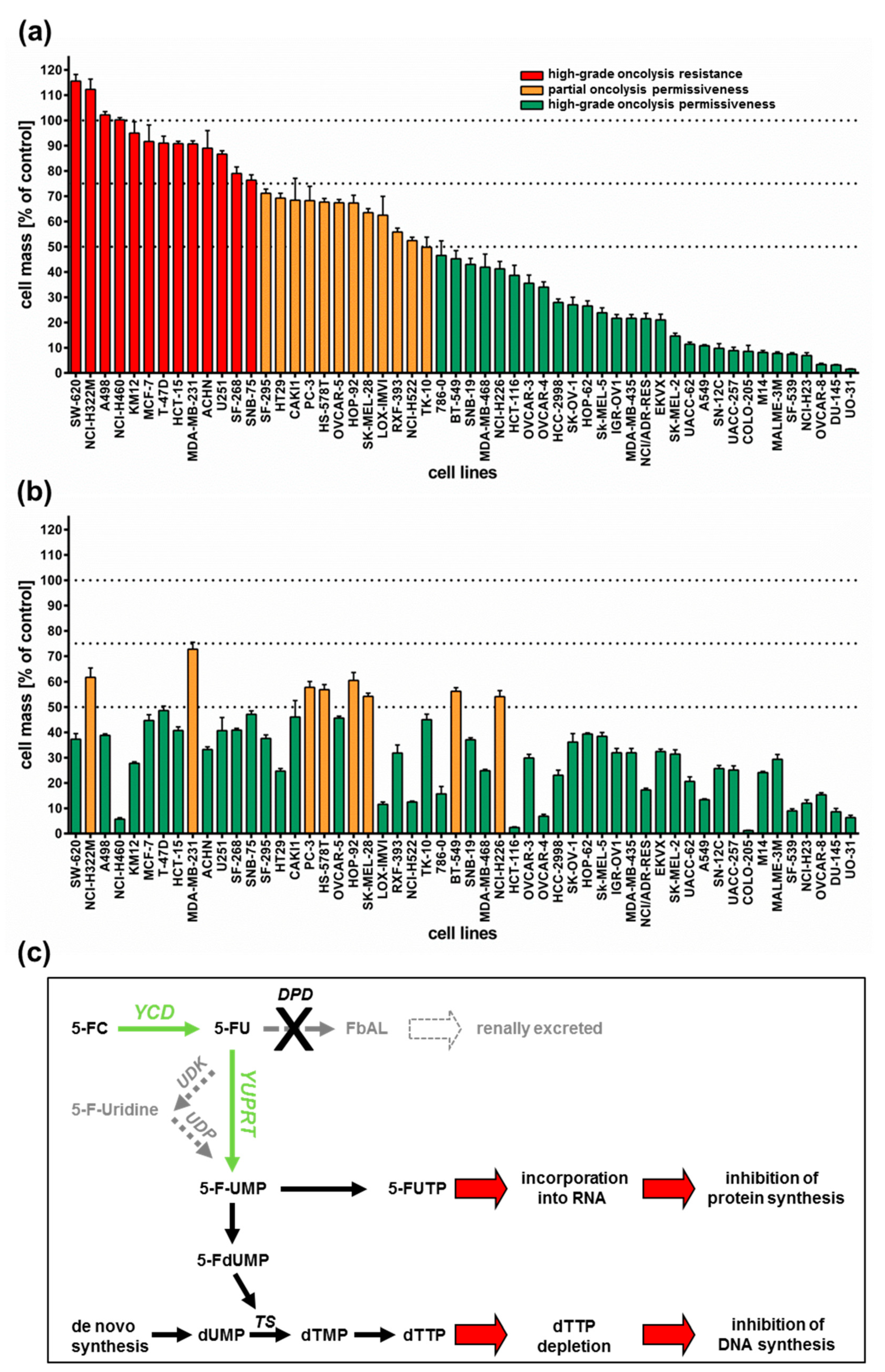
2.1. Screening of the NCI-60 Tumor Panel for Resistances to Oncolysis with GLV-1h94
First, the oncolytic efficacy of the
fcu1 suicide gene-encoding virotherapeutic vector GLV-1h94 alone (i.e., without addition of the prodrug 5-FC) was assessed in a comprehensive and enlarged setting in 54 adherent cell lines derived from solid tumors of the NCI-60 panel, a well-established cancer cell line panel [
14]. Due to great differences in handling, the six leukemia cell lines of the NCI-60 panel were deliberately excluded.
