Exogenous Application of Proteoglycan to the Cell Surface Microenvironment Facilitates to Chondrogenic Differentiation and Maintenance
Abstract
:1. Introduction
2. Results
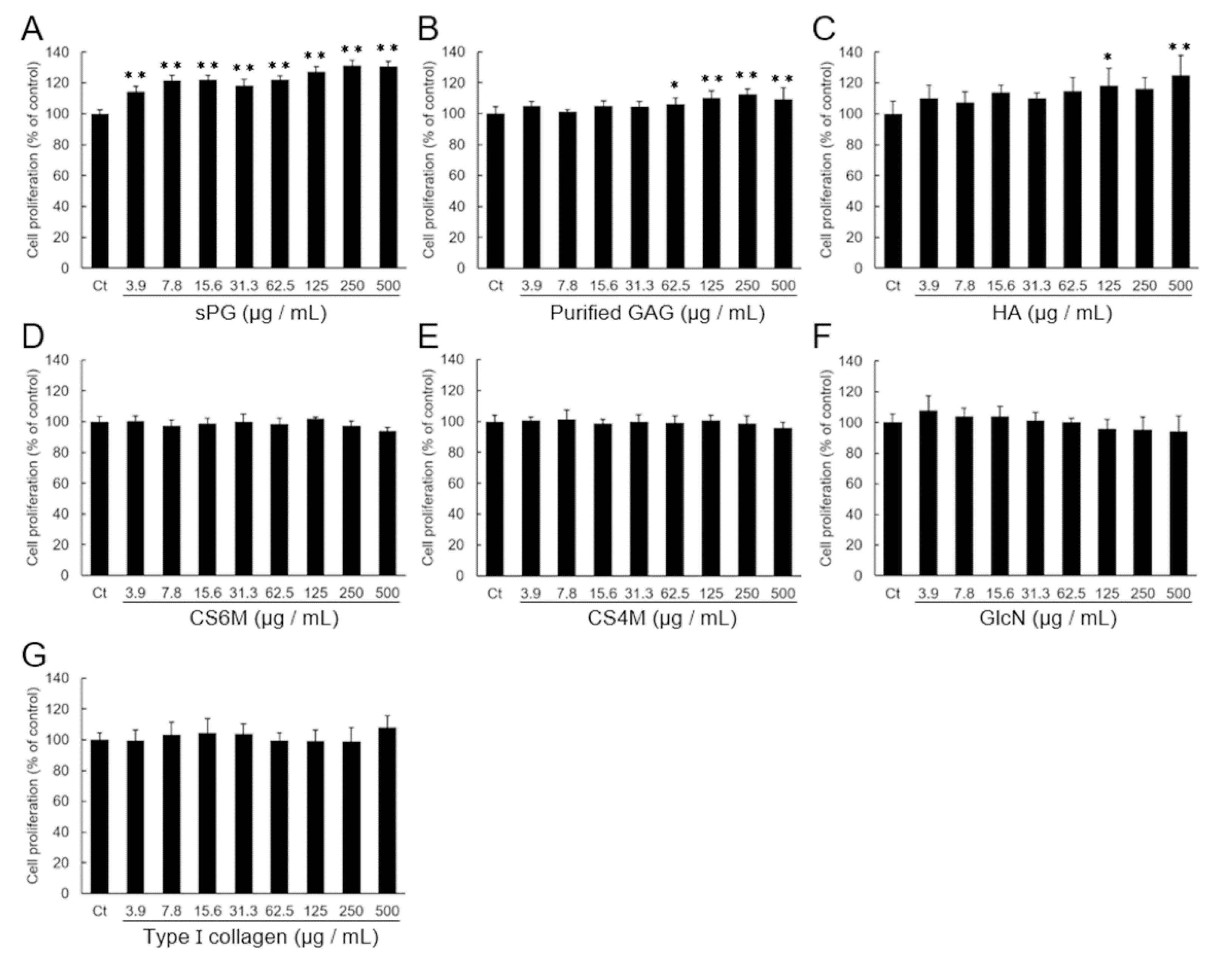
2.1. Effect on the Proliferation of ATDC5 Cells by Addition of ECM Constituting Factor
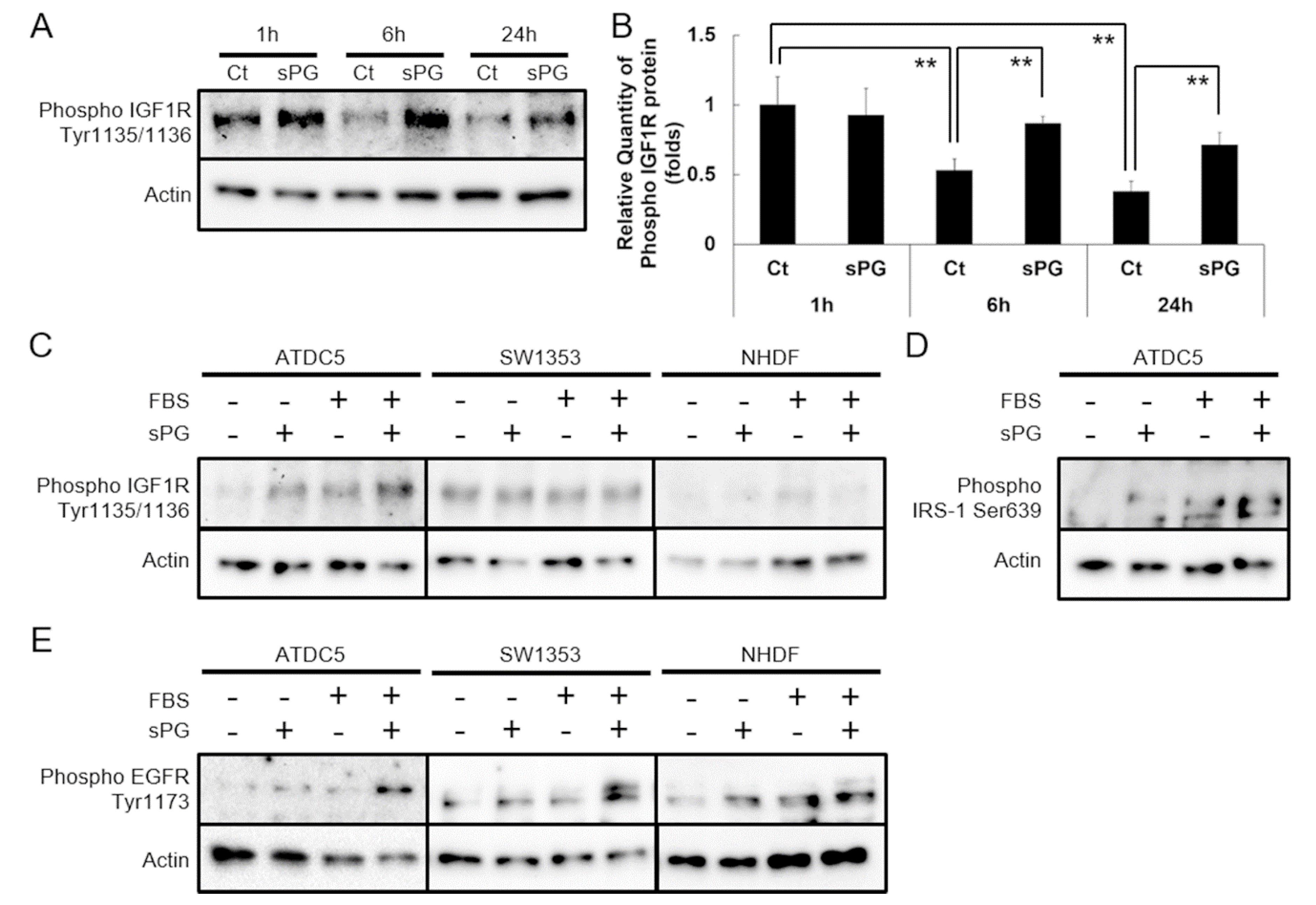
2.2. Effect of sPG on Cellular Proliferation-Related Receptor Activation
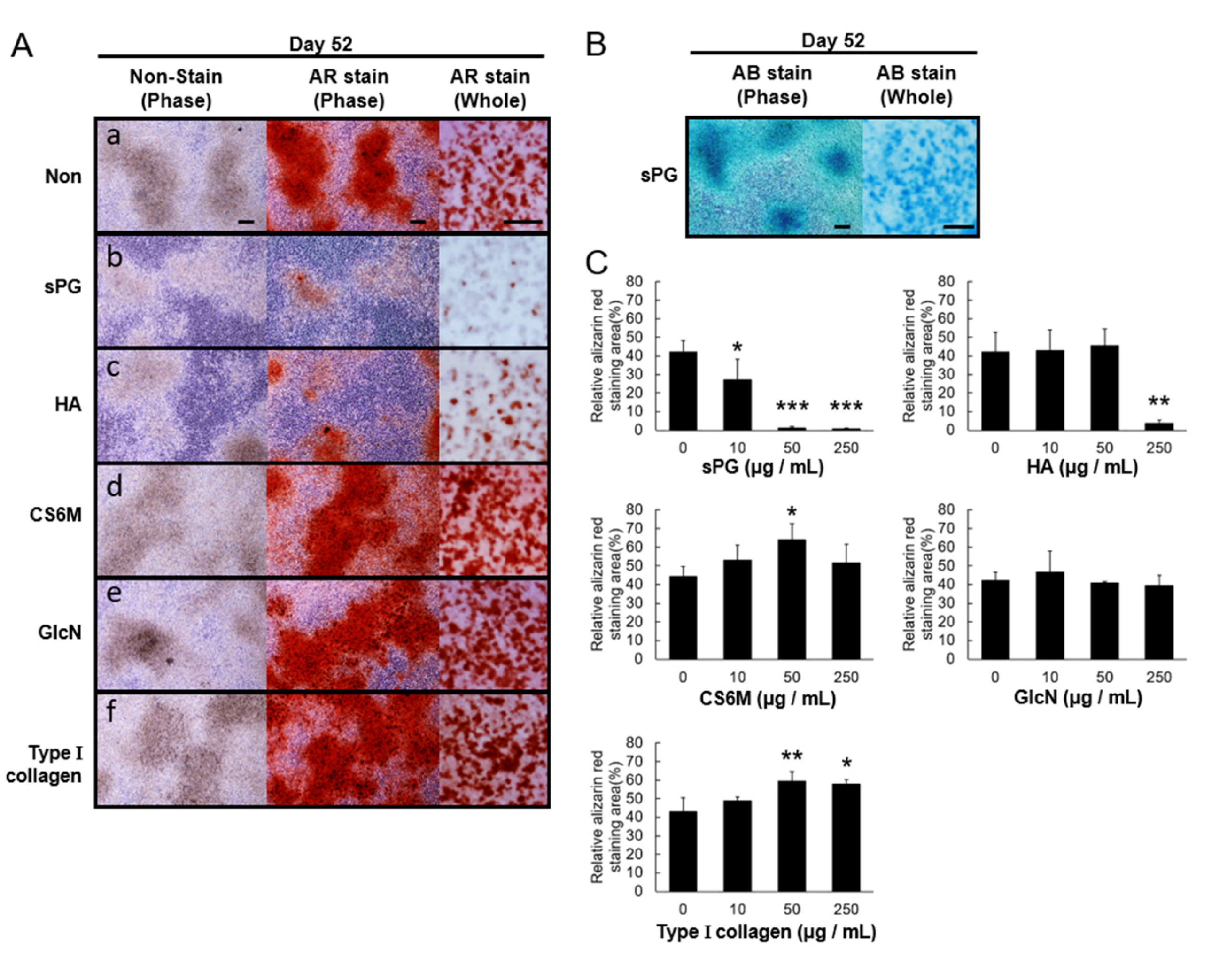
2.3. Effects of ECM Constituents on Chondrogenic Differentiation in ATDC5 Cells
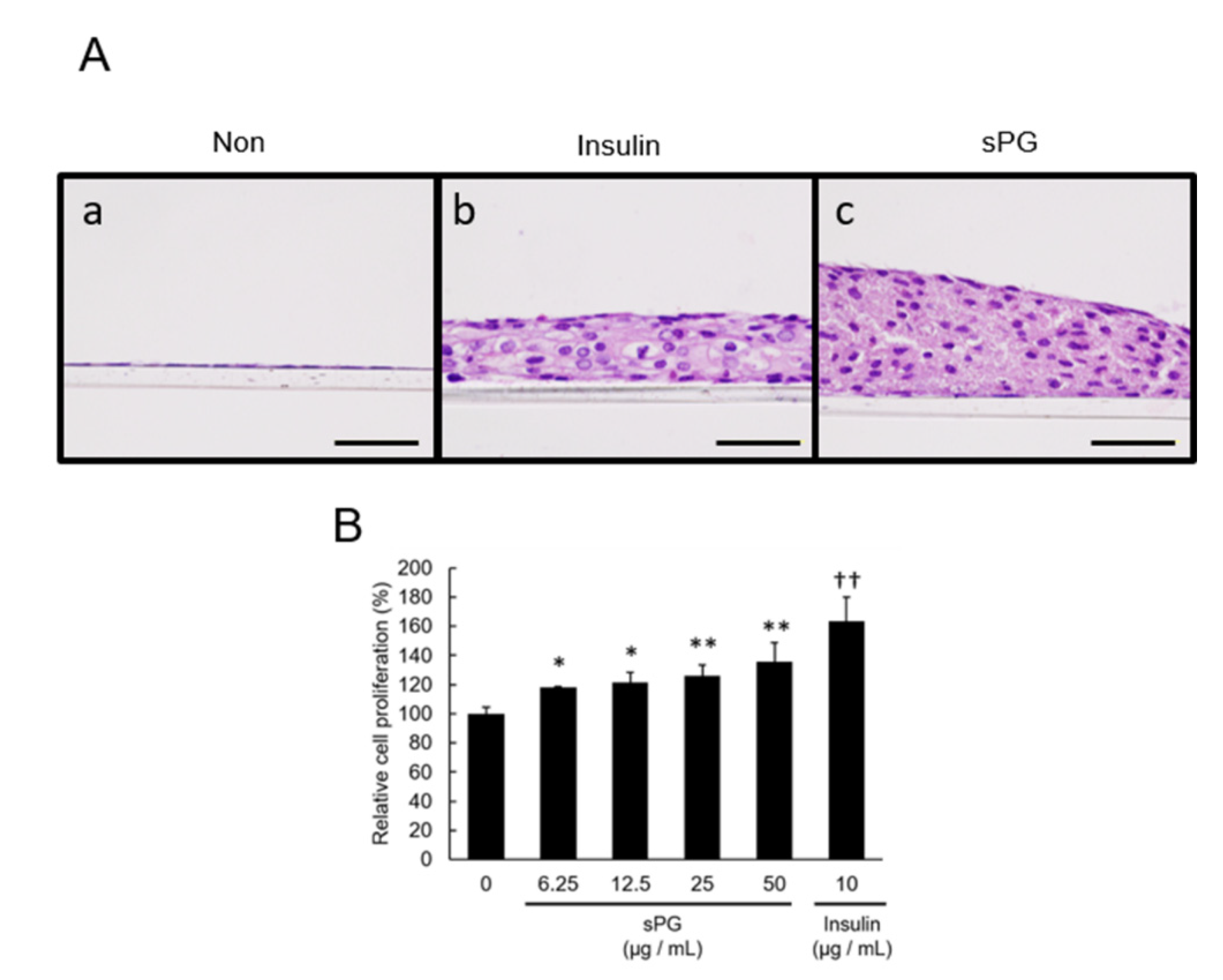
