NGF/TRKA Decrease miR-145-5p Levels in Epithelial Ovarian Cancer Cells
Abstract
1. Introduction
2. Results
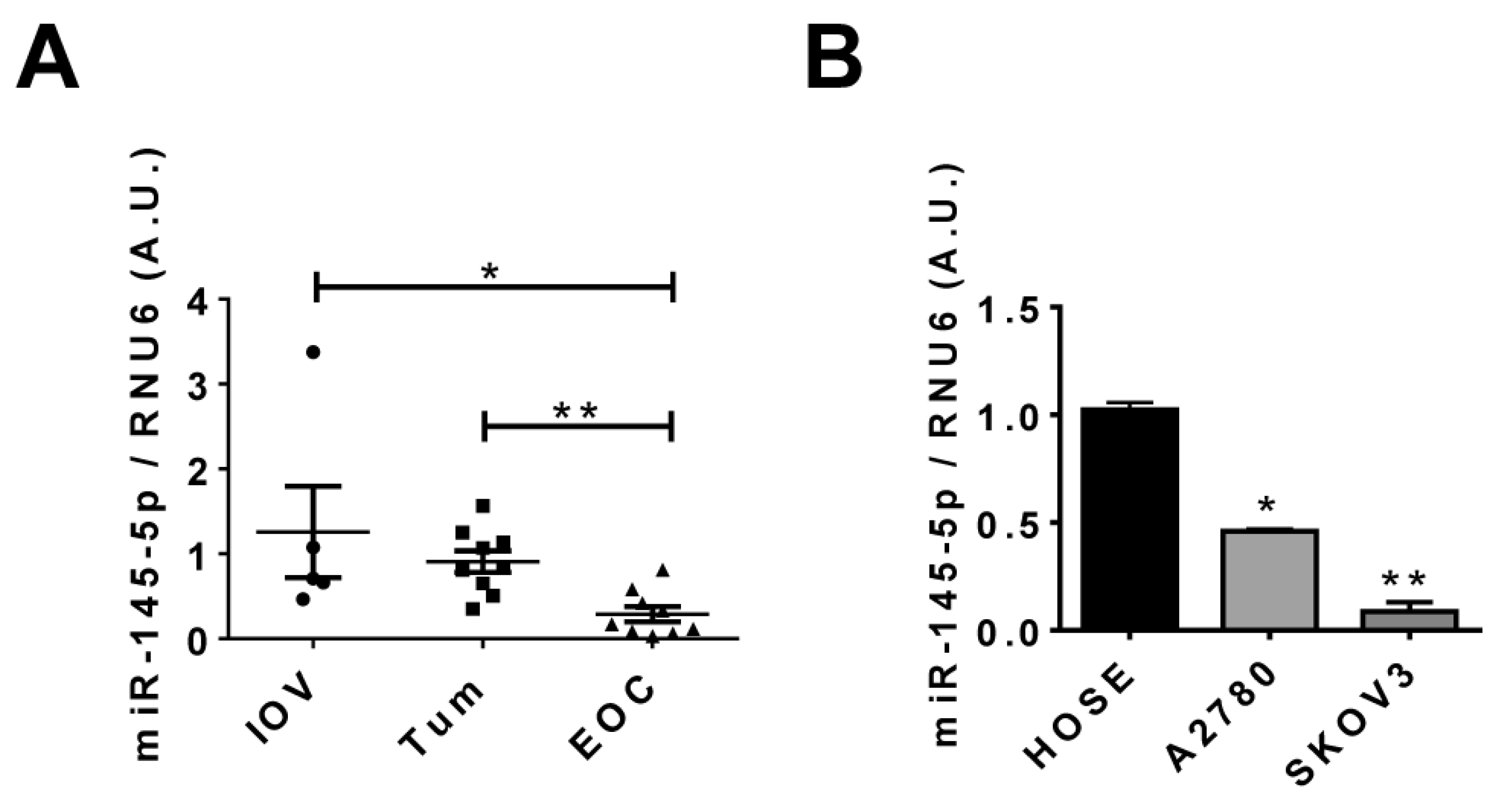
2.1. miR-145-5p Levels Decrease during EOC Progression
2.2. Transient Over-Expression of miR-145 Decreases Cell Proliferation of Ovarian Cells
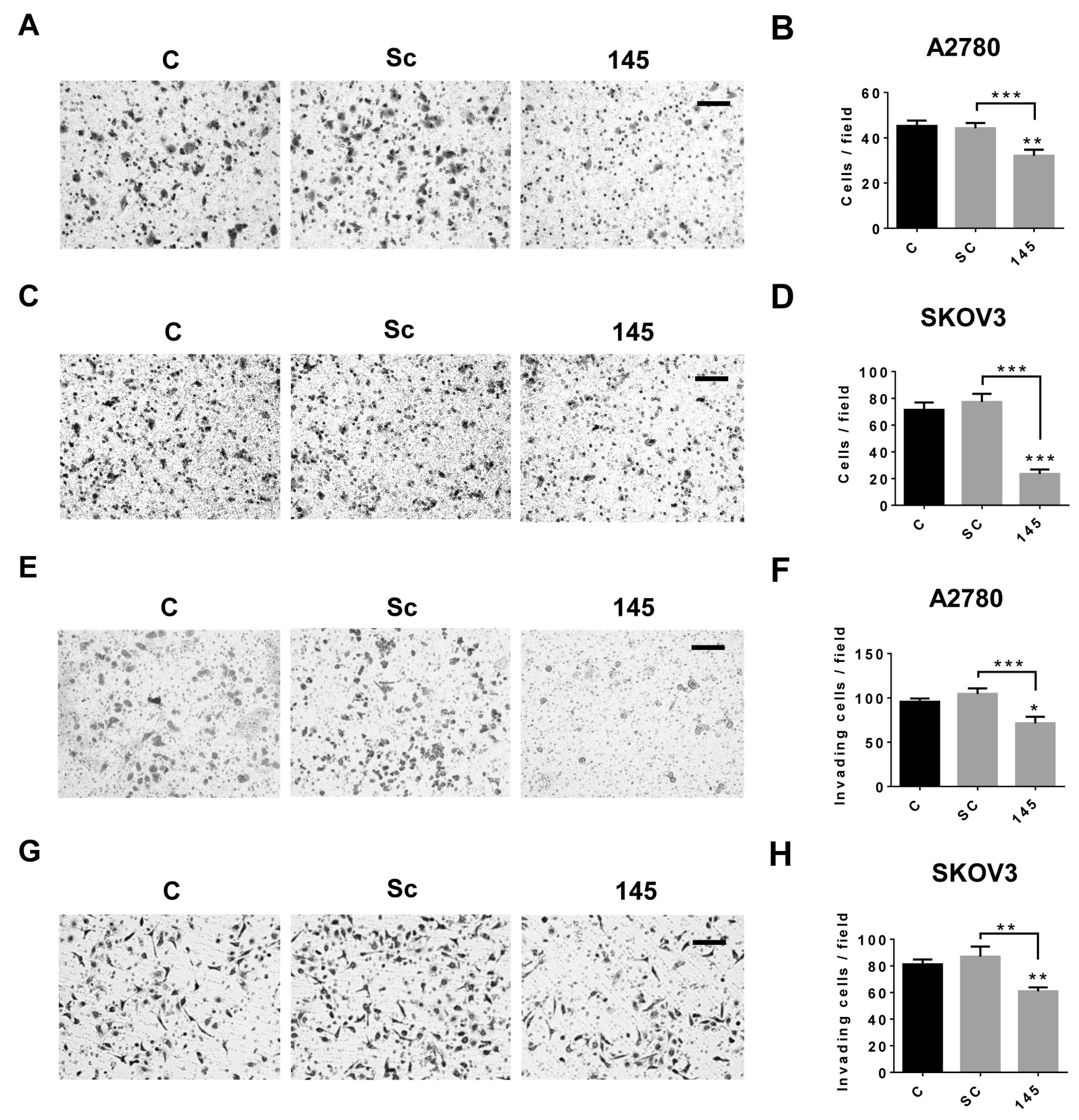
2.3. Over-Expression of miR-145 Decreases Migration and Invasion of EOC Cells
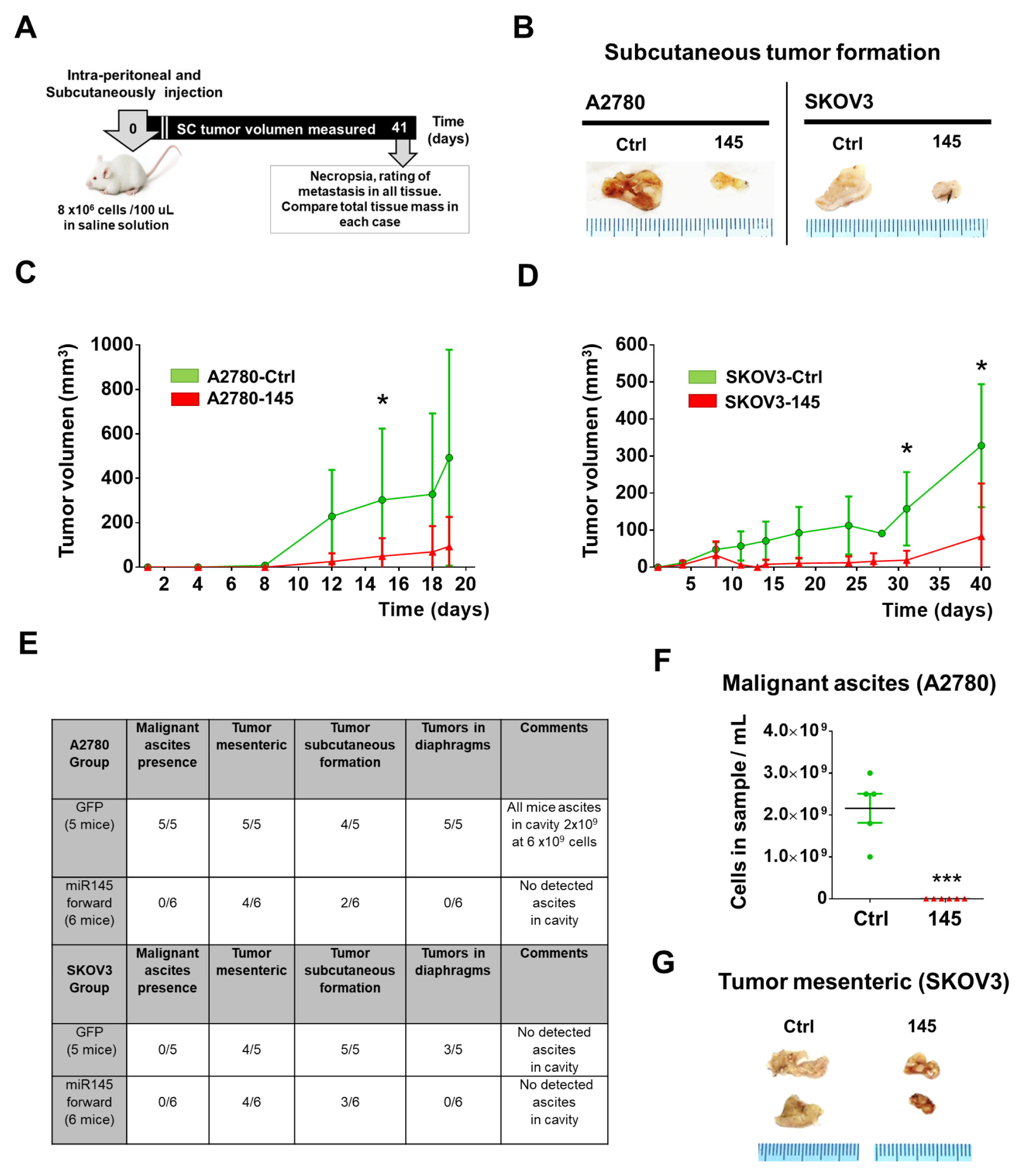
2.4. Over-Expression of miR-145 Decreases Presence of Ascites and Tumor Size of EOC Xenografts
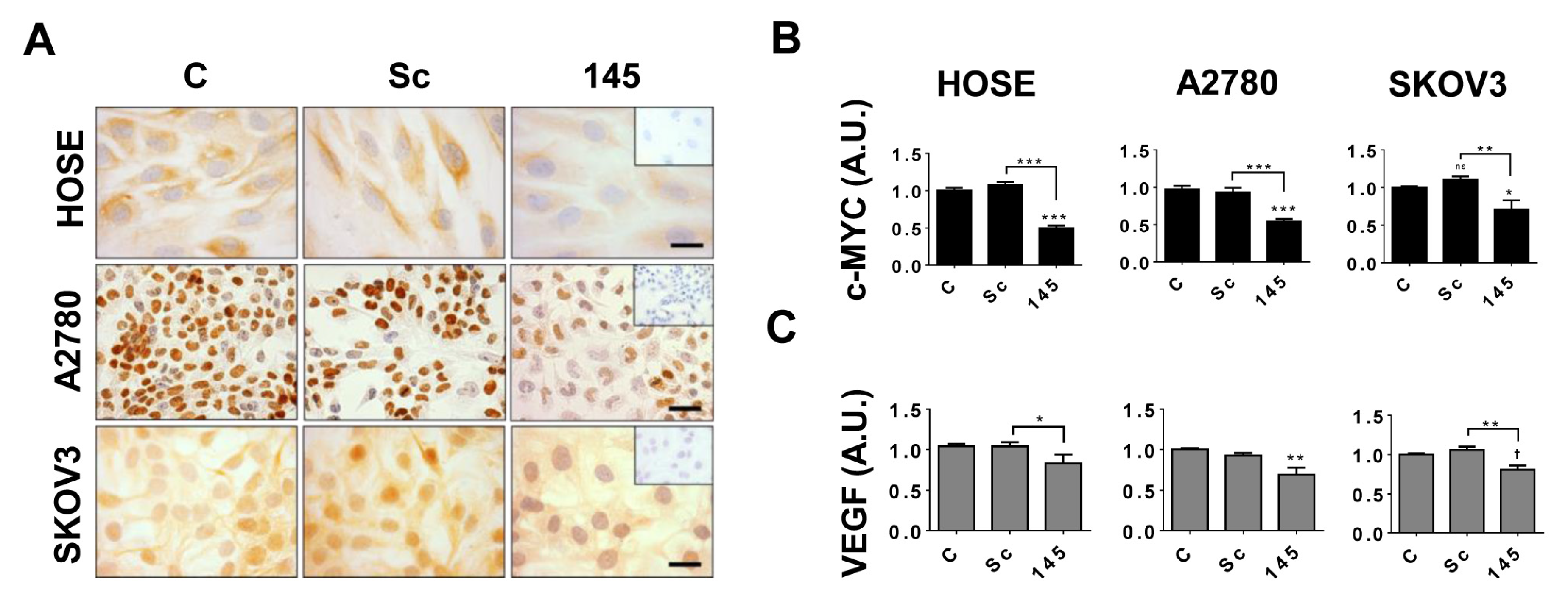
2.5. miR-145 Regulates c-MYC and VEGF Protein Levels in EOC Cells
2.6. In Silico Analysis of miR-145 and NGF/TRKA Targets
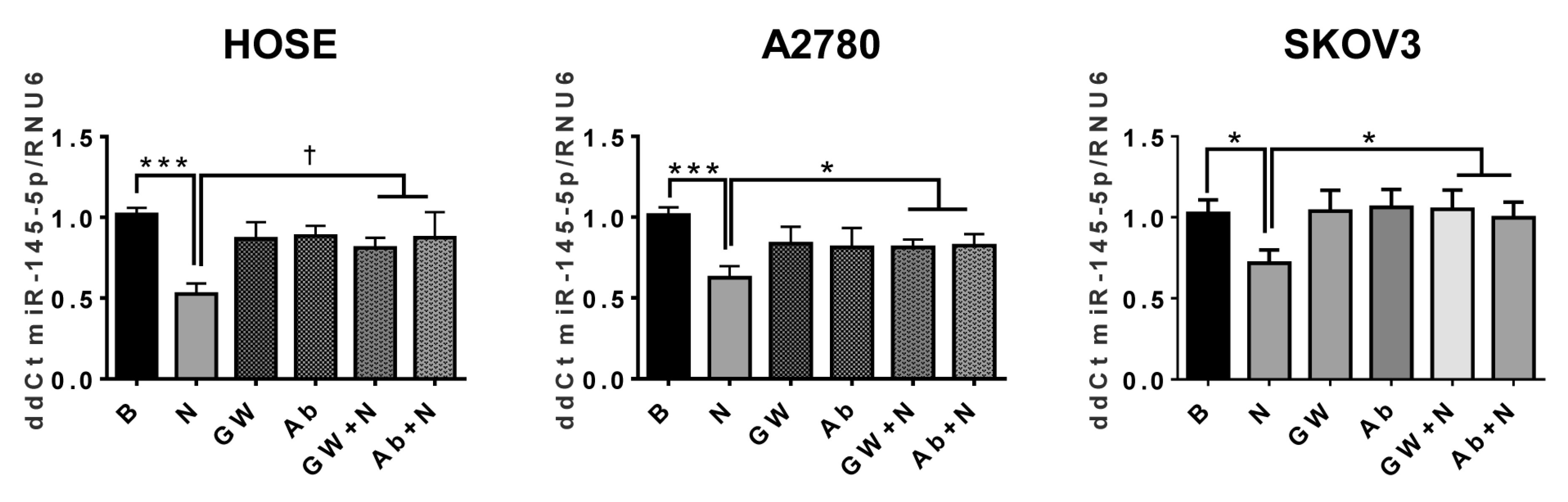
2.7. NGF Decreases miR-145 in Ovarian Cells
2.8. NGF Stimulation Decreases Transcription of miR-145 in EOC Cells
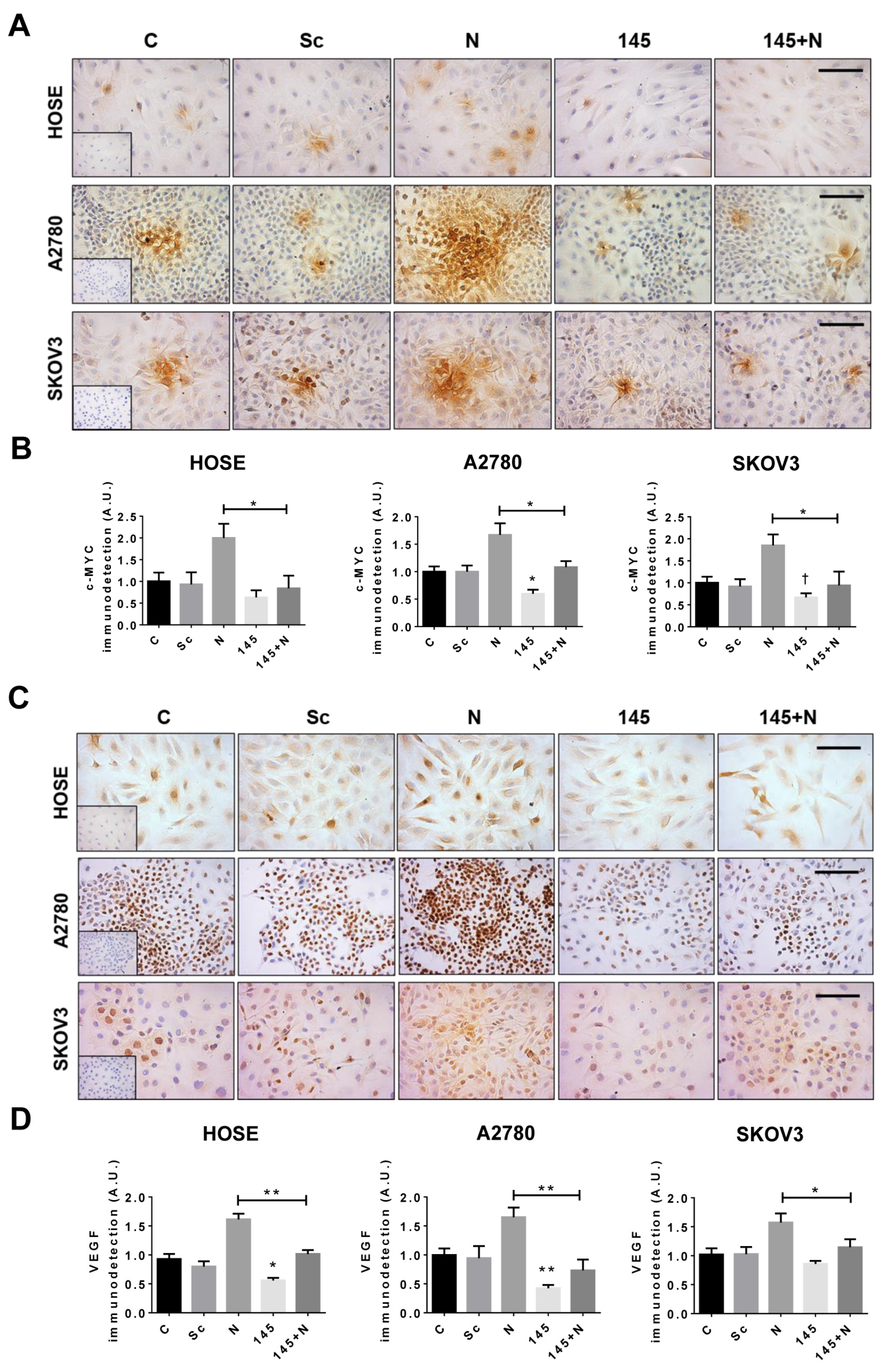
2.9. Overexpression of miR-145 Blocks the NGF-Mediated Effects in Epithelial Ovarian Cells
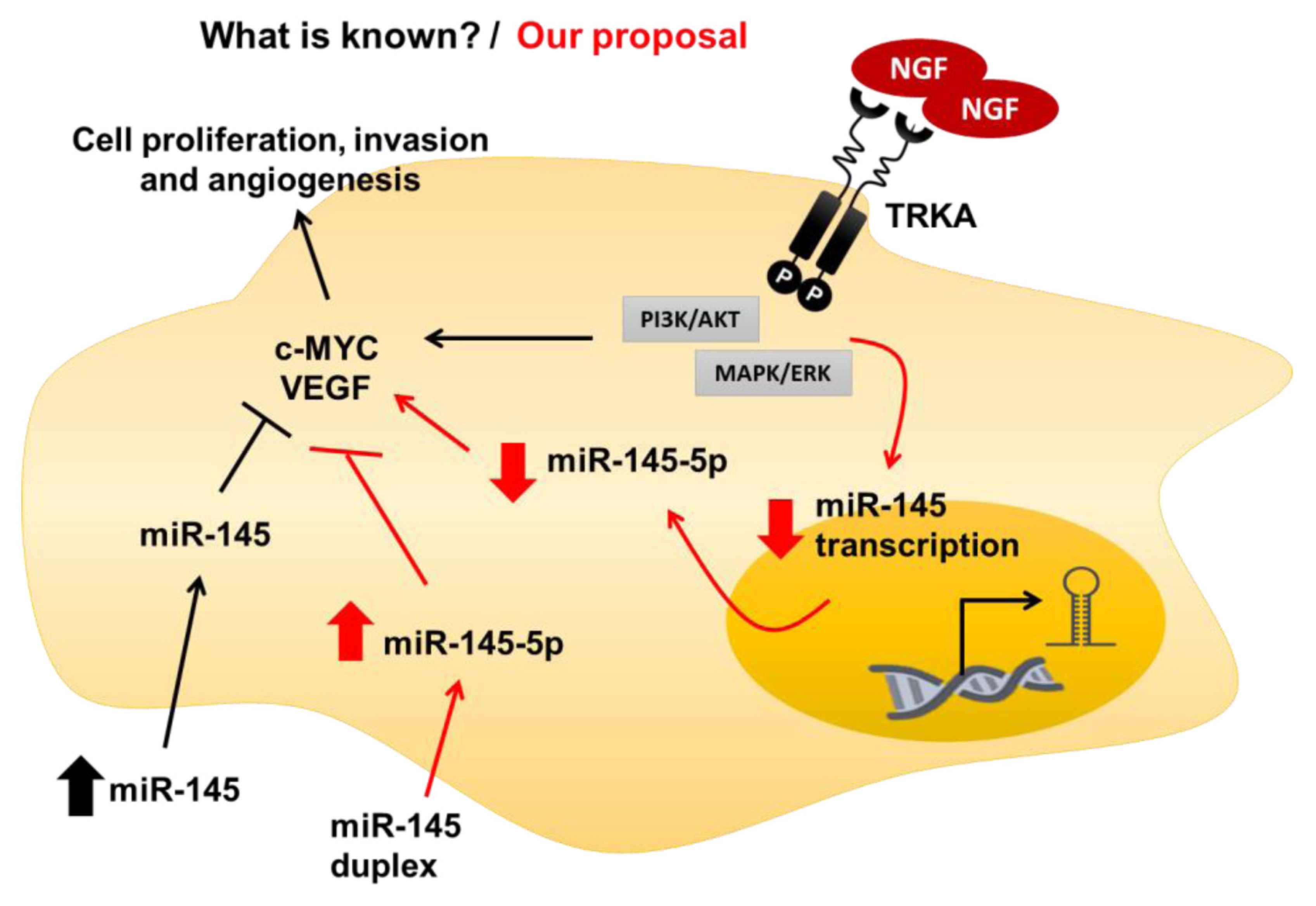
3. Discussion
4. Material and Methods
4.1. Tissue Collection
4.2. Cell Lines
4.3. miR Extraction, RT-PCR and qPCR
4.4. Transfection
4.5. Gen Reporter Assay for miR-145
4.6. Immunocytochemistry
4.7. MTS Assay
4.8. Enzyme-Linked Immunosorbent Assay (ELISA) for VEGF
4.9. Migration Assay
4.10. Invasion Assay
4.11. Over-Expression of miR-145 in EOC Cells by Transduction with Lentiviral Particles
4.12. Mouse Xenografts of EOC Cells with Stable miR-145 Over-Expression
4.13. Subcutaneous Tumor Formation and Peritoneal Carcinomatosis Assay
4.14. In Silico Prediction of miR-145: Target Interactions
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar]
