Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors
Abstract
1. Introduction
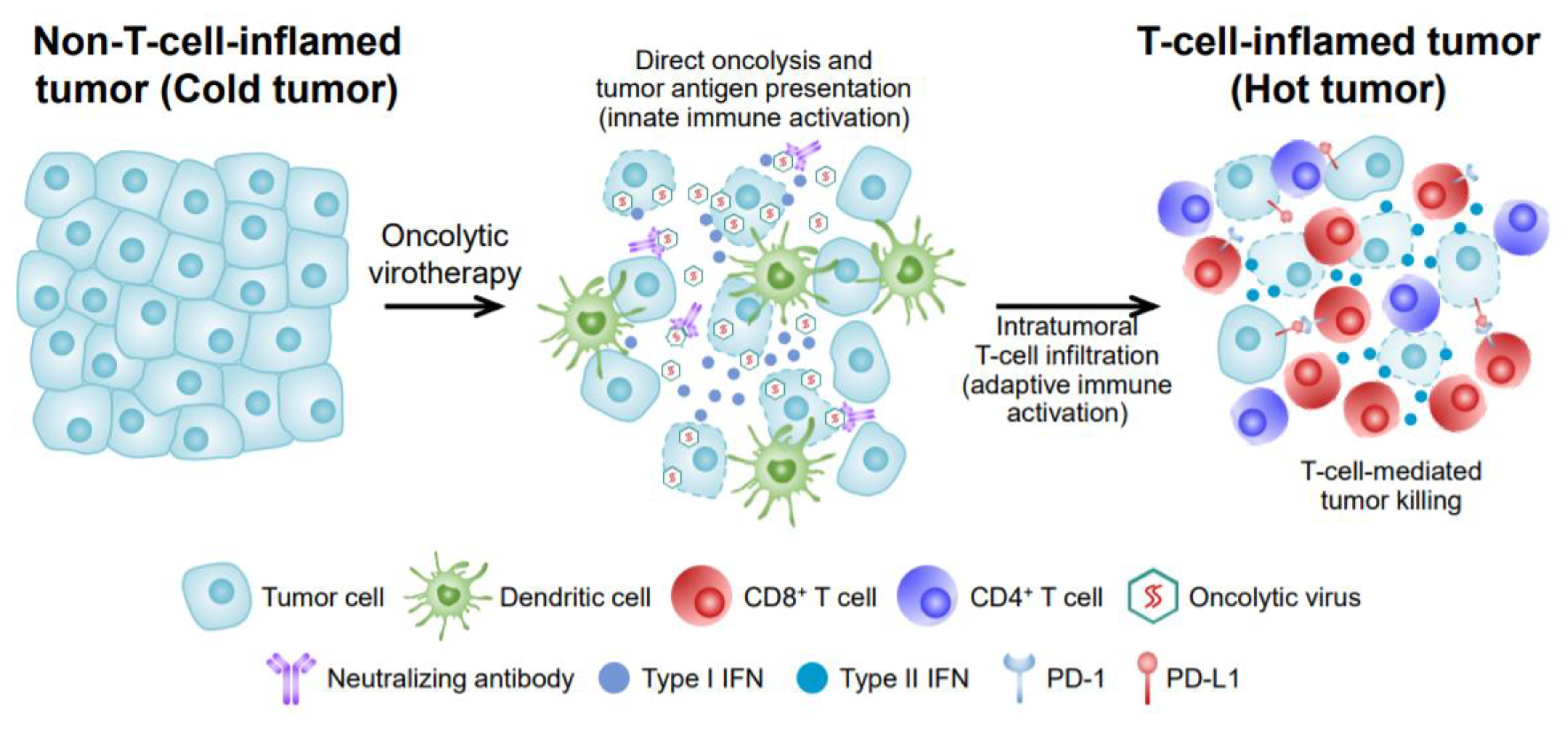
2. Mechanism of OVs’ Anti-Tumor Effects
3. OV Monotherapy
3.1. Melanoma
3.2. OVs against Other Malignant Cancers
4. OV Combination Immunotherapy
4.1. Combination Therapy with OVs and ICIs
4.2. Combination Therapy with OVs and CAR-T Therapy
4.3. Combination Therapy with OVs and BiTEs
5. Current Limitations of OVs for Cancer Treatment
5.1. Choosing Optimal OV Species
5.2. Efficient OV Delivery (Local or Systemic)
5.3. Enhancing Intratumoral OV Infiltration and Diffusion
5.4. Anti-Viral Immunity
5.5. Immunosuppressive TME
6. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Felix, J.; Savvides, S.N. Mechanisms of immunomodulation by mammalian and viral decoy receptors: Insights from structures. Nat. Rev. Immunol. 2017, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.T.; Bell, J.C. Oncolytic virus combination therapy: Killing one bird with two stones. Mol. Ther. 2018, 26, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.J.; Lee, W.S.; Yang, H.; Kong, S.J.; Lee, N.K.; Moon, E.S.; Choi, J.; Han, E.C.; Kim, J.H.; Ahn, J.B.; et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin. Cancer Res. 2019, 25, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Carrascon, V.; Siurala, M.; Santos, J.; Havunen, R.; Tähtinen, S.; Karell, P.; Sorsa, S.; Kanerva, A.; Hemminki, A. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. Oncoimmunology 2018, 7, e1412902. [Google Scholar] [CrossRef]
- Pikor, L.A.; Bell, J.C.; Diallo, J.-S. Oncolytic viruses: Exploiting cancer’s deal with the devil. Trends Cancer 2015, 1, 266–277. [Google Scholar] [CrossRef]
- Shi, T.; Song, X.; Wang, Y.; Liu, F.; Wei, J. Combining oncolytic viruses with cancer immunotherapy: Establishing a new generation of cancer treatment. Front. Immunol. 2020, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Møller, A.-S.W.; Jaderberg, M. Abscopal effect when combining oncolytic adenovirus and checkpoint inhibitor in a humanized NOG mouse model of melanoma. J. Med. Virol. 2019, 91, 1702–1706. [Google Scholar] [CrossRef]
- Kuryk, L.; Møller, A.-S.W.; Jaderberg, M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology 2019, 8, e1532763. [Google Scholar] [CrossRef]
- Siurala, M.; Bramante, S.; Vassilev, L.; Hirvinen, M.; Parviainen, S.; Tähtinen, S.; Guse, K.; Cerullo, V.; Kanerva, A.; Kipar, A.; et al. Oncolytic adenovirus and doxorubicin-based chemotherapy results in synergistic antitumor activity against soft-tissue sarcoma. Int. J. Cancer 2015, 136, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 2016, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Burke, J.; Jonker, D.; Stephenson, J.; Haas, A.R.; Chow, L.Q.M.; Nieva, J.; Hwang, T.-H.; Moon, A.; Patt, R.; et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011, 477, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccines Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef]
- Rehman, H.; Silk, A.W.; Kane, M.P.; Kaufman, H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; López-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C.J.E.; Medicine, M. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Kim, C.; Kim, E.K.; Jung, H.; Chon, H.J.; Han, J.W.; Shin, K.-H.; Hu, H.; Kim, K.S.; Choi, Y.D.; Kim, S.; et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer 2016, 16, 434. [Google Scholar] [CrossRef]