All 54 tumor cell lines were infected with GLV-1h94 at MOI 0.1 and tumor cell masses remaining at 96 h post infection (hpi) were determined by a sulforhodamine B (SRB) assay. With regard to the results, three arbitrary response categories were defined (
Figure 1a). Tumor cell lines in which the cell mass at 96 hpi decreased by less than 25% (in comparison to mock-infected cells) when using an MOI of 0.1 were termed “high-grade resistant” (depicted in red). Tumor cell lines with a remaining cell mass between 50% and 75% were considered to be “partially permissive” (depicted in orange), whereas tumor cell lines with a remaining cell mass of less than 50% were categorized as “high-grade permissive” (depicted in green).
As a result, more than half of the tested tumor cell lines (54%) turned out to be “high-grade permissive” regarding GLV-1h94-mediated oncolysis. Another 22% of the tested tumor cell lines were considered as “partially permissive” and 24% were found to be “high-grade resistant” (
Figure 1a). In each of the eight different tumor entities (breast, central nervous system (CNS), colon, lung, melanoma, ovarian, prostate, and renal cancer), “high-grade permissive”, “partially permissive”, and “high-grade resistant” tumor cell lines could be identified. Interestingly, we did not find any correlation between oncolytic permissiveness and origin/phenotype of the different solid tumor cell lines. However, it was observed that melanoma cell lines in particular reacted very positively to GLV-1h94 infections, seven of nine melanoma cell lines being high-grade permissive. In this context, it is of great interest that an early clinical study already successfully investigated the direct i.t. treatment of malignant melanomas using a wild-type vaccinia virus. In this study, six out of ten patients responded with a complete remission after intralesional (i.l.) virus application [
16]. With regard to the current progress of clinical virotherapy, T-VEC, another genetically modified DNA virus, was approved in the US and in Europe in 2015 as the world’s first viral drug (Imlygic
®) for immunotherapy of patients with unresectable, locally advanced or distant metastatic melanoma [
17]. This data correlation gives reason to believe that malignant melanoma represents a prototypic tumor disease that can be effectively treated by virotherapy.
Next, we investigated whether addition of the prodrug 5-fluorocytosine (5-FC) was able to enhance the anti-tumoral efficacy of GLV-1h94, thus exploiting the
fcu1 suicide gene function (an overview of the 5-FC conversion system is depicted in
Figure 1c). For this purpose, 5-FC was added at 3 hpi and SRB assays were performed at 96 hpi as described (
Figure 1b). Interestingly, all tumor cell lines, which previously were rated as “high-grade resistant” to GLV-1h94 infection now turned out to be “partially permissive” or even “high-grade permissive” tumor cell lines after combined treatment of GLV-1h94 (MOI 0.1) and 5-FC (1 mmol/l) (
Figure 1b; please note that all tumor cell lines are depicted in the same order as used before in
Figure 1a). In particular, administering the prodrug 5-FC post infection changed the pattern of the 13 formerly “red”/“high-grade resistant” tumor cell lines (
Figure 1a) to 11 “high-grade permissive” and two “partially permissive” cell lines (
Figure 1b). Furthermore, out of the tumor cell lines previously classified as “partially permissive” (
n = 12; depicted in yellow in
Figure 1a), four stayed “partially permissive” to combined treatment with no change in the remaining tumor cell masses, whereas eight cell lines turned into “high-grade permissive” tumor cell lines. This observation can be explained by the successful intracellular conversion of the prodrug 5-FC to 5-FU, which exerts its cytotoxic effect not only directly on infected cells but also on uninfected neighboring cells via its strong bystander effect [
18,
19]. Similar results are shown in a recent publication, in which the anti-tumor effect of attenuated vaccinia