- World Ovarian Cancer Coalition. The World Ovarian Cancer Coalition Atlas. Global Trends in Incidence, Mortality and Survival. Available online: https://worldovariancancercoalition.org/wp-content/uploads/2018/10/THE-WORLD-OVARIAN-CANCER-COALITION-ATLAS-2018.pdf (accessed on 7 April 2020).
- Bankhead, C.R.; Collins, C.; Stokes-Lampard, H.; Rose, P.; Wilson, S.; Clements, A.; Mant, D.; Kehoe, S.; Austoker, J. Identifying symptoms of ovarian cancer: A qualitative and quantitative study. BJOG Int. J. Obs. Gynaecol. 2008, 115, 1008–1014. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Gadducci, A.; Guarneri, V.; Peccatori, F.; Ronzino, G.; Scandurra, G.; Zamagni, C.; Zola, P.; Salutari, V. Current strategies for the targeted treatment of high-grade serous epithelial ovarian cancer and relevance of BRCA mutational status. J. Ovarian Res. 2019, 12, 9. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Owens, O.J.; Stewart, C.; Leake, R.E. Growth factors in ovarian cancer. Br. J. Cancer 1991, 64, 1177–1181. [Google Scholar] [CrossRef][Green Version]
- Tapia, V.; Gabler, F.; Muñoz, M.; Yazigi, R.; Paredes, A.; Selman, A.; Vega, M.; Romero, C. Tyrosine kinase A receptor (trkA): A potential marker in epithelial ovarian cancer. Gynecol. Oncol. 2011, 121, 13–23. [Google Scholar] [CrossRef]
- Vera, C.; Tapia, V.; Vega, M.; Romero, C. Role of nerve growth factor and its trka receptor in normal ovarian and epithelial ovarian cancer angiogenesis. J. Ovarian Res. 2014, 7, 82. [Google Scholar] [CrossRef]
- Garrido, M.P.; Torres, I.; Vega, M.; Romero, C. Angiogenesis in Gynecological Cancers: Role of Neurotrophins. Front. Oncol. 2019, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.P.; Hurtado, I.; Valenzuela-Valderrama, M.; Salvatierra, R.; Hernandez, A.; Vega, M.; Selman, A.; Quest, A.F.G.; Romero, C. Ngf-enhanced vasculogenic properties of epithelial ovarian cancer cells is reduced by inhibition of the cox-2/pge2 signaling axis. Cancers (Basel) 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed]