- Tuccitto, A.; Shahaj, E.; Vergani, E.; Ferro, S.; Huber, V.; Rodolfo, M.; Castelli, C.; Rivoltini, L.; Vallacchi, V. Immunosuppressive circuits in tumor microenvironment and their influence on cancer treatment efficacy. Virchows Arch. 2019, 474, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, W.S.; Kong, S.J.; Kim, C.G.; Kim, J.H.; Chang, S.K.; Kim, S.; Kim, G.; Chon, H.J.; Kim, C. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J. Clin. Investig. 2019, 129, 4350–4364. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. The next hurdle in cancer immunotherapy: Overcoming the non-T-cell-inflamed tumor microenvironment. Semin. Oncol. 2015, 42, 663–671. [Google Scholar] [CrossRef]
- Bell, J.C.; Ilkow, C.S. A viro-immunotherapy triple play for the treatment of glioblastoma. Cancer Cell 2017, 32, 133–134. [Google Scholar] [CrossRef] [PubMed]
- De Matos, A.L.; Franco, L.S.; McFadden, G.J.M.T.-M.; Development, C. Oncolytic viruses and the immune system: The dynamic duo. Mol. Ther. Methods Clin. Dev. 2020, 17, 349–358. [Google Scholar] [CrossRef]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 1–15. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic viruses for cancer therapy: Barriers and recent advances. Mol. Ther. Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef]
- Wojton, J.; Kaur, B.J.C. Impact of tumor microenvironment on oncolytic viral therapy. Cytokine Growth Factor Rev. 2010, 21, 127–134. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Arulanandam, R.; De Silva, N.; Thorne, S.H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T.-H.; et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013, 73, 1265–1275. [Google Scholar] [CrossRef]
- Arulanandam, R.; Batenchuk, C.; Angarita, F.A.; Ottolino-Perry, K.; Cousineau, S.; Mottashed, A.; Burgess, E.; Falls, T.J.; De Silva, N.; Tsang, J.; et al. VEGF-mediated induction of PRD1-BF1/Blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell 2015, 28, 210–224. [Google Scholar] [CrossRef]
- Ilkow, C.S.; Marguerie, M.; Batenchuk, C.; Mayer, J.; Ben Neriah, D.; Cousineau, S.; Falls, T.J.; A Jennings, V.; Boileau, M.; Bellamy, D.; et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015, 21, 530. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Ganly, I.; Khuri, F.; Arseneau, J.; Kuhn, J.; Mccarty, T.; Landers, S.; Maples, P.; Romel, L.; Randlev, B.; et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: A phase II trial. Cancer Res. 2000, 60, 6359–6366. [Google Scholar]
- Eissa, I.R.; Villalobos, I.B.; Ichinose, T.; Matsumura, S.; Naoe, Y.; Miyajima, N.; Morimoto, D.; Mukoyama, N.; Zhiwen, W.; Tanaka, M.; et al. The current status and future prospects of oncolytic viruses in clinical trials against melanoma, glioma, pancreatic, and breast cancers. Cancers 2018, 10, 356. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Collichio, F.; Harrington, K.J.; Middleton, M.R.; Downey, G.; Öhrling, K.; Kaufman, H.L. Final analyses of OPTiM: A randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J. Immunother. Cancer 2019, 7, 145. [Google Scholar] [CrossRef]
