Single-Dose Cisplatin Pre-Treatment Enhances Efficacy of ROBO1-Targeted Radioimmunotherapy
Abstract
:1. Introduction
2. Results
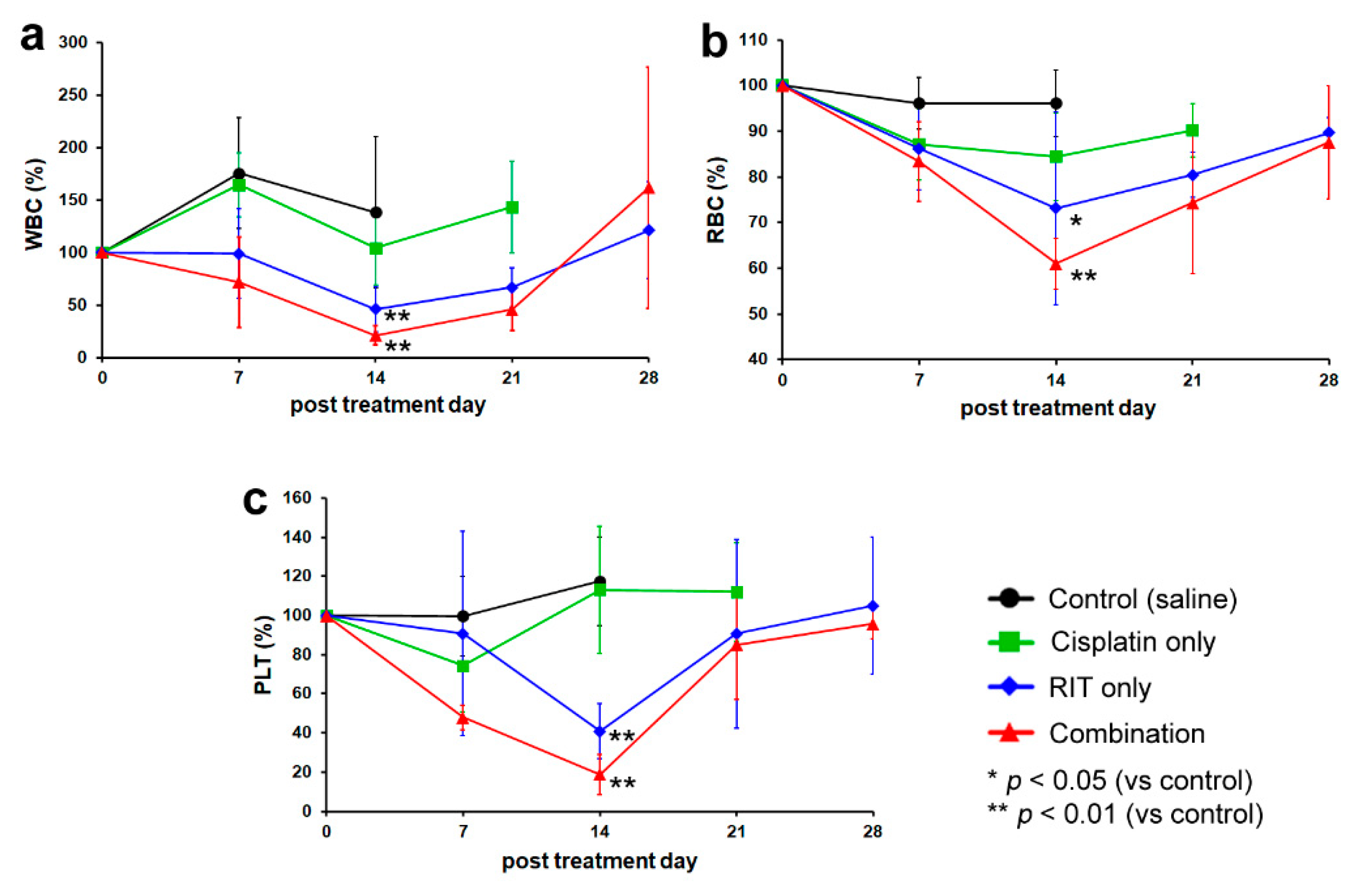
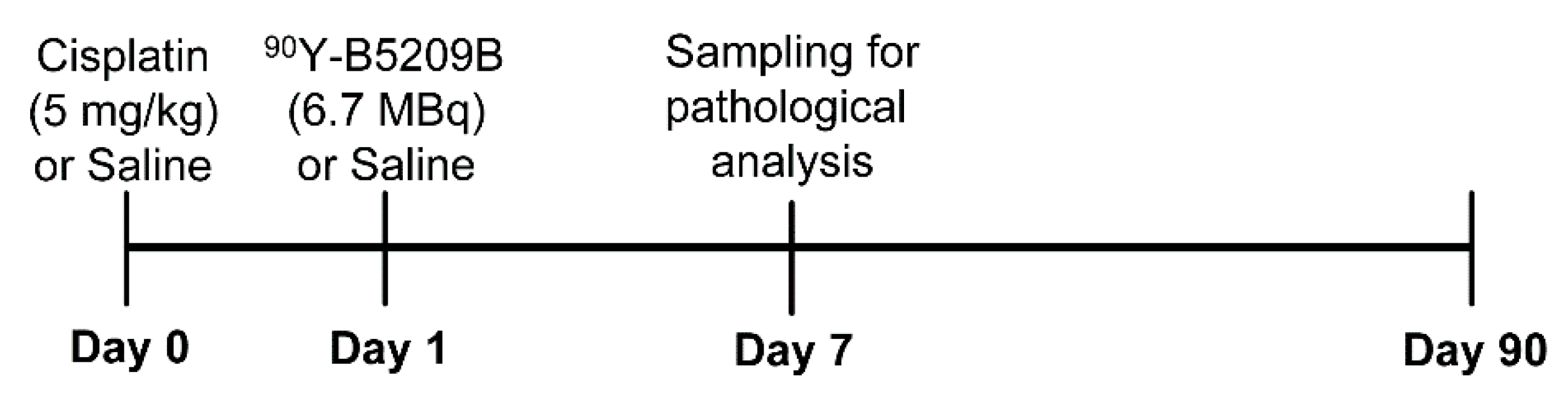
2.1. In Vivo Therapeutic Study
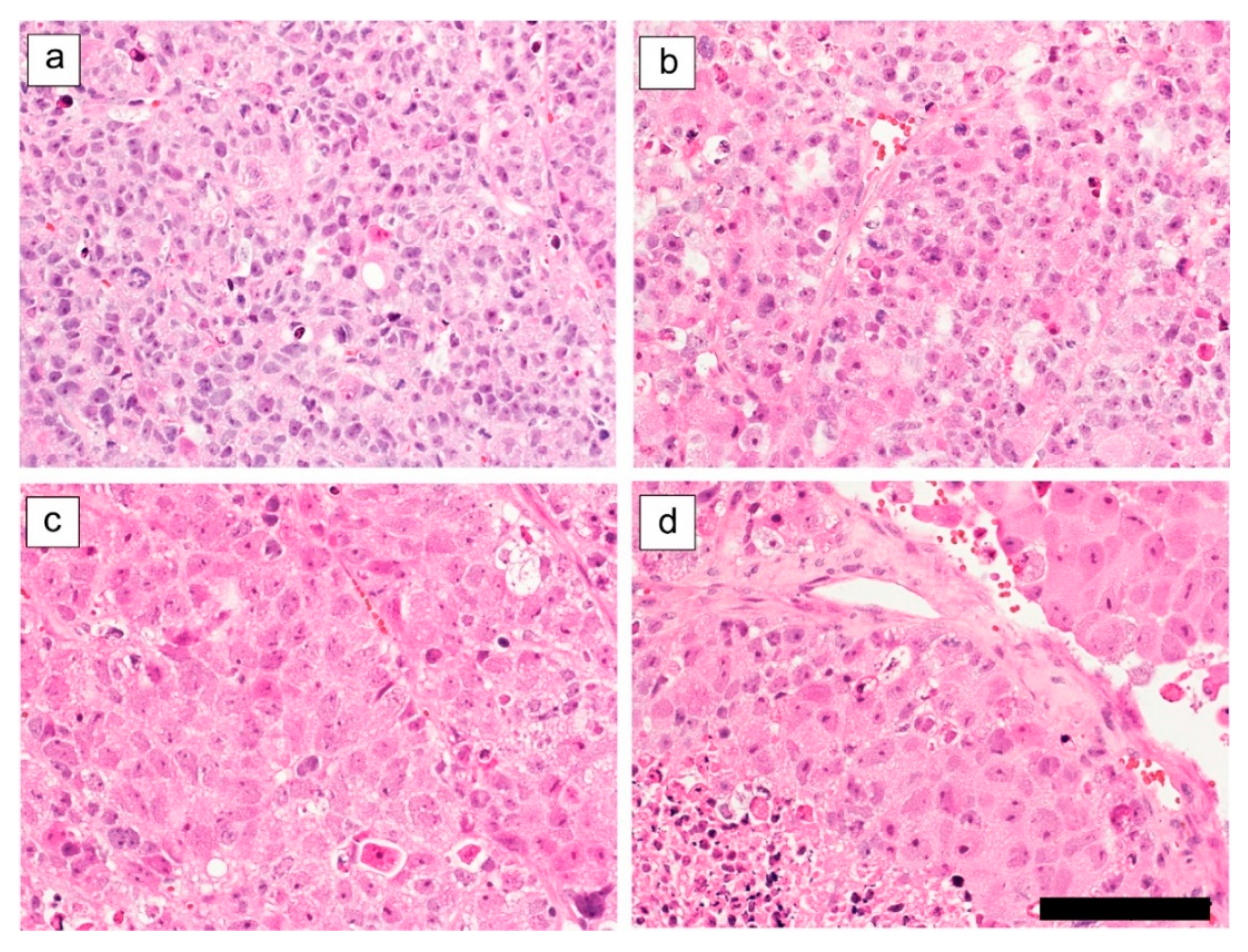
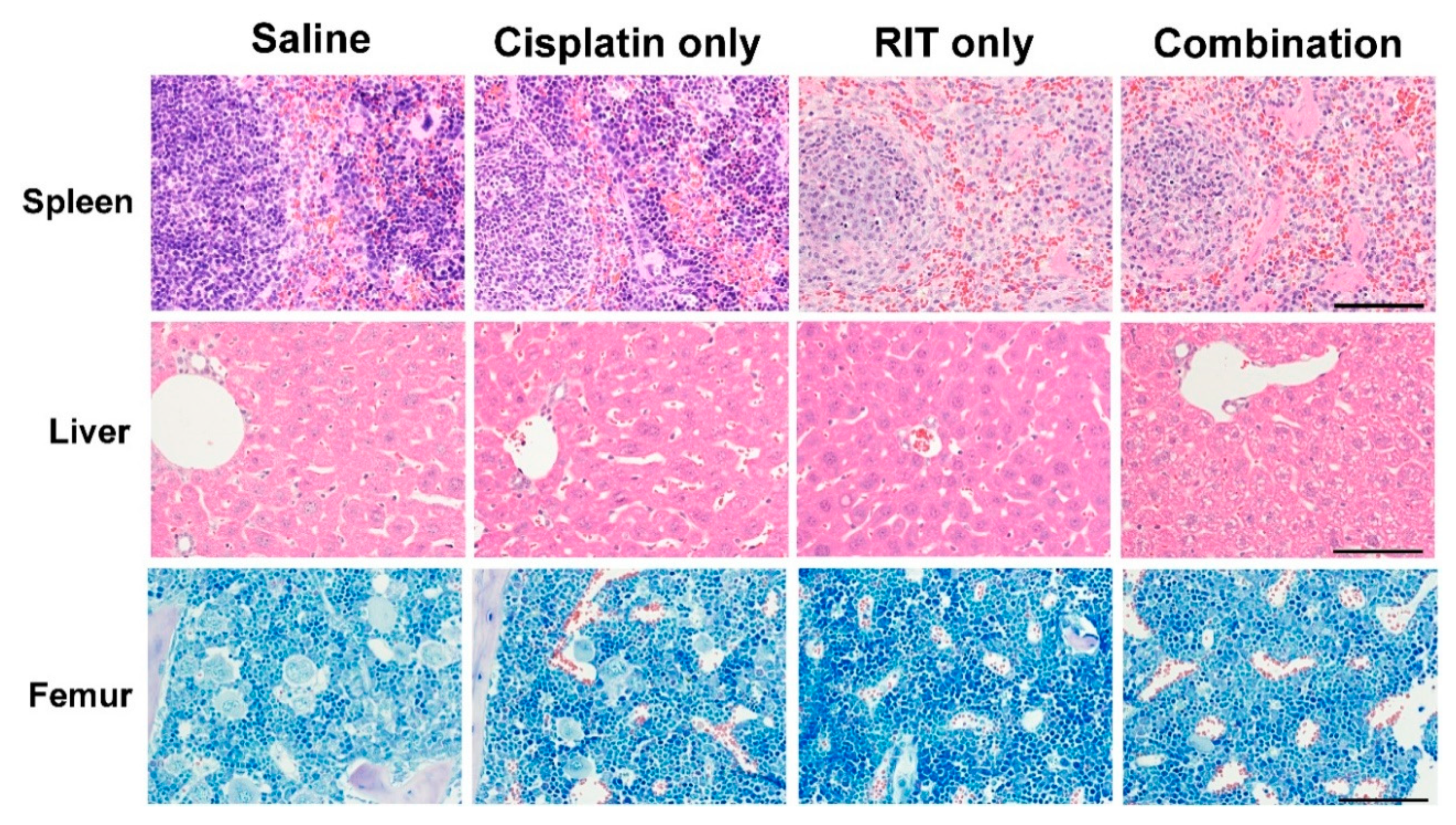
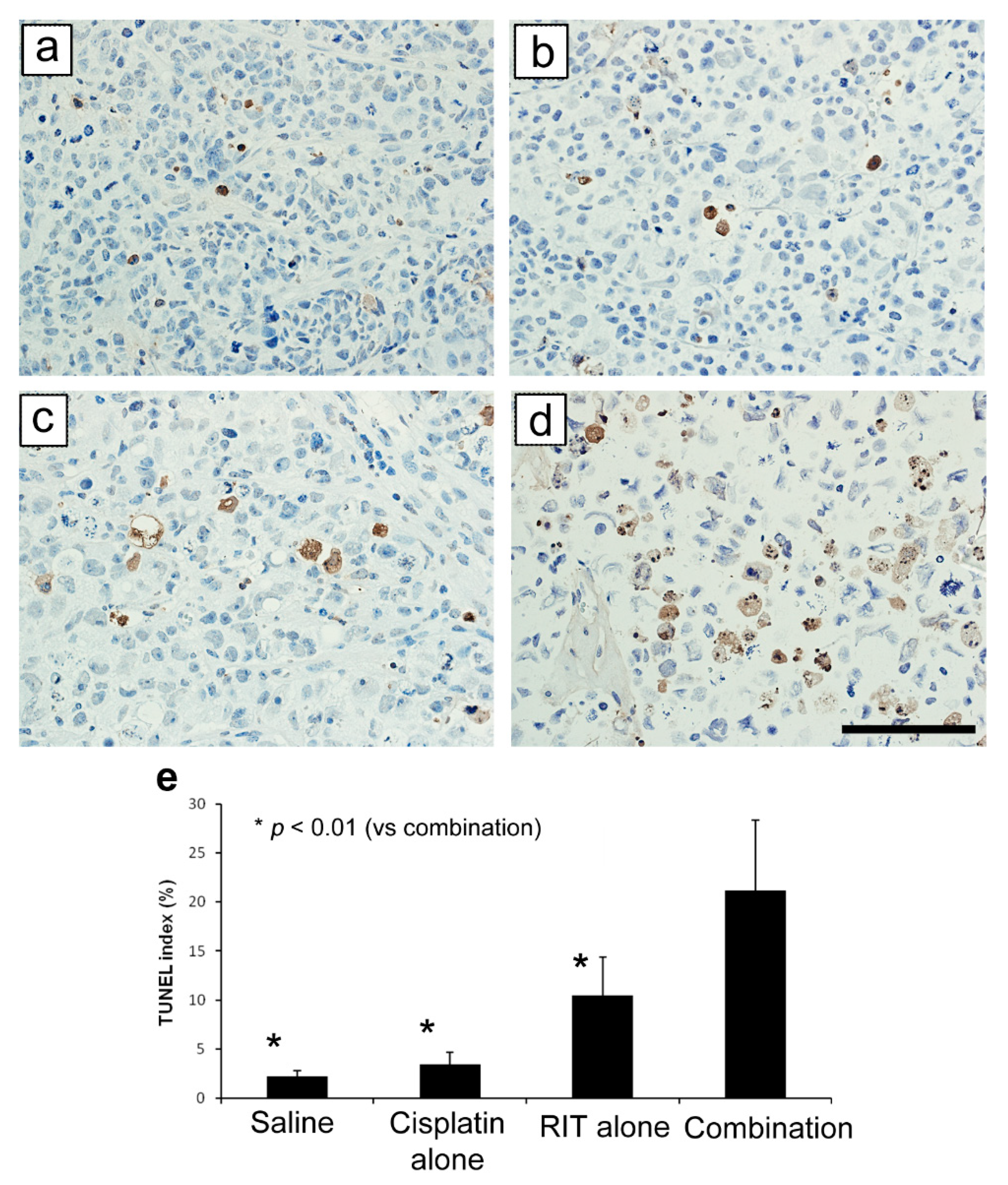
2.2. Ex Vivo Tumor and Organ Histopathology
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Animal Models
4.2. Antibodies
4.3. Chelate Conjugation and Radiolabeling
4.4. Therapeutic Study
4.5. Pathological Study
4.6. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCLC | Small-cell lung cancer |
| RIT | Radioimmunotherapy |
| MST | Median survival time |
| WBC | White blood cell |
| RBC | Red blood cell |
| PLT | Platelet |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| TUNEL | Transferase-mediated dUTP nick end labeling |
| ADC | Antibody–drug conjugate |
| PSMA | Prostate-specific membrane antigen |
References