- Bretones, G.; Delgado, M.D.; León, J. Myc and cell cycle control. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1849, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.P.; Vera, C.; Vega, M.; Quest, A.F.G.; Romero, C. Metformin prevents nerve growth factor-dependent proliferative and proangiogenic effects in epithelial ovarian cancer cells and endothelial cells. Adv. Med. Oncol. 2018, 10, 1758835918770984. [Google Scholar] [CrossRef] [PubMed]
- Campos, X.; Muñoz, Y.; Selman, A.; Yazigi, R.; Moyano, L.; Weinstein-Oppenheimer, C.; Lara, H.E.; Romero, C. Nerve growth factor and its high-affinity receptor trkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, U.; Tapia, V.; Geraldo, M.; Selman, A.; Vega, M.; Romero, C. Nerve Growth Factor Stimulates Cellular Proliferation of Human Epithelial Ovarian Cancer. Horm. Metab. Res. 2012, 44, 656–661. [Google Scholar] [CrossRef]
- Xu, M.; Mo, Y.-Y. The Akt-associated microRNAs. Cell. Mol. Life Sci. 2012, 69, 3601–3612. [Google Scholar] [CrossRef]
- Sun, H.-L.; Cui, R.; Zhou, J.-K.; Teng, K.-Y.; Hsiao, Y.-H.; Nakanishi, K.; Fassan, M.; Luo, Z.; Shi, G.; Tili, E.; et al. ERK Activation Globally Downregulates miRNAs through Phosphorylating Exportin-5. Cancer Cell 2016, 30, 723–736. [Google Scholar] [CrossRef]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.C.; Wolff, R.K.; Stevens, J.R.; Samowitz, W.S.; Herrick, J.S. The PI3K/AKT signaling pathway: Associations of miRNAs with dysregulated gene expression in colorectal cancer. Mol. Carcinog. 2017, 57, 243–261. [Google Scholar] [CrossRef]
