1. Introduction
Coronary artery disease (CAD) is the leading cause of mortality worldwide [
1] and ensues from the narrowing of the blood vessel lumen due to the accumulation of atherosclerotic plaques. A complication of CAD results from the rupture of the developing plaques, leading to a culprit thrombosis that manifests as a myocardial infarction [
2]. The introduction of reperfusion therapy has presented a clinical paradox; on the one hand, it greatly contributes to the increase in survival rates post-acute myocardial infarction (AMI), due to relief of the blockage in the affected coronary artery and resumption of blood flow to the infarcted myocardium [
3]. On the other hand, it results in reperfusion injury, consisting of a series of insults to the myocardium due to the production of reactive oxygen species (ROS), mitochondrial dysfunction, and immune cell infiltration and activation [
4].
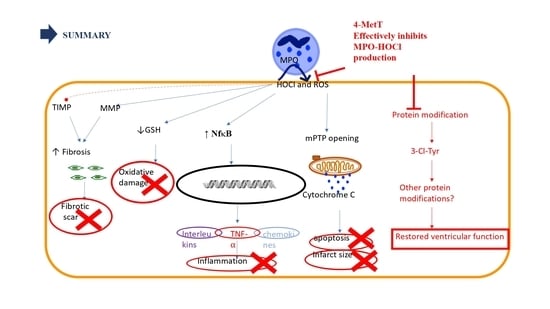
Neutrophils are the predominant infiltrating cell 24–48 h post myocardial reperfusion [
5]; these infiltrating cells remove necrotic and extracellular matrix debris and stimulate the infiltration of inflammatory and then non-inflammatory macrophages, which act to resolve the ongoing inflammation and initiate the process of healing [
6]. Neutrophils also release various proteolytic enzymes that are essential for the formation of non-contractile collagen scars, an essential component of myocardial healing post-AMI. Upon infiltration, neutrophils degranulate and release the enzyme myeloperoxidase (MPO), an enzyme that catalyses the formation of hypohalous acids, including hypochlorous acid (HOCl), the predominant product formed under physiological conditions. HOCl is a contributor to reperfusion injury, as it induces many downstream modifications to cellular targets which have the potential to impair myocardial functioning and increases oxidative stress in the myocardium. For example, the potent oxidant HOCl impairs the bioactivity of nitric oxide (NO) by uncoupling electron flow through damaging eNOS [
7]. Oxidising HOCl also modifies thiol residues and many redox sensitive amino acids (cysteine, tyrosine, and methionine). For example, HOCl induces the chlorination of tyrosine residues, producing the biomarker 3-chlorotyrosine, which is used for quantification of HOCl-induced protein damage [
8,
9].
Neutrophil HOCl also oxidises cysteine thiol residues in the active site of matrix metalloproteinases (MMPs) [
10] and the N-terminal cysteine in their inhibitors (TIMPs) [
11]; these enzymes are essential for extracellular matrix (ECM) remodelling. This results in the overactivation of MMPs and deactivation of TIMPs, causing excessive ECM breakdown which results in excessive fibrosis and tissue remodelling, thereby impairing cardiac contractility and contributing to the progression to heart failure. Similarly, HOCl oxidises thiol residues in the active site of the antioxidants glutathione (GSH) and peroxiredoxins [
12], which impairs the major endogenous antioxidant defence mechanisms in the myocardium, thereby rendering it more susceptible to oxidative stress and damage. Furthermore, HOCl and other hypohalous acids can dysregulate multiple intercellular signalling pathways, including kinase signalling (p38 and Erk2) [
13] and inactivation of protein tyrosine phosphatase (PTP) [
14]. HOCl can also influence cell viability, as cultured cardiomyocytes or endothelial cells demonstrate increased cell apoptosis and necrosis in the presence of HOCl [
15,
16]. Therefore, the modification of amino acid residues, disruption of the phosphoproteome balance, depletion of the antioxidant pool, increase in inflammation and fibrosis, and dysregulation of endothelial function all impair cellular and mitochondrial function and result in cytotoxicity.