Tian Tan strain Guang 9 (VG9), with active yeast cytosine deaminase (CD) expression and thymidine kinase (TK) deficiency, was evaluated in different cancer cell lines. Wild-type VG9 and VG9-CD both showed an identical oncolytic potential, which, however, was significantly different depending on the treated cell line. Their experiments also showed that the addition of 5-FC to VG9-CD-infected cells increased the oncolytic effect significantly compared to VG9-CD treatments alone. The authors postulate that the synergetic effect was more effective when the VG9-CD titers reached at least a level of MOI 1, indicating that the conversion efficiency of CD is only effective above a certain concentration of the expressed CD protein [
20]. In a first-in-human study, a modified vaccinia virus
Ankara (MVA) containing the yeast-originated transgene
fcu1 (TG4023) was investigated in patients with metastatic liver tumors [
8]. The clinical trial aimed to assess the maximum tolerated dose (MTD) of TG4023 and the safety, feasibility, and proof of concept (PoC) of TG4023/5-FC combination to deliver high 5-FU concentrations in tumors. Cancer patients underwent a percutaneous i.t. injection of TG4023 on day 1 using ultrasound guidance followed by i.v. and/or oral 5-FC applications for 14 days. In summary, this phase I study demonstrated that i.t. injection of TG4023 was well tolerated and the MTD was defined as 4 × 10
8 pfu. Therapeutic 5-FU concentrations could be documented in tumors, indicating the proof of concept of virus-directed prodrug therapy. Furthermore, locally measured 5-FU concentrations were higher in tumor tissues compared to blood 5-FU levels, which might explain the reduced systemic toxicity of this special kind of tumor-restricted chemotherapy [
8].
In this study, two of 29 tumor cell lines, which before were classified as “high-grade permissive” to GLV-1h94 monotherapy, now exhibited a “partially permissive” pattern when undergoing combined treatment (see breast cancer cell line BT-549 as well as lung cancer cell line NCI-H226). This phenomenon, on the one hand, might be explained by simple assay variations; on the other hand, it also might be reasonable to believe that converted 5-FU reduces the efficacy of the DNA virus GLV-1h94 due to its function as a thymidylate synthase inhibitor and thus not only prohibits genomic DNA but also viral DNA replication and consequently progeny virus synthesis.
Based on these results, all following experiments were performed exemplarily only with the two “high-grade resistant” tumor cell lines NCI-H460 and HCT-15 as well as with the two “high-grade permissive” cell lines OVCAR-8 and DU-145.
2.2. 5-FU Sensitivity Is Cell Line Specific
Based on the results of the NCI-60 tumor cell screening, which found that all tested cell lines reacted to the addition of 5-FC, but individually to varying extents, the 5-FU sensitivity of selected cell lines was investigated. For this assay, the two “high-grade resistant” tumor cell lines NCI-H460 and HCT-15, which showed significant tumor cell reduction with the addition of 5-FC after infection with GLV-1h94 (
Figure 1b), and the two “high-grade permissive” tumor cell lines OVCAR-8 and DU-145, cell lines whose permissivity hardly changed due to prodrug activation (
Figure 1b), were treated with increasing 5-FU concentrations for 24, 48, 72, and 96 h and cell masses were determined by SRB assays.
When comparing all four cell lines, it could be shown that in NCI-H460 cells even small amounts of 5-FU (10
−3 mM) were sufficient to reduce tumor cells to a remaining cell mass of ~60 % after 48 h and ~30 % (
Figure 2) after 96 h. In contrast, higher concentrations of 5-FU were required for the second “high-grade resistant” cell line HCT-15 (
Figure 2a) as well as for both “high-grade permissive” cell lines (
Figure 2b), to achieve nearly the same cell reduction at both time points. These results indicate a cell line-specific sensitivity against 5-FU. It is known that 5-FU sensitivity is influenced by expression levels of dihydropyrimidine dehydrogenase, the genetic status of p53, and DNA mismatch repair genes [
19]. Furthermore, a study from Wang et al. investigated five pairs of 5-FU-resistant and relevant drug-sensitive parental cancer cell lines to unravel specific molecular factors and cellular pathways mediating and/or predicting 5-FU resistance [
21]. They could show that 5-FU resistance is multifactorial and involves some or all of the following cellular pathways: overproduction of 5-FU targets, up-regulation of specific anti-apoptotic proteins, reduced production of 5-FU–activating enzymes, and increased G1 checkpoint stringency with a reduced cell proliferation rate and reduction in DNA synthetic machinery [
21].