2.4. Evaluation of Three-Dimensional Structure in Cartilage-Like Nodules Induced by sPG in ATDC5 Cells
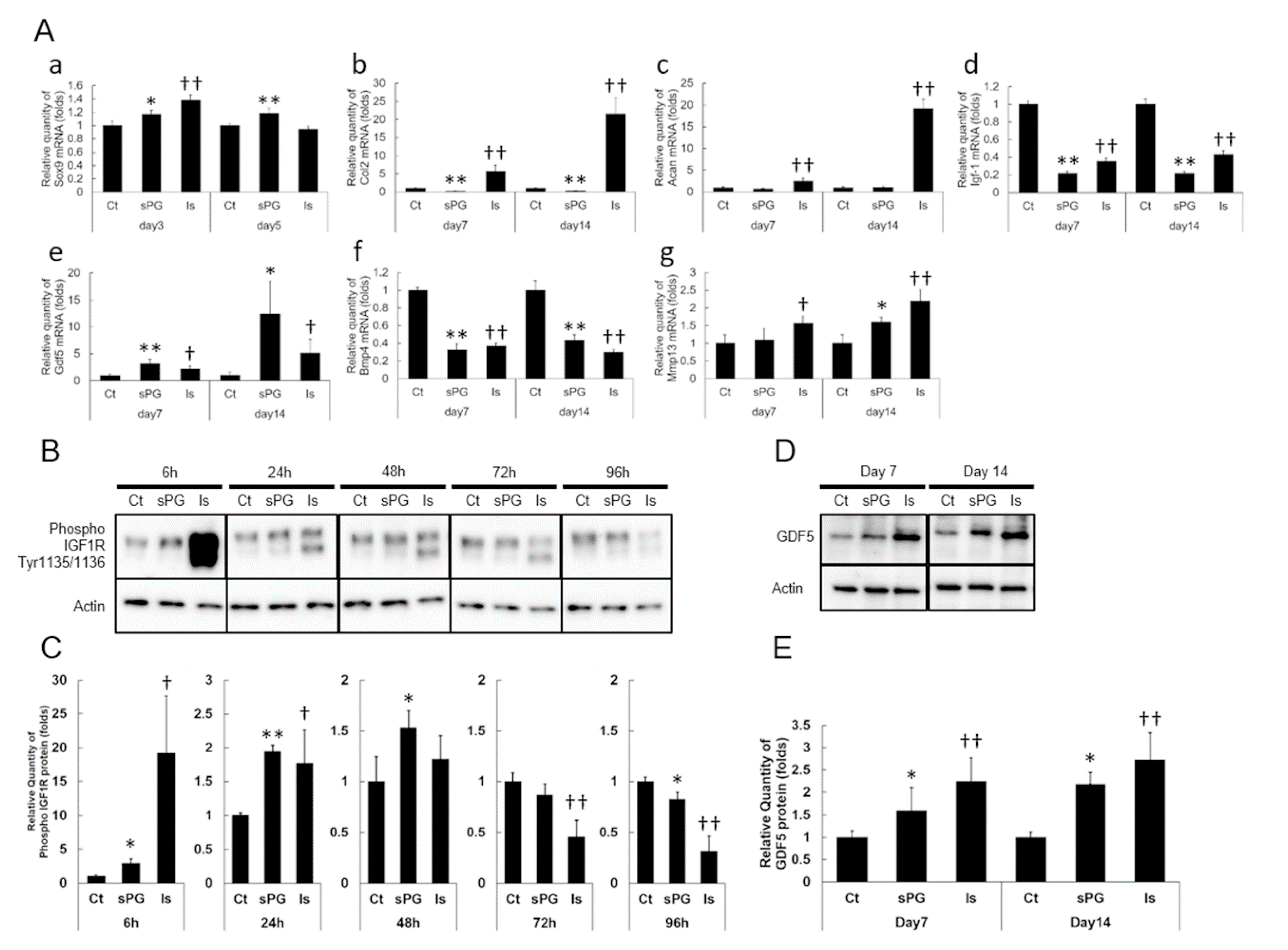
2.5. Effect of sPG on Chondrogenic Differentiation-Related Factor in ATDC5 Cells
2.6. Influence of ECM Constituting Factors on Cartilage Calcification in ATDC5 Cells
2.7. Effect of sPG on Cartilage Calcification-Related Factor (mRNA)
2.8. Influence of sPG on Continued Calcification Conditions
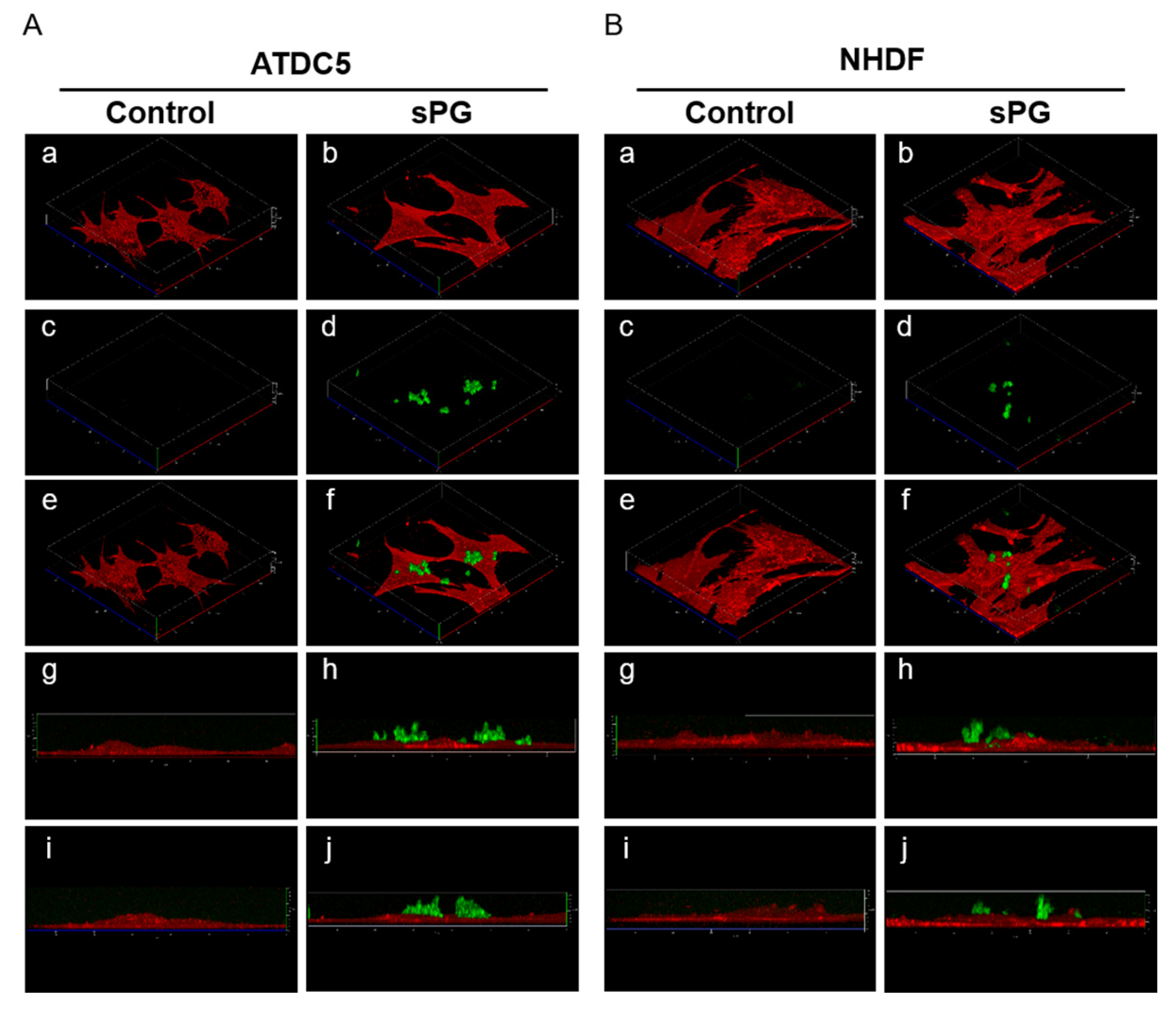
2.9. Localization Analysis of sPG Applied to Living Cells
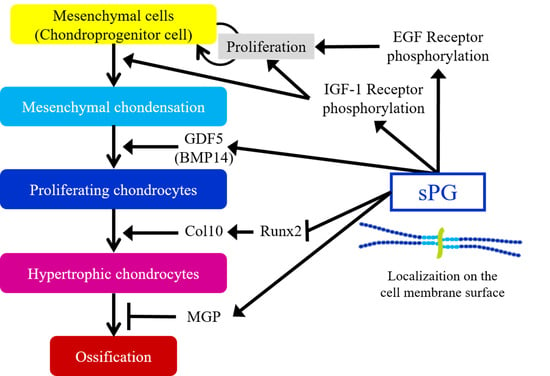
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells and Cell Culture