Clinical studies have reported the role of MPO as a prognostic factor for mortality post-AMI [
17,
18], secondary cardiovascular complications, atrial fibrillation [
19] and heart failure [
20]. As such, due to the contribution of neutrophils to post-AMI injury, targeting this cell type may represent a promising way to limit this form of injury. However, neutrophils also play beneficial roles in the healing of the myocardium post AMI and therefore selective targeting of MPO and its downstream oxidants; most importantly, HOCl provides a better approach to decreasing HOCl-mediated cytotoxicity after reperfusion without impacting scar formation.
Nitroxides are a class of reversible MPO inhibitors that has received wide interest in the field of reperfusion therapy in the kidney [
21], brain [
22], intestinal [
23], and ovarian tissue [
24]. These cell permeable small molecules show low toxicity and are currently being used in vivo as therapeutics for radiation induced macular degeneration and alopecia [
25]. They act via various mechanisms such as superoxide dismutase (SOD) mimetics, free radical scavengers, and reversible MPO inhibitors with catalytic self-regeneration [
26,
27]. Importantly, nitroxides inhibit HOCl production by acting as substrates for MPO compound I [
28]. Nitroxides have been trialled within in vitro cultures of cardiomyoblast H9c2 cells exposed to activated neutrophils, where they were shown to alleviate the HOCl-induced increase in oxidative stress, phosphoproteome dysregulation, and apoptosis [
14]. Accordingly, the current study aims to assess whether the administration of the nitroxide 4-methoxyTEMPO (4-MetT) will provide cardioprotection through inhibiting the neutrophil-MPO activity that occurs following AMI.
2. Materials and Methods
2.1. Chemicals
Chemicals used in the H&E and PSR stains were freshly prepared and of the highest quality. All other chemicals were of the highest purity and sourced from Sigma Aldrich (Castle Hill, NSW, Australia and St Louis, MO, USA), unless otherwise stated.
2.2. AMI Acute and Chronic Model
Male Wistar rats weighing 150–200 g were purchased from the Animal Resources Centre (Perth, WA, Australia) and were fed a diet of standard chow and housed in accordance with the local animal ethics guidelines for the South West Area Health Services Animal Ethics Committee (Protocol approval #2017/032; date of approval: date of approval: 1 November 2017) with an acclimation period of 1 week prior to surgical intervention. All cohorts were provided standard chow and water ad libitum for the duration of the study. Rats were split into respective cohorts, receiving a bolus dose of drug (15 mg/kg 4MetT) or equivalent volume of vehicle (0.9% saline) control (Sham and AMI/R) through oral gavage immediately prior to surgical intervention (acute study) and twice daily at 8 h intervals for the duration of the chronic study. To ensure tolerance of the dosing regimen and repeated animal handling, rat weights were recorded daily.
Anaesthesia was induced in an induction chamber (Advanced Anaesthesia Specialists, Gladesville, NSW, Australia) using 5% (v/v) vaporised isoflurane. The animal was then positioned supine and endotracheal intubation was performed using a blunt-end polyurethane 16-guage intravenous catheter fitted to a graduated 1 mL syringe. Anaesthesia was then maintained with 2% (v/v) isoflurane and the animals were ventilated at a rate of 75 breaths per min with a tidal volume of 10 mL per kg body weight of 0.2 mL O2/min using a small animal ventilator (Harvard, Holliston, MA). Immediately prior to surgery, an intramuscular injection of lignocaine (10 mg/kg body weight) and an i.p. injection of buprenorphine (0.1 mg/kg) were administered.
The upper thorax was then shaved, cleaned using Betadine antiseptic, and the chest opened at the 5th intercostal space with an incision parallel to the ribs. The heart was then exposed by spreading the ribs using a self-retaining retractor (World Precision Instruments, Sarasota, FL, USA). The pericardium was opened using sharp forceps and the left anterior descending (LAD) coronary artery was visualised and ligated using a 6/0 silk suture, which was passed through the myocardium deep to the vessel and through a snare to create a transient ligation for 30 min. Confirmation of vessel ligation was obtained through visualisation of blanching of the left ventricle anterior wall below the stitch. After 30 min, the snare was released, the suture removed from the heart, and reperfusion confirmed by the returned blood flow to the ischemic myocardium. The chest cavity was closed with layered sutures, a subcutaneous injection of lignocaine applied at the wound site, and the animal was revived and placed in an isolated chamber for recovery. Regular pain relief (10% buprenorphine solution in jelly) was provided for up to 72 h, commencing immediately upon the animal awakening. For the surgical Sham Control, the operative technique was identical except that no ligation of the LAD coronary artery was performed. Following either 24 h or 28 days of recovery, rats were anaesthetised as above, echocardiography was performed, and the hearts were excised for biochemical and histological analysis.