- Esaki, S.; Goshima, F.; Ozaki, H.; Takano, G.; Hatano, Y.; Kawakita, D.; Ijichi, K.; Watanabe, T.; Sato, Y.; Murata, T. Oncolytic activity of HF10 in head and neck squamous cell carcinomas. Cancer Gene Ther. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Streby, K.A.; Geller, J.I.; Currier, M.A.; Warren, P.S.; Racadio, J.M.; Towbin, A.J.; Vaughan, M.R.; Triplet, M.; Ott-Napier, K.; Dishman, D.J.; et al. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef]
- Aghi, M.K.; Chiocca, E.A. Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol. Ther. 2009, 17, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Hu, P.; Wu, J.; Wang, J.; Li, J.; Lei, L.; Liu, R. The oncolytic herpes simplex virus vector G47∆ effectively targets breast cancer stem cells. Oncol. Rep. 2013, 29, 1108–1114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, C.; Chi, Z.; Si, L.; Sheng, X.; Li, D.; Wang, X.; Lian, B.; Tang, B.; Mao, L.L.; Yan, X. OrienX010 oncolytic viral therapy in phase Ib trial of intralesional injection in unresected stage IIIC to IV acral melanoma patients in China. In Proceedings of the ASCO Annual Meeting, Chicago, IL, USA, 3–7 June 2016. [Google Scholar]
- Khuri, F.R.; Nemunaitis, J.; Ganly, I.; Arseneau, J.; Tannock, I.F.; Romel, L.; Gore, M.; Ironside, J.; MacDougall, R.H.; Heise, C.; et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 2000, 6, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Haavisto, E.; Garofalo, M.; Capasso, C.; Hirvinen, M.; Pesonen, S.; Ranki, T.; Vassilev, L.; Cerullo, V. Synergistic anti-tumor efficacy of immunogenic adenovirus ONCOS-102 and standard of care chemotherapy in preclinical mesothelioma model. Mol. Ther. 2016, 24, S262. [Google Scholar] [CrossRef]
- Hong, J.; Yun, C.-O. Overcoming the limitations of locally administered oncolytic virotherapy. BMC Biomed. Eng. 2019, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moure, M.; Martinez-Velez, N.; Gonzalez-Huarriz, M.; Marrodán, L.; Cascallo, M.; Alemany, R.; Patiño-García, A.; Alonso, M.M. The oncolytic adenovirus VCN-01 promotes anti-tumor effect in primitive neuroectodermal tumor models. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Milenova, I.; Wenthe, J.; Ståhle, M.; Leja-Jarblad, J.; Ullenhag, G.; Dimberg, A.; Moreno, R.; Alemany, R.; Loskog, A. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin. Cancer Res. 2017, 23, 5846–5857. [Google Scholar] [CrossRef]
- Philbrick, B.; Adamson, D.C. DNX-2401: An investigational drug for the treatment of recurrent glioblastoma. Expert Opin. Investig. Drugs 2019, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Goel, S.; Aparo, S.; Arora, S.P.; Noronha, N.; Tran, H.; Chakrabarty, R.; Selvaggi, G.; Gutierrez, A.; Coffey, M.; et al. A phase II study of pelareorep (REOLYSIN®) in combination with gemcitabine for patients with advanced pancreatic adenocarcinoma. Cancers 2018, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Packiriswamy, N.; Upreti, D.; Zhou, Y.; Khan, R.; Miller, A.; Diaz, R.M.; Rooney, C.M.; Dispenzieri, A.; Peng, K.-W.; Russell, S.J. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia 2020, 1–13. [Google Scholar] [CrossRef]
- Keshavarz, M.; Nejad, A.S.M.; Esghaei, M.; Bokharaei-Salim, F.; Dianat-Moghadam, H.; Keyvani, H.; Ghaemi, A. Oncolytic Newcastle disease virus reduces growth of cervical cancer cell by inducing apoptosis. Saudi J. Biol. Sci. 2020, 27, 47–52. [Google Scholar] [CrossRef]
- Lacroix, J.; Leuchs, B.; Li, J.; Hristov, G.; Deubzer, H.E.; Kulozik, A.E.; Rommelaere, J.; Schlehofer, J.R.; Witt, O. Parvovirus H1 selectively induces cytotoxic effects on human neuroblastoma cells. Int. J. Cancer 2010, 127, 1230–1239. [Google Scholar] [CrossRef]
