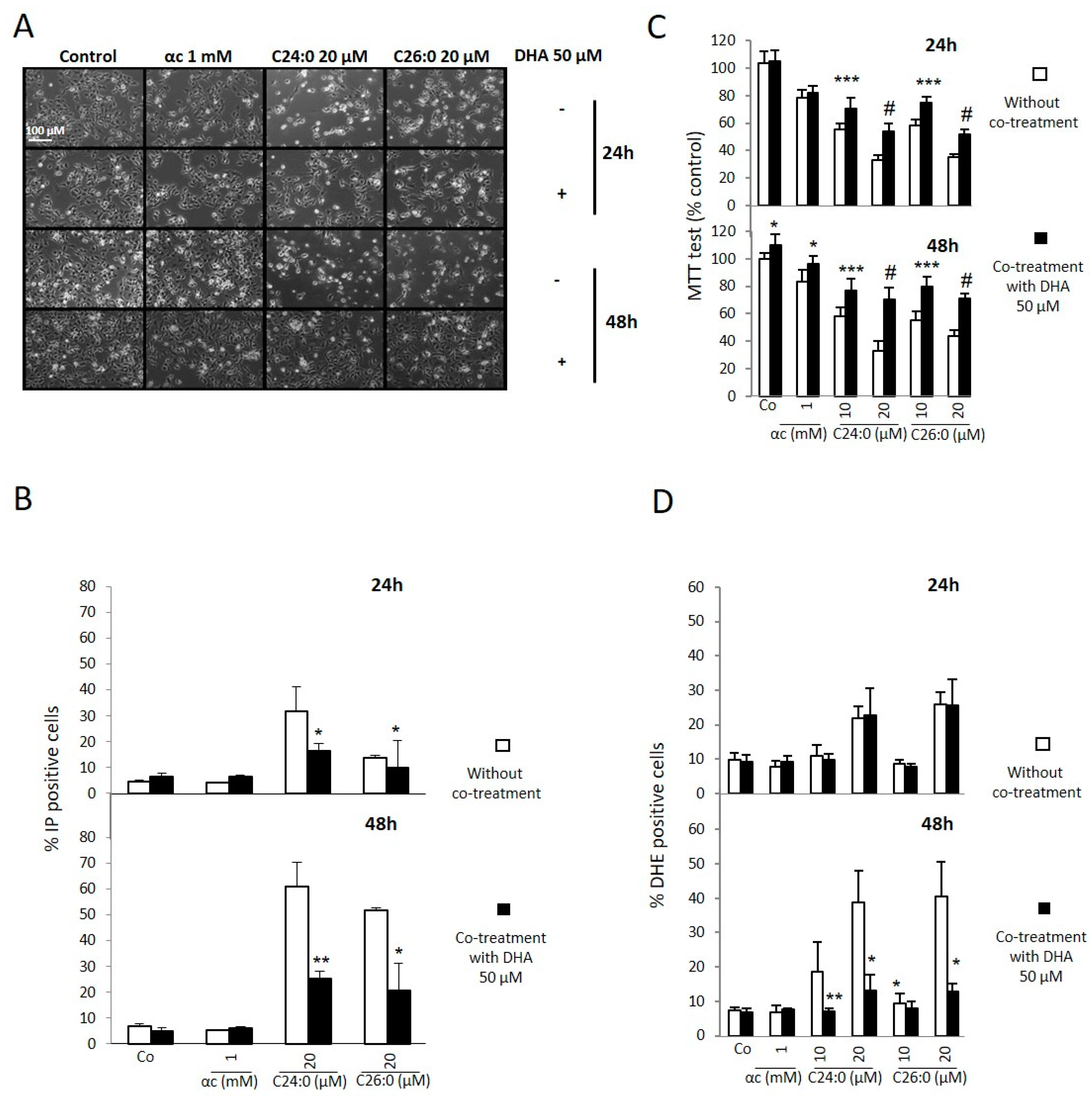
2.1. Effects of DHA on Proliferation, Plasma Membrane, Mitochondria, and Oxidative Stress
2.2. Effects of DHA on Autophagy Process
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Treatment
4.3. Analysis of Cell Morphology by Phase Contrast Microscopy
4.4. Colorimetric MTT Assay
4.5. Flow Cytometric Measurement of Cell Viability with Propidium Iodide
4.6. Flow Cytometric Measurement of ROS Overproduction with Dihydroethidium
4.7. Protein Analysis by Polyacrylamide Gel Electrophoresis and Western Blotting
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCRP | breast cancer resistant protein |
| CNS | central nervous system |
| DHA | Docosahexaenoic acid |
| DHE | dihydroethidium |
| DMSO | dimethylsulfoxide |
| γ-GT | γ-glutamyl transpeptidase |
| MAO | monoamine oxidase |
| MRP | multidrug resistance-associated proteins |
| P-gp | P-glycoprotein |
| PI | propidium iodide |
| VLCFA | very long-chain fatty acid |
References
- Gray, E.; Rice, C.; Hares, K.; Redondo, J.; Kemp, K.; Williams, M.; Brown, A.; Scolding, N.; Wilkins, A. Reductions in neuronal peroxisomes in multiple sclerosis grey matter. Mult. Scler. 2014, 20, 651–659. [Google Scholar] [CrossRef]
- Senanayake, V.K.; Jin, W.; Mochizuki, A.; Chitou, B.; Goodenowe, D.B. Metabolic dysfunctions in multiple sclerosis: Implications as to causation, early detection, and treatment, a case control study. BMC Neurol. 2015, 15, 154. [Google Scholar] [CrossRef]
- Kou, J.; Kovacs, G.G.; Hoftberger, R.; Kulik, W.; Brodde, A.; Forss-Petter, S.; Honigschnabl, S.; Gleiss, A.; Brugger, B.; Wanders, R.; et al. Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol. 2011, 122, 271–283. [Google Scholar] [CrossRef]
- Zarrouk, A.; Riedinger, J.M.; Ahmed, S.H.; Hammami, S.; Chaabane, W.; Debbabi, M.; Ben Ammou, S.; Rouaud, O.; Frih, M.; Lizard, G.; et al. Fatty acid profiles in demented patients: Identification of hexacosanoic acid (C26:0) as a blood lipid biomarker of dementia. J. Alzheimers Dis. 2015, 44, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Doria, M.; Nury, T.; Delmas, D.; Moreau, T.; Lizard, G.; Vejux, A. Protective function of autophagy during VLCFA-induced cytotoxicity in a neurodegenerative cell model. Free Radic. Biol. Med. 2019, 137, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Erauskin, J.; Fourcade, S.; Galino, J.; Ruiz, M.; Schluter, A.; Naudi, A.; Jove, M.; Portero-Otin, M.; Pamplona, R.; Ferrer, I.; et al. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann. Neurol. 2011, 70, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Casasnovas, C.; Ruiz, M.; Schluter, A.; Naudi, A.; Fourcade, S.; Veciana, M.; Castaner, S.; Alberti, A.; Bargallo, N.; Johnson, M.; et al. Biomarker Identification, Safety, and Efficacy of High-Dose Antioxidants for Adrenomyeloneuropathy: A Phase II Pilot Study. Neurotherapeutics 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A. Novel Therapeutic Targets and Drug Candidates for Modifying Disease Progression in Adrenoleukodystrophy. Endocr. Dev. 2016, 30, 147–160. [Google Scholar] [PubMed]