2.3. Echocardiogram Analysis of Functional Parameters
A transthoracic echocardiogram in the parasternal short axis position at the mid-ventricle level was performed at 24 h or 4 weeks post I/R injury prior to the rats being euthanised for subsequent tissue harvesting. Echocardiograms were performed using a SonoSite Edge II Ultrasound System (Bothell, WA, USA) using a HSL25x/13-6 MHz transducer (Fujifilm Sonosite, Bothell, WA, USA), viewed in M-mode to measure the LV end-systolic diameter (LVESD) and LV end-diastolic diameter (LVEDD). The mean measurements of three independent recordings performed in triplicate for each rat were used to calculate the fractional shortening (FS) and ejection fraction (EF) as detailed below:
2.4. Biochemical Assays
Excised hearts were divided, and half the infarct tissue was homogenised using a rotating piston and Teflon coated glass tube (Wheaton, Millville, NJ, USA), as described in detail previously [
29,
30]. Hearts were homogenised in 1 mL lysis buffer [50 mM phosphate buffered saline (pH 7.4), 1 mM EDTA, 10 μm 2,6-Di-tert-butyl-4-methylphenol, 1 tablet/50 mL protease inhibitor cocktail (Roche, Basel, Switzerland), 1 tablet/10 mL PhosStop tablets (Roche, Basel, Switzerland)]. Total protein content of each aliquot was determined using a BCA assay as per the manufacturer’s instructions using Bicinchoninic acid assay [Sigma-Aldrich, Sydney Australia] in test samples and serially diluted bovine serum albumin (BSA)/MilliQ water to construct a standard concentration curve.
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS) of 3-Cl-Tyr/Tyr Ratio
Levels of 3-chlorotyrosine measured relative to total Tyrosine levels (3-Cl-Tyr/Tyr) were measured using liquid chromatography coupled with mass spectrometry as described previously [
31,
32]. Proteins were precipitated with 0.3%
w/v deoxycholic acid and 50%
w/v trichloroacetic acid (TCA) and centrifuged at (7500×
g; 5 °C, 2 min) to remove the supernatant. Pellets were washed in 5%
w/v TCA, centrifuged, washed again in 100% ice cold acetone, and centrifuged. Isolated pellets were treated with 4M methanesulfonic acid and 0.2%
w/v labelled tyrosine (13C9, 15N, L-Tyr; Cambridge isotope laboratories, Inc., Tewksbury, MA, USA) as well as a labelled chloro-tyrsine (13C9, 15N, L-Cl-Tyr; Sigma Aldrich, Sydney, Australia) internal standard mixture.
Next, the samples were placed in hydrolysis vessels (Eldex Laboratories, Napa, CA, USA) degassed with a vacuum pump, then were flushed with argon and heated at 110 °C for 16 h. Solid phase extraction was performed in chromatography extraction cartridges (Supelclean, Envi-Chrom, 250 mg, St. Louis, MO, USA) with 0.1% w/v TFA and 80% methanol v/v as the eluent. The eluate was dried in a vacuum centrifuge (Eppendorf, NSW, Australia, 60 °C, 3 h) then reconstituted in 0.1% v/v formic acid dispersed in HPLC grade water. Quantitative LC/MS was performed using a liquid chromatography triple quadrupole mass spectrometer 8050 (Shimadzu Corporation, Kyoto, Japan). Analyte separation was conducted using Agilent Zorbax Eclipse XDB-C181 (4.6 × 50 mm, 1.8 μm) with a UPLC Zorbax Eclipse XDB-C18 (4.6 × 50 mm, 1.8 μm) guard column. Eluent form the column was directed to a tandem for electrospray ionization MS with a dry gas flow of 10 L/min. All analytes were detected using multiple reaction monitoring (MRM) with using argon as the collision gas. Data was generated and analysed using the LabSolutions quantitative analysis software (Version 5.91, Shimadzu Corp, Kyoto, Japan).