2.3. Prodrug Activation Reduces the Replication of Virus Particles in Tumor Cells Regardless of Their Resistance Classification
To get a closer insight into the differences of “high-grade resistant” and “high-grade permissive” tumor cell lines, viral replication was analyzed in the two “high-grade resistant” cell lines NCI-H460 and HCT-15 as well as in the two “high-grade permissive” cell lines OVCAR-8 and DU-145 (
Figure 3).
Surprisingly, the differences in viral replication of GLV-1h94 alone without prodrug activation in “high-grade permissive” or “high-grade resistant” tumor cells were not as distinct as expected (
Figure 3). However, slightly increased replication tended to occur in OVCAR-8 cells, where viral titers at 48 hpi were more than one log level higher than in the other three cell lines (
Figure 3b, left panel). Usually, after tumor cell infection, oncolytic viruses take complete command of the transcription and translation machinery of the virus host cell to produce the largest possible number of progeny virus particles. If the cellular viral load becomes too large, it will lead to oncolysis and, as a result, to a massive release of newly formed infectious virus particles [
4].
Of note, clinical correlates of vaccinia virus-induced oncolysis were demonstrated in a recent phase I trial [
22]. Cancer patients receiving virus construct GLV-1h68 (closely related to GLV-1h94) exhibited a profound replication of GLV-1h68, which resulted in a distinctive oncolysis, as demonstrated by the release of the GLV-1h68-encoded ß-glucuronidase marker protein as well as of the cell-based enzyme lactate dehydrogenase (LDH), which both became detectable in diverse body fluids (e.g., in the patients´ plasma). Thus, clinical evidence of a substantial vaccinia virus-induced oncolysis was provided.
In consequence, substantially higher titers would be expected in the “high-grade permissive” cancer cell lines and in this study the “high-grade resistance” phenomenon of NCI-H460 and HCT-15 cells against GLV-1h94-mediated oncolysis cannot be explained by a reduced viral replication in these cell lines. On the contrary, in a preclinical study with an oncolytic vesicular stomatitis virus (VSV), it was shown that tumor regression of B16 melanomas was not associated with progressive rounds of virus replication and subsequent oncolysis but rather correlated with viral gene expression and the induction of pro-inflammatory reactions in this tumor model [
23].
When investigating GLV-1h94 replication after prodrug activation by the addition of 5-FC, it became noticeable that in all tested cell lines, the formation of progeny virus particles was massively impaired compared to GLV-1h94 replication alone over the entire observation period of 96 hpi (
Figure 3a,b). Accordingly, prodrug activation led to a reduction of viral replication by approximately three log levels independent of the resistance classification of the cell line used. In addition, it could be shown that, in particular, the cell line NCI-H460, which was classified as “high-grade resistant” to GLV-1h94 monotherapy, became “high-grade permissive” after activation of the prodrug system (
Figure 1a,b), although viral replication was severely restricted. This indicates that cell death does not solely depend on viral replication and oncolysis but that the cytotoxic effect of 5-FU, which is formed by virus-produced SCD from 5-FC, plays a major role. Two scenarios are conceivable in this context; either 5-FU reduces the replication of the DNA virus GLV-1h94 due to its function as a thymidylate synthase inhibitor and thus not only inhibits genomic DNA but also viral DNA replication and consequently progeny virus synthesis [
19] or it also might be reasonable that 5-FU blocks the proliferation of tumor cells which is an important prerequisite for productive virus replication [
24]. To what extent each of the postulated scenarios apply and whether there is a possible additional dependence on the cell line-specific resistance classification and the 5-FU sensitivity must be elucidated in further experiments.