- Martinez, M. Restoring the DHA levels in the brains of Zellweger patients. J. Mol. Neurosci. 2001, 16, 309–316. [Google Scholar] [CrossRef]
- Zarrouk, A.; Nury, T.; Riedinger, J.M.; Rouaud, O.; Hammami, M.; Lizard, G. Dual effect of docosahexaenoic acid (attenuation or amplification) on C22:0-, C24:0-, and C26:0-induced mitochondrial dysfunctions and oxidative stress on human neuronal SK-N-BE cells. J. Nutr. Health Aging 2015, 19, 198–205. [Google Scholar] [CrossRef]
- Hein, S.; Schonfeld, P.; Kahlert, S.; Reiser, G. Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum. Mol. Genet. 2008, 17, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Galino, J.; Ruiz, M.; Fourcade, S.; Schluter, A.; Lopez-Erauskin, J.; Guilera, C.; Jove, M.; Naudi, A.; Garcia-Arumi, E.; Andreu, A.L.; et al. Oxidative damage compromises energy metabolism in the axonal degeneration mouse model of X-adrenoleukodystrophy. Antioxid. Redox Signal. 2011, 15, 2095–2107. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, S.; Lopez-Erauskin, J.; Galino, J.; Duval, C.; Naudi, A.; Jove, M.; Kemp, S.; Villarroya, F.; Ferrer, I.; Pamplona, R.; et al. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum. Mol. Genet. 2008, 17, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Keeley, T.P.; Mann, G.E. Defining Physiological Normoxia for Improved Translation of Cell Physiology to Animal Models and Humans. Physiol. Rev. 2019, 99, 161–234. [Google Scholar] [CrossRef]
- Stuart, J.A.; Fonseca, J.; Moradi, F.; Cunningham, C.; Seliman, B.; Worsfold, C.R.; Dolan, S.; Abando, J.; Maddalena, L.A. How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxid. Med. Cell Longev. 2018, 2018, 8238459. [Google Scholar] [CrossRef]
- Ferguson, D.C.J.; Smerdon, G.R.; Harries, L.W.; Dodd, N.J.F.; Murphy, M.P.; Curnow, A.; Winyard, P.G. Altered cellular redox homeostasis and redox responses under standard oxygen cell culture conditions versus physioxia. Free Radic. Biol. Med. 2018, 126, 322–333. [Google Scholar] [CrossRef]
- Launay, N.; Aguado, C.; Fourcade, S.; Ruiz, M.; Grau, L.; Riera, J.; Guilera, C.; Giros, M.; Ferrer, I.; Knecht, E.; et al. Autophagy induction halts axonal degeneration in a mouse model of X-adrenoleukodystrophy. Acta Neuropathol. 2015, 129, 399–415. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Mackrill, J.J.; Samadi, M.; Durand, P.; Riedinger, J.M.; Doria, M.; Vejux, A.; Limagne, E.; Delmas, D.; et al. Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7beta-hydroxycholesterol-, or 24(S)-hydroxycholesterol: Protective effects of alpha-tocopherol and docosahexaenoic acid (DHA.; C22:6 n-3). Steroids 2015, 99, 194–203. [Google Scholar] [CrossRef]