2.6. Protein Tyrosine Phosphatase Activity Assay
Phosphatase activity was measured by a spectrophotometric assay as described in detail elsewhere [
33]. This assay monitors the conversion of the colourless p-nitrophenyl phosphate (p-NPP) substrate to the yellow p-nitrophenyl (p-NP) product. Equal amounts of homogenate protein (5 μg from each sample as determined by BCA assay above) were diluted in 0.5 mM MgCl
2 in PBS in a COSTAR 96 well flat bottom plate. p-NPP substrate (pH 7.4) was added to each well and absorbance readings were obtained at 405nm (every 20 min for 10 h, 37 °C) with a TECAN M200 PRO plate reader (Tecan, Austria). The concentration of p-NP produced was calculated using the Beer Lambert law: ΔA = εΔC
pNPl with the path length (l) being 1 cm and the extinction coefficient for p-NP at 405 nm ε = 18 mM
−1 cm
−1. The rate of p-NP production in relation to time was plotted and phosphatase activity (nM/s) was determined by calculating the gradient of the plotted line during the first 80 min of the reaction.
2.7. Western Blot Analysis of p-p38
Levels of phosphorylated p38 were determined by Western blotting following protein separation by SDS-PAGE. Equal amounts of protein (5 μg) from each sample were diluted in MilliQ water, mixed with loading buffer (1/10 ß-mercaptoethanol in 4 × Laemmli sample buffer (Bio-Rad, Sydney, Australia), denatured at 95 °C for 5 min and loaded onto fast cast SDS-PAGE gels (TGX Stain-Free FastCast Acrylamide Kit; thickness 1 mm), which were prepared according to the manufacturer’s instructions. Proteins were resolved at 200 V for 45 min in resolving buffer [composition: 25 mM Tris, 192 mM glycine and 0.1% SDS]. Resolved proteins were imaged on a Bio-Rad ChemiDoc imaging system with Image LabTM software (version 5.2), which was used to calculate total protein content. Next, the resolved proteins were transferred onto an Immobilon Polyvinylidene Fluoride (PVDF; Millipore) membrane using a Bio-Rad Trans-Blot Turbo blotting system (Bio-Rad, Sydney, Australia) at (1 A; 25 V; 30 min) in transfer buffer [composition: 25 mM Tris, 190 mM glycine, 20% (v/v) methanol in 1 L of MilliQ water]. PVDF membranes were imaged on the Bio-Rad ChemiDoc imaging system.
Non-specific binding was minimised by incubating the membranes with 5% (w/v) BSA in Tris Buffered Saline with Tween-20 (TBST) [composition: 25 mM Tris base, 140 mM sodium chloride (NaCl), 4 mM potassium chloride (KCl), 0.1% Tween 20 in 1 L of MilliQ water] blocking solution on a rocker at 22 °C for 4 h. p-p38 was detected by incubation of the membrane with 1:1000 (v/v) MAPK phospho-p38 (p-p38; Cell Signalling technology) primary antibody in 1% (w/v) BSA in TBST. Excess primary antibody was washed, and binding antibody was labelled through incubation with 1:10,000 peroxidase labelled anti-rabbit IgG secondary antibody in 1% w/v BSA in TBST with rocking (22 °C; 1 h). Following washing steps, membranes were dipped in Immobilion Forte Western HRP substrate (Millipore) and proteins were visualised by chemiluminescence on the Bio-Rad ChemiDoc imaging system. Western Blot quantification was performed by using quantitative densitometry using Image Lab software by normalising p-p38 levels against corresponding total protein levels measured in the same lane imaged directly from membranes prior to transfer to PVDF, as described above. A ratio of the volume of p38 to total protein volume was calculated for each sample and divided by the protein-normalised ratio detected in the surgical SHAM group to obtain the fold-change in p38 phosphorylation in treatment groups relative to the surgical SHAM group.
2.8. GSH/GSSG Assessment
Levels of reduced glutathione (GSH) and glutathione disulphate (GSSG) were measured spectrophotometrically as described previously [
34] by reaction of homogenates with 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB, 1.5 mg/mL) at 412 nm. To determine GSH levels; serial dilutions of GSH and GSSG were prepared from stock solutions in potassium phosphate EDTA buffer (KPE) and used to create a standard curve. Cell homogenates were prepared in 0.1 M KPE (pH 7.5). Blank KPE, GSH, and GSSG standards and cell homogenates in KPE were loaded into a COSTAR 96 well flat bottom plate. A mixture of DTNB: glutathione reductase (GR) solution (1:1 ratio) was added to each well for 30 s followed by β-NADPH; absorbance was read at 412 nm every 30 s for 2 min.