- Deb, B.; Uddin, A.; Chakraborty, S. miRNAs and ovarian cancer: An overview. J. Cell. Physiol. 2017, 233, 3846–3854. [Google Scholar] [CrossRef]
- Chen, S.-N.; Chang, R.; Lin, L.-T.; Chern, C.-U.; Tsai, H.-W.; Wen, Z.-H.; Li, Y.-H.; Li, C.-J.; Tsui, K.-H. MicroRNA in Ovarian Cancer: Biology, Pathogenesis, and Therapeutic Opportunities. Int. J. Environ. Res. Public Health 2019, 16, 1510. [Google Scholar] [CrossRef]
- Bartel, D.P. Micrornas: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, B. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, C.G.L. MicroRNA and cancer—Focus on apoptosis. J. Cell. Mol. Med. 2008, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Bras-Rosario, L.; Matsuda, A.; Pinheiro, A.I.; Gardner, R.; Lopes, T.; Amaral, A.J.; Gama-Carvalho, M. Expression Profile of microRNAs Regulating Proliferation and Differentiation in Mouse Adult Cardiac Stem Cells. PLoS ONE 2013, 8, e63041. [Google Scholar] [CrossRef]
- Ivey, K.N.; Srivastava, D. microRNAs as Developmental Regulators. Cold Spring Harb. Perspect. Biol. 2015, 7, a008144. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Z.; Unruh, A.K.; Ivan, C.; Baggerly, K.A.; Calin, G.A.; Li, Z.; Bast, R.C.; Le, X.-F. Clinically Relevant microRNAs in Ovarian Cancer. Mol. Cancer Res. 2014, 13, 393–401. [Google Scholar] [CrossRef]
- Katz, B.; Trope, C.G.; Reich, R.; Davidson, B. MicroRNAs in Ovarian Cancer. Hum. Pathol. 2015, 46, 1245–1256. [Google Scholar] [CrossRef]
- Retamales-Ortega, R.; Oróstica, L.; Vera, C.; Cuevas, P.; Hernández, A.; Hurtado, I.; Vega, M.; Romero, C. Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 507. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J. Prognostic role of microRNA-145 in prostate cancer: A systems review and meta-analysis. Prostate Int. 2015, 3, 71–74. [Google Scholar] [CrossRef]