2.4. Conversion Rate of 5-FC into 5-FU Depends on the Cell Line-Specific Infection Rate of GLV-1h94
To further elucidate the impact of the chemotherapeutic compound 5-FU, the metabolic conversion rate of 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU) after infection of the “high-grade resistant” cell line NCI-H460 and the “high-grade permissive” cell line OVCAR-8 with GLV-1h94 and the addition of 5-FC was analyzed by mass spectrometry (
Figure 4). Here, we deliberately investigated solely the cell lines NCI-H460 and OVCAR-8, because the metabolic conversion rate is only interesting for cell lines that show strong differences in 5-FU sensitivity, which is not the case for HCT-15 and DU-145.
As expected, the conversion rates in both cell lines differed greatly from each other. Accordingly, the conversion rate in NCI-H460 cells at 96 hpi was below 50% (
Figure 4a), whereas, at the same time, a conversion rate of 100% was detected in OVCAR-8 cells (
Figure 4b). These findings correlate directly with the results of the infection and cell mass studies of the NCI-60 panel (
Figure 1a, b) as well as with the 5-FU sensitivity testing (
Figure 2). The cell line NCI-H460 displayed a very high resistance to GLV-1h94-mediated oncolysis with nearly 100% remaining cell mass after infection with GLV-1h94 alone at 96 hpi (
Figure 1a). Therefore, it can be assumed that only a few cells are infected and only a small amount of SCD is produced. This lack of prodrug-converting enzyme in turn means that little 5-FC can be converted to 5-FU, which explains the conversion rate of less than 50% (
Figure 4a). However, NCI-H460 cells responded very efficiently to the combination of viro- and chemotherapy with a remaining cell mass of 5% after prodrug activation (
Figure 1b). This effect can be explained by the cell line’s very pronounced sensitivity to 5-FU (
Figure 2), indicating that the conversion rate of 5-FC to 5-FU is still high enough to result in massive chemotoxic tumor cell lysis. In contrast, OVCAR-8 is a “high-grade permissive” cell line (3% remaining cell mass 96 hpi with GLV-1h94;
Figure 1a), which is why a large number of cells are initially infected and, therefore, a large amount of prodrug-converting enzyme is expressed in the cells. Hence, the added 5-FC was completely converted into 5-FU and a conversion rate of 100% (
Figure 4b) was measured, which confirms that the conversion rate depends on the cell line-specific infection rate of GLV-1h94. Since a large number of cells are already initially infected and OVCAR-8 cells are less sensitive to 5-FU, the large amount of converted 5-FU does not cause an additional cytotoxic effect. It rather seems that in this context 5-FU has a negative influence on viral replication due to its function as a thymidylate synthase inhibitor, which would explain the slight increase in cell mass 96 hpi after combination therapy (15% remaining cell mass;
Figure 1b).
These complex correlations indicate that the chemotoxic effect of converted 5-FU is directed against the tumor cells as well as against the virus particles. The balance between cell line-specific susceptibility to GLV-1h94-induced oncolysis and 5-FU sensitivity determines which effect predominates.