To determine GSSG levels, cell homogenates were pre-treated with 10% v/v 2-vinylpydrine (Sigma Aldrich, USA; 1 h; room temperature) then 10% v/v triethanolamine (TEAM, Sigma Aldrich, USA; pH 6–7; 10 min); absorbance was measured at 412 nm every 30 s for 2 min.
2.9. Histological Staining and Quantification of Infarct Size
Heart blocks were fixed in formalin, embedded in paraffin and sectioned on a rotary paraffin microtome (5 μm sections) to be used in all histological analysis. Slides were stained with haematoxylin and eosin to allow visualisation of infarcts and associated neutrophil infiltration. Slides were scanned using Zeiss AxioScan.Z1 at 20× magnification to obtain an image of the compete heart section. The infarct areas were quantified using QuPath software (v0.1.2). The infarct was outlined, and the total area of the infarct section was digitally determined by the software in μm2. The area of the ventricles was subtracted from the total area of the stained heart section to generate a relative area value for the full myocardial section. A normalised ratio of the infarct area: relative area was compared between all 3 groups. Infarct area quantification was performed by using two independent double-blinded assessors.
2.10. PSR Staining and Collagen Scar Quantification
Tissue sections were deparaffinised and rehydrated through a series of graded alcohols, immersed in a Picro Sirius red solution (22 °C; 1 h) and de-differentiated by dipping 3 times in acidified water. The slides were dehydrated, coverslipped, imaged on the ZEISS Axio Scan.Z1 Slide Scanner at 20× magnification, and collagen scar area was quantified using QuPath Software as described above.
2.11. TUNEL Staining of Non-Viable/Apoptotic Cell Levels
Cell viability in tissue sections was detected using a TUNEL fluorometric kit (Promega, NSW, Australia) according to the protocol supplied by the manufacturer. The slides were deparaffinised in xylene then rehydrated by immersion through a series of graded alcohols, washed in 0.85% v/v NaCl then PBS, fixed in 4% v/v paraformaldehyde in PBS, washed in PBS, and permeabilised in 20 μg/mL proteinase K (22 °C; 10 min).
Next, the slides were washed in PBS then fixed in 4% v/v paraformaldehyde, washed in PBS, and incubated with equilibration buffer (22 °C; 10 min) then with rTdT incubation buffer [90% Equilibration Buffer, 10% Nucleotide Mix, 2% rTdT Enzyme (37 °C; 1 h)]. Reaction was stopped by immersion in 2xSSC, slides were washed in PBS, and nuclei were counterstained in 1:600 Spectral DAPI (PerkinElmer, USA) in TBST. Fluorescent slides were imaged on the ZEISS AXIO SCOPE upright fluorescent microscope (Zeiss, NSW, Australia) and the level of cardiomyocyte viability was quantified using MetaMorph® image analysis software (version 7.6; Molecular Devices, San Jose, CA, USA). An intensity threshold was manually selected to include all fluorescent apoptotic cells. The intensity of fluorescence was measured as integrated OD and normalised against the section area.
2.12. OPAL Multiplexing of Inflammatory Markers
OPAL multiplex immunohistochemistry allows the simultaneous staining of 6 different antigens of interest using the OPAL kit supplied by the manufacturer (PerkinElmer, Billerica, MA, USA). Thin tissue sections were deparaffinised in xylene then rehydrated by immersion through a series of graded alcohols. Antigen retrieval was performed in a pH6 retrieval buffer using a pressure cooker (125 °C, 30 s). The slides were washed in water and TBST, endogenous peroxidase activity was blocked (3% v/v H2O2; 22 °C; 5 min) and slides were washed again in TBST. Non-specific protein binding was minimised by incubation with a DAKO casein blocking agent (Agilent Technology, USA, 22 °C; 30 min) then slides were incubated with anti-MDA primary antibody (Abcam, VIC, Australia; 1:1000 v/v in antibody diluent; 22 °C; 1 h). Unbound antibody was washed with TBST (3 × 3 min) and primary antibody was labelled with Envision secondary antibody (DAKO, USA; 22 °C; 30 min). Slides were washed in TBST and incubated with the TSA fluorophore; OPAL690 (1:50 v/v in TSA diluent; 22 °C; 10 min). All subsequent steps were performed in the dark.