- Tang, L.; Wei, D.; Yan, F. MicroRNA-145 functions as a tumor suppressor by targeting matrix metalloproteinase 11 and Rab GTPase family 27a in triple-negative breast cancer. Cancer Gene. 2016, 23, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, X.; Hu, X.-L.; Cheng, L.-Z.; Yu, J.-Y.; Wei, Z.-B. Downregulation of miR-145-5p correlates with poor prognosis in gastric cancer. Eur. Rev. Med. Pharm. Sci. 2016, 20, 3026–3030. [Google Scholar]
- Li, C.; Yan, G.; Yin, L.; Liu, T.; Li, C.; Wang, L. Prognostic roles of microRNA 143 and microRNA 145 in colorectal cancer: A meta-analysis. Int. J. Biol. Mark. 2019, 34, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. P53 represses c-myc through induction of the tumor suppressor mir-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Xu, Q.; Mao, F.; Li, D.; Bian, C.; Liu, L.-Z.; Jiang, Y.; Chen, X.; Qi, Y.; Zhang, X.; et al. MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle 2012, 11, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Tang, J.; Wang, P.; Li, L.; Yan, X.; Zheng, X.; Ren, S.; Zhang, M.; Xu, M. The Prognostic Value and Regulatory Mechanisms of microRNA-145 in Various Tumors: A Systematic Review and Meta-analysis of 50 Studies. Cancer Epidemiol. Biomark. Prev. 2019, 28, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Jiang, Z.; Xie, G.; Lu, Y. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumor Biol. 2015, 36, 5305–5313. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xiao, Z.; Wang, K.; Liu, W.; Hao, Q. MiR-145 is downregulated in human ovarian cancer and modulates cell growth and invasion by targeting p70S6K1 and MUC1. Biochem. Biophys. Res. Commun. 