- Bradley, S.; Jakes, A.D.; Harrington, K.; Pandha, H.; Melcher, A.; Errington-Mais, F. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. 2014, 3, 47. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Whitley, R.; Kimberlin, D.W.; Prober, C.G. Pathogenesis and disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kohlhapp, F.J.; Kaufman, H.L. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin. Cancer Res. 2016, 22, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Collichio, F.A.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.; Spitler, L.; Puzanov, I.; Agarwala, S.; Milhem, M.; et al. Final planned overall survival (OS) from OPTiM, a randomized Phase III trial of talimogene laherparepvec (T-VEC) versus GM-CSF for the treatment of unresected stage IIIB/C/IV melanoma (NCT00769704). J. Immunother. Cancer 2014, 2, P263. [Google Scholar] [CrossRef]
- Todo, T. Results of Phase II Clinical Trial of Oncolytic Herpes Virus G47 Delta in Patients with Glioblastoma. Neuro-Oncology 2019, 21, vi4. [Google Scholar] [CrossRef]
- Pourshams, A.; Sepanlou, S.G.; Ikuta, K.S.; Bisignano, C.; Safiri, S.; Roshandel, G.; Sharif, M.; Khatibian, M.; Fitzmaurice, C.; Nixon, M.R.; et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef]
- Skelton, R.A.; Javed, A.; Zheng, L.; He, J. Overcoming the resistance of pancreatic cancer to immune checkpoint inhibitors. J. Surg. Oncol. 2017, 116, 55–62. [Google Scholar] [CrossRef]
- Koujima, T.; Tazawa, H.; Ieda, T.; Araki, H.; Fushimi, T.; Shoji, R.; Kuroda, S.; Kikuchi, S.; Yoshida, R.; Umeda, Y.; et al. Oncolytic Virus-Mediated Targeting of the ERK Signaling Pathway Inhibits Invasive Propensity in Human Pancreatic Cancer. Mol. Ther. Oncolytics 2020, 17, 107–117. [Google Scholar] [CrossRef]
- Ghouse, S.M.; Nguyen, H.-M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Nair, V.S.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B.J.C. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2018, 36, 1658. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017, 170, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Talimogene laherparepvec (T-VEC) in combination (combo) with ipilimumab (ipi) versus ipi alone for advanced melanoma: 3-year landmark analysis of a randomized, open-label, phase II trial. Ann. Oncol. 2019, 30. [Google Scholar] [CrossRef]
- Hecht, J.R.; Pless, M.; Cubillo, A.; Calvo, A.; Raman, S.; Liu, C.; Chan, E.; Chesney, J.A.; Prat, A. Early safety from a phase 1, multicenter, open-label clinical trial of talimogene laherparepvec (T-VEC) injected into liver tumors. J. Clin. Oncol. 2018, 36, 438. [Google Scholar] [CrossRef]
- Hecht, J.R.; Pless, M.; Cubillo, A.; Calvo, A.; Chon, H.J.; Liu, C.; Snyder, W.; Chan, E.; Chaney, M.F.; Chesney, J.A. Early safety from a phase I, multicenter, open-label clinical trial of talimogene laherparepvec (T-VEC) injected (inj) into liver tumors in combination with pembrolizumab (pem). J. Clin. Oncol. 2020, 38, 3015. [Google Scholar] [CrossRef]
- Rha, S.Y.; Merchan, J.; Oh, S.Y.; Kim, C.; Bae, W.K.; Lee, H.W.; Dillon, M.; Deering, R.; Kroog, G.; Ha, K.S. A phase Ib study of recombinant vaccinia virus in combination with immune checkpoint inhibition (ICI) in advanced renal cell carcinoma (RCC). In Proceedings of the AACR Annual Meeting 2020, Philadelphia, PA, USA, 22–24 June 2020. [Google Scholar]