Excess TSA was washed with TBST and slides were microwaved in a pH9 retrieval buffer positioned in a water bath. The apparatus was heated (1000 W microwave, 100% power, 2 min), followed by 20 min of heating at 20% power. This microwaving process removed preformed complexes while leaving the bound fluorophore intact and the tissue ready for addition of the next antibody. This process was repeated for the following primary antibody and OPAL-fluorophore combinations: (i) pH9 retrieval buffer for the anti-p-p65 primary antibody (Abcam, Australia; 1:400 v/v in antibody diluent; 22 °C; 1 h) with OPAL620 (1:50 v/v in TSA diluent; 22 °C; 10 min); (ii) pH9 retrieval buffer for the anti-MAPK p-p38 primary antibody (Cell signalling, QLD, Australia; 1:200 v/v in antibody diluent; 22 °C; 1 h) with OPAL 570 (1:50 v/v in TSA diluent; 22 °C; 10 min) (iii) pH6 retrieval buffer for the anti-TNF primary antibody (Abcam, Australia; 1:500 v/v in antibody diluent; 22 °C; 1 h) with OPAL540 (1:50 v/v in TSA diluent; 22 °C; 10 min) employed as the secondary agent and (iv) pH6 retrieval buffer for the anti-MPO primary antibody (Abcam, Australia; 1:200 v/v in antibody diluent; 22 °C; 1 h) with OPAL520 (1:50 v/v in TSA diluent; 22 °C; 10 min).
Following a final wash, slides were incubated with 0.5% w/v Sudan Black B in 70% v/v ethanol (22 °C; 5 min) to decrease autofluorescence. Finally, slides were washed, and nuclei were counterstained with spectral DAPI (1:1000 v/v in TBST; 22 °C; 10 min), mounted with an aqueous mounting medium (DAKO, USA) and coverslips were applied. OPAL imaging was performed on a PerkinElmer MANTRA microscope (PerkinElmer, MA, USA) with a personal library that was developed using singleplex trials with the fluorophores to be imaged. The recorded spectrum for each fluorophore: OPAL690, OPAL620, OPAL570, OPAL540, OPAL520, and DAPI, was saved to be used when staining test tissue. Furthermore, an autofluorescence spectrum from each fluorophore was saved in the library. Upon image capture, a scale bar was added using the Fiji ImageJ software (version 1.52a) and OPAL quantification was performed using the ImageJ software. The number of cells expressing each marker was quantified and divided by the area of the imaged section expressed in pixels. However, due to the inconsistent staining of the marker MAPK p-p38 between the various myocardial samples from different treatment groups, it was not quantified.
2.13. Statistical Analysis
Statistical analysis of all experiments was performed on GraphPad Prism®; version 7. Where required raw data was tested for normality (distribution) using the Shapiro–Wilk test and Kolmogorov–Smirnov tests embedded in GraphPad with the alpha-value set to 0.05; these analyses indicated that data from AMI groups were normally distributed. Subsequently, all values were compared using one-way ANOVA with a post hoc Tukey test to correct for multiple comparisons or for pairwise comparisons, a Mann–Whitney test was used. Statistical significance was determined as p < 0.05. All graphed results were displayed as mean and standard deviation (SD) unless specified otherwise.
4. Discussions
One of the main contributors towards reperfusion injury is neutrophil infiltration that occurs within 24–48 h post AMI [
5]. Upon activation, infiltrating neutrophils release MPO and this enzyme catalyses the formation of the oxidant HOCl that can elicit protein modifications, dysregulates the cellular phosphoproteome, deplete the GSH antioxidant pool, increases MMP activity, and subsequently promote fibrosis. As such, inhibiting MPO should alleviate HOCl mediated damage to the myocardium post-AMI. This study tested the efficacy of the MPO inhibitor and antioxidant 4-MetT in an experimental model of myocardial infarction. Echocardiogram analysis in rats revealed a significant restoration in EF and FS following daily administration of 4-MetT over 28 days. However, this functional restoration was evident irrespective of infarct size and degree of fibrosis seen in the collagen scar that remained the same in the presence or absence of the drug. Indeed, staining with Picro Sirius Red revealed that chronic administration of the drug failed to decrease the size of the collagen scar post-AMI.