2013, 441, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qin, X.; Song, H.; Li, Y.; Qiu, Y.; Cui, S.; Wang, Y.; Wang, H.; Gong, J. Upregulated microRNA-15b alleviates ovarian cancer through inhitbition of the PI3K/Akt pathway by targeting LPAR3. J. Cell. Physiol. 2019, 234, 22331–22342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pei, M.; Li, Z.; Li, H.; Liu, Y.; Li, J. Double-negative feedback interaction between DNA methyltransferase 3A and microRNA-145 in the Warburg effect of ovarian cancer cells. Cancer Sci. 2018, 109, 2734–2745. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Qin, Y.; Sheng, M.; Cui, X.; Chen, W.; Zhong, J.; Yan, J.; Chen, Y. miR-145 suppresses ovarian cancer progression via modulation of cell growth and invasion by targeting CCND2 and E2F3. Mol. Med. Rep. 2019, 19, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, X.; Li, W.; Chen, Y. MicroRNA-145-5p regulates the proliferation of epithelial ovarian cancer cells via targeting SMAD4. J. Ovarian Res. 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maines-Bandiera, S.L.; Kruk, P.A.; Auersperg, N. Simian virus 40-transformed human ovarian surface epithelial cells escape normal growth controls but retain morphogenetic responses to extracellular matrix. Am. J. Obs. Gynecol. 1992, 167, 729–735. [Google Scholar] [CrossRef]
- The European Collection of Authenticated Cell Cultures (ECACC). Cell Line Profile: Ovarian Cancer Cell Line a2780. Available online: https://www.phe-culturecollections.org.uk/media/113526/a2780-cell-line-profile.pdf (accessed on 8 June 2020).
- American Type Culture Collection (ATCC). Sk-ov-3 [skov-3; skov3] (atcc® htb-77™). Available online: https://www.atcc.org/Products/All/HTB-77.aspx#documentation (accessed on 8 June 2020).