2.5. Visualization of the Different Targets of 5-FU by Fluorescence Microscopy
To confirm and, more importantly, visualize the developed hypotheses, fluorescence and brightfield images of NCI-H460 and OVCAR-8 (
Figure 5 and
Figure 6) as well as of HCT-15 and DU-145 cells (
Figure 7 and
Figure 8) were taken at 24, 48, 72, and 96 hpi or solely at 96 hpi after GLV-1h94 monotherapy as well as after prodrug activation by the addition of 5-FC. Since the viral construct GLV-1h94 encodes the marker protein GFP, it is possible to observe virus infection by fluorescence microscopy. In the “high-grade resistant” cell lines NCI-H460 (
Figure 5a, left panel) and HCT-15 (
Figure 7a, left panel), infection with GLV-1h94 alone could be proven by detection of GFP expression starting at 24 hpi and increasing until 96 hpi. However, when looking at the cell layer in the corresponding brightfield images, in both cell lines, almost no oncolysis could be detected over the course of infection (
Figure 5a and
Figure 6a, upper panel). This result confirms that both NCI-H460 and HCT-15 are “high-grade resistant” cell lines, which indeed allow infection with GLV-1h94 and corresponding protein expression to a restricted extent but do not respond to virus replication with oncolysis. Interestingly, when activating the prodrug system by adding 5-FC at 3 hpi, no GFP expression could be observed in NCI-H460 cells as early as 24 hpi and throughout the entire course of infection (
Figure 5a, right panel), indicating a reduced viral infection. However, when looking at the brightfield images of the NCI-H460 cell layers, especially at 96 hpi (
Figure 6a, lower panel), it is noticeable that their cell mass was strongly reduced, also confirmed by SRB analysis which revealed a cell mass reduction of ~95 % (
Figure 1b). Based on these results, one could speculate that in this specific cell line, which is very sensitive to 5-FU (
Figure 2), converted 5-FU inhibits cell proliferation itself, which indirectly also stops viral replication, rather than directly inhibits virus replication. Remarkably, when focusing on the brightfield images of the HCT-15 cell layers after prodrug activation over the time course of 96 hpi (
Figure 7a, right panel), and particularly at 96 hpi (
Figure 8a, lower panel), it is noticeable that their cell mass was only reduced to a small extent. This result indicates that, specifically in HCT-15 cells, which have a reduced 5-FU sensitivity compared to NCI-H460, converted 5-FU partially inhibits both cell proliferation and virus replication, resulting in a “combined” cell mass reduction of only about 57 % (
Figure 1b).
When observing the “high-grade permissive” cell lines OVCAR-8 (
Figure 5b, left panel) and DU-145 (
Figure 7b, left panel), it was found that infection with GLV-1h94 alone leads in both cell lines to a massive GFP expression starting at 24 hpi which increases until 96 hpi. In addition, a progressive destruction of both OVCAR-8 and DU-145 cell layers could be observed (
Figure 5b and
Figure 7b, left panel). These microscopic images, especially the close-ups of the 96 hpi time points (
Figure 6b, upper panel;
Figure 8b, upper panel), confirm that both cell lines are “high-grade permissive” cell lines, in which an increase in GLV-1h94 virus replication also led to efficient oncolysis. Surprisingly, when adding 5-FC to the infected cells, no GFP expression and, therefore, reduced viral infection could be observed at 24 hpi and throughout the entire course of infection as in NCI-H460 cells (
Figure 5b and
Figure 7b, right panel). Interestingly, when looking at the close-ups of the 96 hpi time points, both the OVCAR-8 as well as the DU-145 cell layers were significantly less destroyed than after GLV-1h94 monotherapy (
Figure 6b and
Figure 8b, lower panel). These results suggest that in both “high-grade permissive” cell lines, compared to the “high-grade resistant” cell line NCI-H460, the primary infection rate is high, resulting in high amounts of prodrug convertase. Therefore, added 5-FC is completely converted to 5-FU, thus achieving high levels that could actually inhibit cell proliferation. However, both OVCAR-8 and DU-145 are cell lines with low 5-FU sensitivity, so it can be speculated that the high 5-FU concentrations in these specific cell lines directly inhibit virus replication (as reflected by a lack of GFP expression) rather than cell proliferation.
In conclusion, the microscopic analysis of GLV-1h94 infection either as monotherapy or with prodrug activation as combined virochemotherapy confirms that the cytotoxic effect of converted 5-FU indeed depends on the balance between cell line-specific susceptibility to GLV-1h94-induced oncolysis and cell line-inherent 5-FU sensitivity. Thus, in a cell line like OVCAR-8 or DU-145, which are permissive to GLV-1h94 infection and at the same time less sensitive to 5-FU, the cytostatic effect is more likely to be directed towards direct virus replication. If, however, a cell line is more resistant to GLV-1h94, like NCI-H460, but shows high 5-FU sensitivity, converted 5-FU (now in lower amounts) tends to inhibit cell proliferation and thus indirectly virus replication, as the breeding ground for the production of progeny virus particles is missing.