By contrast, LC-MS analysis revealed that 3-Cl-tyrosine levels increased significantly 24 h post-experimental AMI, while 4-MetT administration significantly decreased this biomarker of MPO activity to levels below baseline (SHAM) levels. Given that 3-chlorotyrosine is an established marker of HOCl mediated damage, these results indicate that: (i) reperfusion post experimental AMI induces the activation of neutrophil-MPO to yield HOCl-mediated damage to cardiac tissue, and (ii) the supplemented nitroxide successfully reaches the myocardium and exerts its inhibitory activity on MPO, thereby decreasing MPO-activity (and HOCl production) and validating the study design. HOCl induces several protein modifications which alter the protein structure and result in altered protein function, which could result in depressed myocardial function. This is supported by previous studies which have shown that HOCl induces modifications of several proteins resulting in loss of function of these proteins. For example, HOCl oxidatively modifies horse heart myoglobin in vitro and results in loss of reduction of myoglobin from the ferric to the ferrous state caused by cytochrome b5 reductase, an event essential for oxygen transport caused by myoglobin [
9]. Furthermore, HOCl and another MPO oxidant—HOSCN—modify low density lipoprotein (LDL), thereby interfering with endothelial vasorelaxation and inducing endothelial dysfunction [
35]. Therefore, the drug might ameliorate these protein modifications, and this may account for the restoration of ventricular function observed 28 days after AMI was reported herein.
Infarct visualization by H&E revealed that specimens showed two different phenotypes: infarcts with a mild phenotype, and infarcts with a severe phenotype characterised by intramyocardial haemorrhage (IMH). Infarcts showing haemorrhage displayed excessive tissue damage and larger infarct sizes. This is consistent with studies reporting that IMH is associated with larger infarcts sizes, decreased myocardial salvage and, impaired left ventricular function in patients showing ST-elevation myocardial infarction (STEMI) and was associated with increased adverse cardiovascular events [
36,
37], however the small sample size in this study was insufficient to extrapolate any significant findings from this cohort and as such these specimens were excluded from all subsequent experiments. The pathogenesis of IMH remains unknown, however it has been suggested that the microvascular blockage of the vasculature during reperfusion injury decreases the integrity of the vascular walls, while reperfusion aggravates this permeability and enhances endothelial leakage into the infarcted tissue [
38]. Nonetheless, 4-MetT failed to decrease the infarct size irrespective of the presence or absence of IMH. The effect of MPO inhibitors on modulating infarct size has been controversial as some MPO inhibitors decreased infarct size [
39] while others failed to do so [
40]. Given the association between infarct size and the degree of tissue apoptosis, myocardial viability was assessed by using TUNEL. Interestingly, the drug non-significantly restored myocardial viability and apoptosis levels tended to decrease in infarcts without IMH.
Other downstream markers dysregulated by HOCl were then tested and revealed that the reperfusion significantly depleted total thiols in the myocardium, while drug administration marginally restored levels close to baseline and in turn this correlated with slightly decreased p38 activation, although drug-mediated alterations in these parameters did not reach significance. Notably, MPO
+-staining co-localised with MDA
+ staining, thereby validating the role of neutrophils and HOCl in activating oxidative pathways in the myocardium post-AMI. However, the drug did not show any potential in decreasing the levels of oxidative damage and pro-inflammatory activation. A lack of efficacy for 4MetT in modulating myocardial inflammation and oxidative damage is somewhat contradictory to previous studies that have shown that the MPO inhibitor PF-1355 [
40] and nitroxides [
41] can decrease inflammation in models of ischemia and reperfusion. This discrepancy between the results could be attributed to the drug administration regimen, as the MPO inhibitor PF-1355 was administered for 7 and 21 days [
40] while the nitroxide in the Zhu study discussed below was conjugated to a hydrogel ensuring longevity of the drug for a period of 1 week. In this study, the drug was administered once by oral gavage prior to surgery in the immunofluorescence analysed specimens.