- Tiwari, A.; Mukherjee, B.; Dixit, M. MicroRNA Key to Angiogenesis Regulation: MiRNA Biology and Therapy. Curr. Cancer Drug Targets 2018, 18, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Z.; Yan, S.; Gao, F. Effect of miR-145 on gastric cancer cells. Mol. Med. Rep. 2019, 19, 3403–3410. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Zhang, J.; Zhang, N.; Li, T.; Fang, J.; Zhang, Y.; Zuo, F.; Tao, Z.; Tang, S.; et al. Mir-145 inhibits proliferation and migration of breast cancer cells by directly or indirectly regulating tgf-beta1 expression. Int. J. Oncol. 2017, 50, 1701–1710. [Google Scholar] [CrossRef]
- Cristóbal, I.; Sanz-Álvarez, M.; Torrejón, B.; Santos, A.; Luque, M.; Rojo, F.; García-Foncillas, J. Potential Therapeutic Impact of miR-145 Deregulation in Colorectal Cancer. Molecules 2018, 26, 1399–1400. [Google Scholar] [CrossRef]
- Zheng, T.-L.; Li, D.-P.; He, Z.-F.; Zhao, S. miR-145 sensitizes esophageal squamous cell carcinoma to cisplatin through directly inhibiting PI3K/AKT signaling pathway. Cancer Cell Int. 2019, 19, 215–250. [Google Scholar] [CrossRef]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.-G.; Alder, H.; et al. MicroRNA Signatures in Human Ovarian Cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef]
- Chen, X.; Dong, C.; Law, P.T.; Chan, M.T.V.; Su, Z.; Wang, S.; Wu, W.K.; Xu, H. MicroRNA-145 targets TRIM2 and exerts tumor-suppressing functions in epithelial ovarian cancer. Gynecol. Oncol. 2015, 139, 513–519. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Xie, C.; Yin, X.; Liu, Y.; Cao, Y.; Fang, Y.; Lin, X.; Xu, Y.; Xu, W.; et al. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. Int. J. Cancer 2014, 135, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, J.; Ye, Z.; Han, X.; Zheng, X.; Hou, H.; Chen, W.; Li, X.; Zhao, L. 20(s)-rg3 blocked epithelial-mesenchymal transition through dnmt3a/mir-145/fscn1 in ovarian cancer. Oncotarget 2017, 8, 53375–53386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massagué, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Drug Approval Package: Kynamro (Mipomersen Sodium) Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203568Orig1s000TOC.cfm (accessed on 7 April 2020).
- Filipów, S.; Łaczmański, Ł. Blood Circulating miRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front. Genet. 2019, 10, 169. [Google Scholar]
- Peng, Z.; Duan, F.; Yin, J.; Feng, Y.; Yang, Z.; Shang, J. Prognostic values of microRNA-130 family expression in patients with cancer: A meta-analysis and database test. J. Transl. Med. 2019, 17, 314–347. [Google Scholar] [CrossRef]
- Huang, C.; Yu, M.; Yao, X. MicroRNA-17 and the prognosis of human carcinomas: A systematic review and meta-analysis. BMJ Open 2018, 8, e018070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Pan, S.H.; Yan, J.J.; Xu, G. The prognostic value of microrna-183 in human cancers: A meta-analysis. Medicine (Baltimore) 2018, 97, e11213. [Google Scholar] [CrossRef] [PubMed]
- Slack, F.J.; Weidhaas, J.B. Microrna in cancer prognosis. N. Engl. J. Med. 2008, 359, 2720–2722. [Google Scholar] [CrossRef]
- Chung, Y.-W.; Bae, H.S.; Song, J.-Y.; Lee, J.K.; Lee, N.W.; Kim, T.; Lee, K.-W. Detection of MicroRNA as Novel Biomarkers of Epithelial Ovarian Cancer From the Serum of Ovarian Cancer Patient. Int. J. Gynecol. Cancer 2013, 23, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal. Transduct. Target. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Shi, J. Regulatory networks between neurotrophins and miRNAs in brain diseases and cancers. Acta Pharm. Sin. 2014, 36, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Schratt, G.M.; Nigh, E.A.; Chen, W.G.; Hu, L.; Greenberg, M.E. BDNF Regulates the Translation of a Select Group of mRNAs by a Mammalian Target of Rapamycin-Phosphatidylinositol 3-Kinase-Dependent Pathway during Neuronal Development. J. Neurosci. 2004, 24, 7366–7377. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.A.; Ruiz, C.R.; Eyler, E.C.; Lin, K.; Meffert, M.K. Dual regulation of miRNA biogenesis generates target specificity in neurotrophin-induced protein synthesis. Cell 2012, 148, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S.; Tavazoie, S.F.; Maloratsky, A.; Jacobs, K.M.; Harris, K.M.; E Greenberg, M. CREB: A Major Mediator of Neuronal Neurotrophin Responses. Neuron 1997, 19, 1031–1047. [Google Scholar] [CrossRef]
- Riccio, A.; Pierchala, B.A.; Ciarallo, C.L.; Ginty, D.D. An NGF-TrkA-Mediated Retrograde Signal to Transcription Factor CREB in Sympathetic Neurons. Science 1997, 277, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Maggirwar, S.B.; Sarmiere, P.D.; Dewhurst, S.; Freeman, R.S. Nerve growth factor-dependent activation of nf-kappab contributes to survival of sympathetic neurons. J. Neurosci. 1998, 18, 10356–10365. [Google Scholar] [CrossRef]
- Descamps, S.; Toillon, R.-A.; Adriaenssens, E.; Pawlowski, V.; Cool, S.M.; Nurcombe, V.; Le Bourhis, X.; Boilly, B.; Peyrat, J.-P.; Hondermarck, H. Nerve Growth Factor Stimulates Proliferation and Survival of Human Breast Cancer Cells through Two Distinct Signaling Pathways. J. Biol. Chem. 2001, 276, 17864–17870. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; Adriaenssens, E.; Yazidi-Belkoura, I.; Bourhis, X.; Nurcombe, V.; Hondermarck, H. Nerve Growth Factor Receptors and Signaling in Breast Cancer. Curr. Cancer Drug Targets 2004, 4, 463–470. [Google Scholar] [CrossRef]
- Ikemura, K.; Yamamoto, M.; Miyazaki, S.; Mizutani, H.; Iwamoto, T.; Okuda, M. MicroRNA-145 Post-transcriptionally Regulates the Expression and Function of P-glycoprotein in Intestinal Epithelial Cells. Mol. Pharm. 2012, 83, 399–405. [Google Scholar] [CrossRef]