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 725–737. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Akerley, W.; Bogner, P.; Borghaei, H.; Chow, L.Q.; Downey, R.J.; Gandhi, L.; Ganti, A.K.; Govindan, R.; Grecula, J.C.; et al. Small cell lung cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 78–98. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zimmermann, S.; Parikh, K.; Mansfield, A.S.; Adjei, A.A. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin. Proc. 2019, 94, 1599–1622. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Goldenberg, D.M. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J. Nucl. Med. 2005, 46 (Suppl. S1), 115s–127s. [Google Scholar] [PubMed]
- Goldenberg, D.M. Monoclonal antibodies in cancer detection and therapy. Am. J. Med. 1993, 94, 297–312. [Google Scholar] [CrossRef]
- Fujiwara, K.; Koyama, K.; Suga, K.; Ikemura, M.; Saito, Y.; Hino, A.; Iwanari, H.; Kusano-Arai, O.; Mitsui, K.; Kasahara, H.; et al. 90Y-Labeled Anti-ROBO1 Monoclonal Antibody Exhibits Antitumor Activity against Small Cell Lung Cancer Xenografts. PLoS ONE 2015, 10, e0125468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brose, K.; Bland, K.S.; Wang, K.H.; Arnott, D.; Henzel, W.; Goodman, C.S.; Tessier-Lavigne, M.; Kidd, T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999, 96, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Kidd, T.; Brose, K.; Mitchell, K.J.; Fetter, R.D.; Tessier-Lavigne, M.; Goodman, C.S.; Tear, G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998, 92, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xiao, Y.; Ding, B.B.; Zhang, N.; Yuan, X.; Gui, L.; Qian, K.X.; Duan, S.; Chen, Z.; Rao, Y.; et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 2003, 4, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Mertsch, S.; Schmitz, N.; Jeibmann, A.; Geng, J.G.; Paulus, W.; Senner, V. Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J. Neuro-Oncol. 2008, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, W.L.; Fu, L.; Gu, F.; Ma, Y.J. Slit2/Robo1 signaling in glioma migration and invasion. Neurosci. Bull. 2010, 26, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Funahashi, S.; Yamauchi, N.; Shibahara, J.; Midorikawa, Y.; Kawai, S.; Kinoshita, Y.; Watanabe, A.; Hippo, Y.; Ohtomo, T.; et al. Identification of ROBO1 as a novel hepatocellular carcinoma antigen and a potential therapeutic and diagnostic target. Clin. Cancer Res. 2006, 12, 3257–3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Hamakubo, T.; Mitsui, K.; Yagami, R.; Fujiyoshi, Y.; Ajioka, Y.; Naito, M. Roundabout1 distribution in neoplastic and non-neoplastic diseases with a focus on neoangiogenesis. Int. J. Clin. Exp. Pathol. 2018, 11, 5755–5764. [Google Scholar]
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Früh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012, 30, 1692–1698. [Google Scholar] [CrossRef]
- Kvols, L.K. Radiation sensitizers: A selective review of molecules targeting DNA and non-DNA targets. J. Nucl. Med. 2005, 46 (Suppl. S1), 187s–190s. [Google Scholar] [PubMed]
- Gill, M.R.; Vallis, K.A. Transition metal compounds as cancer radiosensitizers. Chem. Soc. Rev. 2019, 48, 540–557. [Google Scholar] [CrossRef]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 2005, 3, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Sears, C.R.; Cooney, S.A.; Chin-Sinex, H.; Mendonca, M.S.; Turchi, J.J. DNA damage response (DDR) pathway engagement in cisplatin radiosensitization of non-small cell lung cancer. DNA Repair 2016, 40, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.; Schittenhelm, M.; Honecker, F.; Malenke, E.; Lauber, K.; Wesselborg, S.; Hartmann, J.T.; Bokemeyer, C.; Mayer, F. Cell-cycle progression and response of germ cell tumors to cisplatin in vitro. Int. J. Oncol. 2006, 29, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Koyama, K.; Suga, K.; Ikemura, M.; Saito, Y.; Hino, A.; Iwanari, H.; Kusano-Arai, O.; Mitsui, K.; Kasahara, H.; et al. A (90)Y-labelled anti-ROBO1 monoclonal antibody exhibits antitumour activity against hepatocellular carcinoma xenografts during ROBO1-targeted radioimmunotherapy. EJNMMI Res. 2014, 4, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Kim, Y.-S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release 2008, 127, 41–49. [Google Scholar] [CrossRef]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody-drug conjugates for cancer therapy. Lancet. Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Sharkey, R.M.; Karacay, H.; Govindan, S.V.; Goldenberg, D.M. Combination radioimmunotherapy and chemoimmunotherapy involving different or the same targets improves therapy of human pancreatic carcinoma xenograft models. Mol. Cancer Ther. 2011, 10, 1072–1081. [Google Scholar] [CrossRef] [Green Version]
- Couturier, O.; Supiot, S.; Degraef-Mougin, M.; Faivre-Chauvet, A.; Carlier, T.; Chatal, J.F.; Davodeau, F.; Cherel, M. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 601–614. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, K.; Koyama, K.; Tsuji, A.B.; Iwanari, H.; Kusano-Arai, O.; Higashi, T.; Momose, T.; Hamakubo, T. Single-Dose Cisplatin Pre-Treatment Enhances Efficacy of ROBO1-Targeted Radioimmunotherapy. Int. J. Mol. Sci. 2020, 21, 7728. https://doi.org/10.3390/ijms21207728
Fujiwara K, Koyama K, Tsuji AB, Iwanari H, Kusano-Arai O, Higashi T, Momose T, Hamakubo T. Single-Dose Cisplatin Pre-Treatment Enhances Efficacy of ROBO1-Targeted Radioimmunotherapy. International Journal of Molecular Sciences. 2020; 21(20):7728. https://doi.org/10.3390/ijms21207728
Chicago/Turabian StyleFujiwara, Kentaro, Keitaro Koyama, Atsushi B. Tsuji, Hiroko Iwanari, Osamu Kusano-Arai, Tatsuya Higashi, Toshimitsu Momose, and Takao Hamakubo. 2020. "Single-Dose Cisplatin Pre-Treatment Enhances Efficacy of ROBO1-Targeted Radioimmunotherapy" International Journal of Molecular Sciences 21, no. 20: 7728. https://doi.org/10.3390/ijms21207728