- Nishio, N.; Diaconu, I.; Liu, H.; Cerullo, V.; Caruana, I.; Hoyos, V.; Bouchier-Hayes, L.; Savoldo, B.; Dotti, G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor–modified T cells in solid tumors. Cancer Res. 2014, 74, 5195–5205. [Google Scholar] [CrossRef]
- Shaw, A.R.; Porter, C.E.; Watanabe, N.; Tanoue, K.; Sikora, A.; Gottschalk, S.; Brenner, M.K.; Suzuki, M. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol. Ther. 2017, 25, 2440–2451. [Google Scholar] [CrossRef]
- Park, A.K.; Fong, Y.; Kim, S.-I.; Yang, J.; Murad, J.P.; Lu, J.; Jeang, B.; Chang, W.-C.; Chen, N.G.; Thomas, S.H.; et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020, 12, eaaz1863. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Brinkmann, U. Bispecific antibodies. Drug Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef]
- Suryadevara, C.M.; Gedeon, P.C.; Sanchez-Perez, L.; Verla, T.; Alvarez-Breckenridge, C.; Choi, B.D.; Fecci, P.E.; Sampson, J.H. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology 2015, 4, e1008339. [Google Scholar] [CrossRef]
- Yu, S.; Li, A.; Liu, Q.; Yuan, X.; Xu, H.; Jiao, D.; Pestell, R.G.; Han, X.; Wu, K. Recent advances of bispecific antibodies in solid tumors. J. Hematol. Oncol. 2017, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.A.; Guedan, S.; Rojas, L.A.; Moreno, R.; Arias-Badia, M.; de Sostoa, J.; June, C.H.; Alemany, R. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017, 77, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.; Fajardo, C.A.; Posey, A.D.; Shaw, C.; Da, T.; Young, R.M.; Alemany, R.; June, C.H.; Guedan, S. Improving CART-cell therapy of solid tumors with oncolytic virus–driven production of a bispecific T-cell engager. Cancer Immunol. Res. 2018, 6, 605–616. [Google Scholar] [CrossRef]
- Flemming, A. Trifunctional antibodies unleash NK cells. Nat. Rev. Cancer 2019, 19, 369. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 2019, 177, 1701–1713. [Google Scholar] [CrossRef]
- Hagedorn, C.; Kreppel, F. Capsid Engineering of Adenovirus Vectors: Overcoming Early Vector–Host Interactions for Therapy. Hum. Gene Ther. 2017, 28, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002, 9, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.S.; Lemoine, N.R.; Wang, Y. Systemic delivery of oncolytic viruses: Hopes and hurdles. Adv. Virol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Martinez-Quintanilla, J.; Seah, I.; Chua, M.; Shah, K. Oncolytic viruses: Overcoming translational challenges. J. Clin. Investig. 2019, 129, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Doronin, K.; Shashkova, E.V.; May, S.M.; Hofherr, S.E.; Barry, M.A. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum. Gene Ther. 2009, 20, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Green, N.; Herbert, C.W.; Hale, S.; Hale, A.; Mautner, V.; Harkins, R.; Hermiston, T.; Ulbrich, K.; Fisher, K.; Seymour, L. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004, 11, 1256–1263. [Google Scholar] [CrossRef]