4.3. Cell Proliferation Assay
4.4. Immunoblotting
4.5. Chondrogenic Differentiation
4.6. Cartilage Calcification
4.7. RNA Isolation and Reverse Transcription (RT)-PCR
4.8. Real-Time Quantitative PCR
4.9. Alcian Blue Staining
4.10. Alizarin Red Staining
4.11. Histology of Chondrogenic Differentiation
4.12. Fluorescent Labeling of sPG
4.13. Confocal Fluorescence Live Cell Imaging
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMP | Bone morphogenetic protein |
| CLSM | Confocal laser scanning microscopy |
| CS | Chondroitin sulfate |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| FGF | Fibroblast growth factor |
| GAG | Glycosaminoglycan |
| GDF5 | Growth differentiation factor 5 |
| GlcN | Glucosamine hydrochloride |
| HA | Hyaluronan |
| IGF-1 | Insulin-like growth factor 1 |
| IRS-1 | Insulin receptor substrate 1 |
| MGP | Matrix gla protein |
| OA | Osteoarthritis |
| PCM | Pericellular matrix |
| PG | Proteoglycan |
| PTHrP | Parathyroid hormone-related protein |
| Runx2 | Runt-related transcription factor 2 |
| SOX9 | SRY-box transcription factor 9 |
| TGF-β | Transforming growth factor beta |
References
- Chronic Diseases and Health Promotion, World Health Organization. Available online: https://www.who.int/chp/topics/rheumatic/en/ (accessed on 26 August 2020).