- Zhan, M.; Zhao, X.; Wang, H.; Chen, W.; Xu, S.; Wang, W.; Shen, H.; Huang, S.; Wang, J. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumor Biol. 2016, 37, 10553–10562. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Hallas-Potts, A.; Dawson, J.C.; Herrington, C.S. Ovarian cancer cell lines derived from non-serous carcinomas migrate and invade more aggressively than those derived from high-grade serous carcinomas. Sci. Rep. 2019, 9, 5515. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Papagiannakopoulos, T.; Pan, G.; Thomson, J.A.; Kosik, K.S. Microrna-145 regulates oct4, sox2, and klf4 and represses pluripotency in human embryonic stem cells. Cell 2009, 137, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.A.; Tapia, J.C.; Fernandez, J.G.; Torres, V.A.; Munoz, N.; Galleguillos, D.; Leyton, L.; Quest, A.F. Caveolin-1-mediated suppression of cyclooxygenase-2 via a beta-catenin-tcf/lef-dependent transcriptional mechanism reduced prostaglandin e2 production and survivin expression. Mol. Biol. Cell 2009, 20, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Ata, H.; Rawat, D.K.; Gulati, S.; Kahn, A.G.; Edwards, J.G.; Gupte, S.A. Contractile protein expression is upregulated by reactive oxygen species in aorta of Goto-Kakizaki rat. Am. J. Physiol. Circ. Physiol. 2013, 306, H214–H224. [Google Scholar] [CrossRef]
- Marty, G.D. Blank-field correction for achieving a uniform white background in brightfield digital photomicrographs. Biotechniques 2007, 42, 716–720. [Google Scholar] [CrossRef]
- Dubois-Camacho, K.; Diaz-Jimenez, D.; De La Fuente, M.; Quera, R.; Simian, D.; Martínez, M.; Landskron, G.; Olivares-Morales, M.; Cidlowski, J.A.; Xu, X.; et al. Inhibition of miR-378a-3p by Inflammation Enhances IL-33 Levels: A Novel Mechanism of Alarmin Modulation in Ulcerative Colitis. Front. Immunol. 2019, 10, 2449. [Google Scholar] [CrossRef]
- Lobos-González, L.; Silva, V.; Araya, M.; Restovic, F.; Echenique, J.; Oliveira-Cruz, L.; Fitzpatrick, C.; Briones, M.; Villegas, J.; Villota, C.; et al. Targeting antisense mitochondrial ncRNAs inhibits murine melanoma tumor growth and metastasis through reduction in survival and invasion factors. Oncotarget 2016, 7, 58331–58350. [Google Scholar] [CrossRef]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. MIENTURNET: An interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinform. 2019, 20, 545. [Google Scholar] [CrossRef] [PubMed]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef] [PubMed]


| miR-145-3p/5p | Target Gene | Protein Affected (Downregulated) | Tumorigenic/ Metastatic Effect | NGF-Mediated Effect |
|---|---|---|---|---|
| miR-145-3p/5p | MYC * | c-MYC | Transcription of proliferation genes | Upregulation |
| miR-145-3p/5p | MYCBP2 | MYC Binding Protein 2 | Transcription of proliferation genes | Upregulation |
| miR-145-3p/5p | VEGFA * | Vascular Endothelial Growth Factor | Tumor angiogenesis | Upregulation |
| miR-145-5p | ADAM17 | ADAM metallopeptidase domain 17 | ECM remodeling | Upregulation |
| miR-145-5p | AKT1 | AKT Serine/Threonine Kinase 1 | PI3K/Akt signaling pathway | Upregulation |
| miR-145-3p | PIK3C2A | Phosphoinositide-3-kinase, class 2, alpha polypeptide | PI3K/Akt signaling pathway | Upregulation |
| miR-145-5p | CAV1 | Caveolin 1 | Cell migration/invasion | Upregulation |
| miR-145-5p | NGF | Nerve Growth Factor | NGF signaling pathway | - |
| miR-145-5p | NTRK1 | High-affinity Nerve Growth Factor Receptor (TRKA) | NGF signaling pathway | - |
| miR-145-5p | BEX3 | Nerve Growth Factor Receptor Associated Protein 1 | NGF signaling pathway Apoptosis regulation | Upregulation |
| miR-145-3p/5p | MAPK1 | Mitogen-activated Protein kinase 1 (ERK) | Cell proliferation signaling | Upregulation |
| miR-145-3p/5p | MYCT1 | MYC Target 1 (MYC target protein 1) | Cell proliferation signaling | Downregulation |
| miR-145-3p/5p | MAPK10 | Mitogen-activated protein kinase 10 (JNK3) | Cell proliferation signaling | Downregulation |
| miR-145-3p/5p | CDH5 | Cadherin-5 | Cell adhesion | Downregulation |
| miR-145-3p/5p | BCL2 | B-cell CLL/lymphoma 2 | Anti-apoptosis Apoptosis regulation | Downregulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, M.P.; Torres, I.; Avila, A.; Chnaiderman, J.; Valenzuela-Valderrama, M.; Aramburo, J.; Oróstica, L.; Durán-Jara, E.; Lobos-Gonzalez, L.; Romero, C. NGF/TRKA Decrease miR-145-5p Levels in Epithelial Ovarian Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7657. https://doi.org/10.3390/ijms21207657
Garrido MP, Torres I, Avila A, Chnaiderman J, Valenzuela-Valderrama M, Aramburo J, Oróstica L, Durán-Jara E, Lobos-Gonzalez L, Romero C. NGF/TRKA Decrease miR-145-5p Levels in Epithelial Ovarian Cancer Cells. International Journal of Molecular Sciences. 2020; 21(20):7657. https://doi.org/10.3390/ijms21207657
Chicago/Turabian StyleGarrido, Maritza P., Ignacio Torres, Alba Avila, Jonás Chnaiderman, Manuel Valenzuela-Valderrama, José Aramburo, Lorena Oróstica, Eduardo Durán-Jara, Lorena Lobos-Gonzalez, and Carmen Romero. 2020. "NGF/TRKA Decrease miR-145-5p Levels in Epithelial Ovarian Cancer Cells" International Journal of Molecular Sciences 21, no. 20: 7657. https://doi.org/10.3390/ijms21207657
APA StyleGarrido, M. P., Torres, I., Avila, A., Chnaiderman, J., Valenzuela-Valderrama, M., Aramburo, J., Oróstica, L., Durán-Jara, E., Lobos-Gonzalez, L., & Romero, C. (2020). NGF/TRKA Decrease miR-145-5p Levels in Epithelial Ovarian Cancer Cells. International Journal of Molecular Sciences, 21(20), 7657. https://doi.org/10.3390/ijms21207657