- Hill, C.; Carlisle, R. Achieving systemic delivery of oncolytic viruses. Expert Opin. Drug Deliv. 2019, 16, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Choi, I.-K.; Lee, H.-S.; Yan, H.H.; Son, M.K.; Ahn, H.M.; Hong, J.; Yun, C.-O.; Hong, S.-S. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. 2017, 396, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Lee, Y.; Yoo, J.; Yoon, A.; Kim, H.; Kim, D.; Seidler, D.; Kim, J.H.; Yun, C.O. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 2010, 17, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Guedan, S.; Rojas, J.J.; Gros, A.; Mercade, E.; Cascallo, M.; Alemany, R.J.M.T. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol. Ther. 2010, 18, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, N.; Poirier, J.T.; Burga, L.N.; Bostina, M. Virus–Receptor Interactions and Virus Neutralization: Insights for Oncolytic Virus Development. Oncolytic Virother. 2020, 9, 1. [Google Scholar] [CrossRef]
- Davies, D.H.; McCausland, M.M.; Valdez, C.; Huynh, D.; Hernandez, J.E.; Mu, Y.; Hirst, S.; Villarreal, L.; Felgner, P.L.; Crotty, S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 2005, 79, 11724–11733. [Google Scholar] [CrossRef]
- Chakradhar, S. Viral vanguard: Designing cancer-killing viruses to chase metastatic tumors. Nat. Med. 2017, 23, 652–655. [Google Scholar] [CrossRef]
- Guedan, S.; Alemany, R. CAR-T cells and oncolytic viruses: Joining forces to overcome the solid tumor challenge. Front. Immunol. 2018, 9, 2460. [Google Scholar] [CrossRef]
- Sharp, D.W.; Lattime, E.C. Recombinant poxvirus and the tumor microenvironment: Oncolysis, immune regulation and immunization. Biomedicines 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
| Type of Virus | OV | Genetic Modifications | Cancer Type |
|---|---|---|---|
| Herpes simplex virus-1 (HSV-1, DNA virus) | T-VEC (talimogene laherparepvec, Imlygic) | ICP34.5 and ICP47 deletion, GM-CSF insertion | Melanoma [34] |
| HF10 (canerpaturev, C-REV) | Loss of expression of UL43, Ul49.5, UL55, UL56, and LAT | Head and neck cancer [35] | |
| HSV1716 (Seprehvir) | ICP34.5 deletion | Extracranial cancers [36] | |
| G207 | ICP34.5 deletion, ICP6 deletion, and LacZ insertion | Glioblastoma [37] | |
| G47∆ | ICP34.5 deletion, ICP6 deletion, ICP47 deletion, and LacZ insertion | Breast cancer [38] | |
| OrienX010 | ICP34.5 and ICP47 deletion, GM-CSF insertion | Melanoma [39] | |
| Vaccinia viruses (DNA virus) | Pexastimogene devacirepvec (Pexa-Vec) | Thymidine kinase deletion, GM-CSF insertion | Hepatocellular carcinoma, renal cell carcinoma [5] |
| Adenoviruses (DNA virus) | H101 (Oncorine) | E1B deletion and E3 partial deletion | Head and neck cancer [39] |
| ONYX-015 | E1B-55 KDa gene deletion | Head and neck cancer [40] | |
| ONCOS-102 (formerly named CGTG-102) | adeno∆24-RGD-GM-CSF insertion | Mesothelioma [41] Prostate cancer [42] Melanoma [10] Ovarian and colorectal cancer [31] | |
| VCN-01 | pRb-dependent; loaded with genes encoding PH20 hyaluronidase | Primitive neuroectodermal tumor [43] | |
| LOAd-703 | pRb-dependent; loaded with genes encoding CD40L and 4-1BBL | Pancreatic cancer [44] | |
| DNX-2401 | Deletion in 24bp in EIA and RGD-motif was engineered into the fiber H-loop, enabling the virus to use αvβ3 or αvβ5 an integrins to enter cells | Recurrent glioblastoma [45] | |
| Reovirus (RNA virus) | Pelareorep (Reolysin) | Natural virus | Pancreatic cancer [46] |
| Paramyxoviridae (RNA virus) | Measles virus | hNIS insertion for MV-NIS and CEA insertion for MV-CEA | Multiple myeloma [47] |
| Newcastle disease virus (NDV) | Natural virus | Cervical cancer [48] | |