While the nitroxide failed to reduce infarct size, the extent of myocardial fibrosis, and the inflammatory activation and oxidative damage, it significantly restored left ventricular function. This could be explained by the significant decrease in 3-chlorotyrosine levels post-drug administration. As explained above, tyrosine chlorination is a non-specific protein modification which could change the structure of a protein. If this modification is in a site essential for the activity of that protein, then such modification could result in increased or decreased activity. It is also important to consider that HOCl induces many other protein modifications upon release from activated neutrophils [
42], and inhibition of these protein modifications and their disturbance of the protein function could account for the observed restoration of left ventricular function by 4-MetT. In support of this, Vasilyev et al. [
43] reported that MPO induces the oxidation of amino acids such as glycine and threonine into cytotoxic aldehydes in vivo, which are involved in decreasing cardiac function and worsening of left ventricular remodelling. The authors further reported that genetic deletion of MPO in MPO knockout mice restored left ventricular function and attenuated LV remodelling. As such, this suggests that the functional restoration of ventricular function upon inhibition of MPO could be associated with a decrease in the oxidative modification of proteins essential for ventricular function as determined here.
Nitroxides are rapidly reduced to the hydroxylamine form [
44] in vivo; however, in the presence of an oxidative environment such as post-AMI/R the hydroxylamine form can recycle back to the active nitroxide moiety. It is worth noting that in the acute model employed in this study, the drug was only delivered once by oral gavage, suggesting that a proportion could have been rapidly metabolised before being redistributed to the heart. This approach may have resulted in lower concentrations of active drug reaching the heart than established with the cellular model of cardio protection [
14]. Therefore, future studies in this model could increase the oral drug dosage to achieve higher concentrations of active drug in the heart prior to assessing cardio-protection through the antioxidant and anti-inflammatory pathways we have assessed. This assertion is further supported by a novel study which has compared modes of delivery of a nitroxide either directly injected into the heart or delivered covalently bonded to a thermally responsive hydrogel which ensured drug longevity and prolonged drug scavenging properties [
41]. The authors reported that the hydrogel conjugated nitroxide showed a decrease in IL-1b secretion, a decrease in apoptosis, and improved cardiac functioning measured as increased left ventricular end diastolic volume [
41]. This suggests that a strategy to increase drug concentration and half-life results in an improved cardio-protection, and many studies are currently researching new ways to increase the potency of nitroxides in vivo through this mechanism [
44,
45].
Study Limitations
Myocardial stunning (and preconditioning of myocardial tissue) may be an important issue that was not assessed here. Assessing myocardial stunning would require a functional heart assessment soon after reperfusion. However, in the current study design, ultrasound imaging to assess cardiac function was not performed until 24 h after reperfusion (at the earliest), corresponding to a time period where neutrophils are present in the cardiac tissue and the hypothesis that the drug 4-MetT acts to inhibit MPO can be reasonably tested.
Notably, elegant work by Roberto Bolli has suggested that free radical scavenging antioxidants can play a role in minimising myocardial stunning [
46], so there is merit in testing whether 4-MetT diminishes myocardial stunning in addition to inhibiting MPO and corresponding HOCl-mediated tissue damage; the study could then test an additive protective effect (if any impact on stunning were evident). Therefore, an expanded study is warranted and should be considered in future experimental design. Alternative mechanisms to explain cardio-protection also include maintenance of the K(ATP) channel function [
47], a role for nitric oxide [
48], and protein tyrosine kinases [
49] through a nuclear factor-kappaB pathway [
50]. Thus, the current study is somewhat limited in scope and additional studies aiming to combine aspects of radical scavenging that impact on myocardial stunning, maintenance of K(ATP), assessment of NO levels, kinase activity, and transcriptional pathways that link with MPO-inhibition may well identify a complete and novel mechanism of action for this class of cyclic nitroxides.
Another limitation includes the lack of a scrambled (non-MPO targeting) treatment arm in the study design, although physiological saline was included as a non-drug (vehicle) control in the SHAM and AMI groups. Finally, although we reviewed PSR staining as a surrogate for the extent of myocardial scarring after AMI, it would be prudent to review whether 4-MetT acts directly on myofibroblasts that heavily populate the primary infarct zone and are responsible for the overproduction of extracellular matrix through a mechanism involving TGF-beta [
51].