- Paker, A.M.; Sunness, J.S.; Brereton, N.H.; Speedie, L.J.; Albanna, L.; Dharmaraj, S.; Moser, A.B.; Jones, R.O.; Raymond, G.V. Docosahexaenoic acid therapy in peroxisomal diseases: Results of a double-blind, randomized trial. Neurology 2010, 75, 826–830. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimizu, T.; Ohtsuka, Y.; Yamashiro, Y.; Oshida, K. Early dietary treatments with Lorenzo’s oil and docosahexaenoic acid for neurological development in a case with Zellweger syndrome. Brain Dev. 2007, 29, 586–589. [Google Scholar] [CrossRef]
- Arai, Y.; Kitamura, Y.; Hayashi, M.; Oshida, K.; Shimizu, T.; Yamashiro, Y. Effect of dietary Lorenzo’s oil and docosahexaenoic acid treatment for Zellweger syndrome. Congenit. Anom. 2008, 48, 180–182. [Google Scholar] [CrossRef]
- Singh, I.; Kishimoto, Y. Effect of cyclodextrins on the solubilization of lignoceric acid, ceramide, and cerebroside, and on the enzymatic reactions involving these compounds. J. Lipid Res. 1983, 24, 662–665. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yeh, C.J.; Hsi, B.L.; Faulk, W.P. Propidium iodide as a nuclear marker in immunofluorescence. II. Use with cellular identification and viability studies. J. Immunol. Methods 1981, 43, 269–275. [Google Scholar] [CrossRef]
- Yazdani, M. Concerns in the application of fluorescent probes DCDHF-DA, DHR 123 and DHE to measure reactive oxygen species in vitro. Toxicol. In Vitro 2015, 30, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A. American Heart Association Council on Basic Cardiovascular, S., Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nury, T.; Doria, M.; Lizard, G.; Vejux, A. Docosahexaenoic Acid Attenuates Mitochondrial Alterations and Oxidative Stress Leading to Cell Death Induced by Very Long-Chain Fatty Acids in a Mouse Oligodendrocyte Model. Int. J. Mol. Sci. 2020, 21, 641. https://doi.org/10.3390/ijms21020641
Nury T, Doria M, Lizard G, Vejux A. Docosahexaenoic Acid Attenuates Mitochondrial Alterations and Oxidative Stress Leading to Cell Death Induced by Very Long-Chain Fatty Acids in a Mouse Oligodendrocyte Model. International Journal of Molecular Sciences. 2020; 21(2):641. https://doi.org/10.3390/ijms21020641
Chicago/Turabian StyleNury, Thomas, Margaux Doria, Gérard Lizard, and Anne Vejux. 2020. "Docosahexaenoic Acid Attenuates Mitochondrial Alterations and Oxidative Stress Leading to Cell Death Induced by Very Long-Chain Fatty Acids in a Mouse Oligodendrocyte Model" International Journal of Molecular Sciences 21, no. 2: 641. https://doi.org/10.3390/ijms21020641
APA StyleNury, T., Doria, M., Lizard, G., & Vejux, A. (2020). Docosahexaenoic Acid Attenuates Mitochondrial Alterations and Oxidative Stress Leading to Cell Death Induced by Very Long-Chain Fatty Acids in a Mouse Oligodendrocyte Model. International Journal of Molecular Sciences, 21(2), 641. https://doi.org/10.3390/ijms21020641