| Parvovirus (RNA virus) | Parvovirus H-1 (ParvOryx) | Natural virus | Neuroblastoma [49] |
| Picornaviruses (RNA virus) | CVA21 (Cavatak) | Natural virus | Melanoma, breast cancer [50] |
| PVSRIPO | CD155/Necl5-dependent poliovirus; the internal ribosome entry site (IRES) of the poliovirus replaced with the IRES from human rhinovirus type 2 (HRV2) | Glioblastoma [51] |
| Type of Virus | OV | Immune Checkpoint Inhibitor | Phase | Cancer Type | Route of OV Administration | NCT Number |
|---|---|---|---|---|---|---|
| HSV-1 | Talimogene laherparepvec, Imlygic (T-VEC) | Ipilimumab | I/II | Melanoma | IT | NCT01740297 |
| Pembrolizumab | III | Melanoma | IT | NCT02263508 | ||
| Pembrolizumab | I | Head and neck cancer | IT | NCT02626000 | ||
| Nivolumab | II | Lymphoma and non-melanoma skin cancers | IT | NCT02978625 | ||
| HF10 (canerpaturev, C-REV) | Ipilimumab | II | Melanoma | IT | NCT02272855 | |
| Ipilimumab | II | Melanoma | IT | NCT03153085 | ||
| Vaccinia virus | Pexastimogene devacirepvec (Pexa-Vec) | Ipilimumab | I | Advanced solid tumors | IT | NCT02977156 |
| Durvalumab/tremelimumab | I | Colorectal cancer | IV | NCT03206073 | ||
| Nivolumab | I/II | Hepatocellular carcinoma | IT | NCT03071094 | ||
| Cemiplimab | I | Renal cell carcinoma | IV, IT | NCT03294083 | ||
| Adenovirus | ONCOS-102 | Pembrolizumab | I | Advanced or unresectable melanoma | IT | NCT03003676 |
| Durvalumab | I/II | Advanced peritoneal cancers | IP | NCT02963831 | ||
| LOAd703 | Atezolizumab | I/IIa | Pancreatic cancer | IT | NCT02705196 | |
| p53 transduced adenovirus (Ad-p53) | Pembrolizumab | I/II | Head and neck cancer | IA | NCT02842125 | |
| Nivolumab | II | Head and neck cancer | IT | NCT03544723 | ||
| Adenovirus vaccine expressing MAGE-A3 (Ad-MAGEA3) | Pembrolizumab | I/II | Non-small cell lung cancer | IM | NCT02879760 | |
| Pembrolizumab | I | Metastatic melanoma and Squamous cell skin carcinoma | IM | NCT03773744 | ||
| Coxsackie virus | CVA21 (Cavatak) | Pembrolizumab | I | Melanoma | IT | NCT02565992 |
| Pembrolizumab | I | Non-small cell lung cancer and bladder cancer | IV | NCT02043665 | ||
| Ipilimumab | I | Melanoma | IV | NCT03408587 | ||
| Reovirus | Pelareorep (Reolysin) | Pembrolizumab | I | Advanced pancreatic adenocarcinoma | IV | NCT02620423 |
| Nivolumab | I | Relapsed/refractory multiple myeloma | IV | NCT03605719 | ||
| VSV | VSV-hIFNbeta-sodium iodide | Avelumab | I | Malignant solid tumor | IT | NCT02923466 |
| VSV-IFNβ-NIS | Pembrolizumab | I | Non-small cell lung cancer and hepatocellular carcinoma | IV | NCT03647163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, C.-M.; Chon, H.J.; Kim, C. Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors. Int. J. Mol. Sci. 2020, 21, 7743. https://doi.org/10.3390/ijms21207743
Oh C-M, Chon HJ, Kim C. Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors. International Journal of Molecular Sciences. 2020; 21(20):7743. https://doi.org/10.3390/ijms21207743
Chicago/Turabian StyleOh, Chang-Myung, Hong Jae Chon, and Chan Kim. 2020. "Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors" International Journal of Molecular Sciences 21, no. 20: 7743. https://doi.org/10.3390/ijms21207743
APA StyleOh, C.-M., Chon, H. J., & Kim, C. (2020). Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors. International Journal of Molecular Sciences, 21(20), 7743. https://doi.org/10.3390/ijms21207743