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Lotz, M.; Loeser, R.F. Effects of aging on articular cartilage homeostasis. Bone 2012, 51, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grogan, S.P.; D’Lima, D.D. Joint aging and chondrocyte cell death. Int. J. Clin. Rheumtol. 2010, 5, 199–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ikebuchi, M.; Saeki, S.; Furukawa, D.; Orita, K.; Niimi, N.; Tsukahara, Y.; Nakamura, H. Changes in viscoelastic properties of articular cartilage in early stage of osteoarthritis, as determined by optical coherence tomography-based strain rate tomography. BMC Musculoskelet. Disord. 2019, 20, 417. [Google Scholar] [CrossRef] [Green Version]
- Mariani, E.; Pulsatelli, L.; Facchini, A. Signaling pathways in cartilage repair. Int. J. Mol. Sci. 2014, 15, 8667–8698. [Google Scholar] [CrossRef]
- Atsumi, T.; Miwa, Y.; Kimata, K.; Ikawa, Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ. Dev. 1990, 30, 109–116. [Google Scholar] [CrossRef]
- Gu, Y.T.; Chen, J.; Meng, Z.L.; Ge, W.Y.; Bian, Y.Y.; Cheng, S.W.; Xing, C.K.; Yao, J.L.; Fu, J.; Peng, L. Research progress on osteoarthritis treatment mechanisms. Biomed. Pharm. 2017, 93, 1246–1252. [Google Scholar] [CrossRef]
- Appel, B.; Baumer, J.; Eyrich, D.; Sarhan, H.; Toso, S.; Englert, C.; Skodacek, D.; Ratzinger, S.; Grassel, S.; Goepferich, A.; et al. Synergistic effects of growth and differentiation factor-5 (GDF-5) and insulin on expanded chondrocytes in a 3-D environment. Osteoarthr. Cartil. 2009, 17, 1503–1512. [Google Scholar] [CrossRef] [Green Version]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [Green Version]
- Tsezou, A. Osteoarthritis year in review 2014: Genetics and genomics. Osteoarthr. Cartil. 2014, 22, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, A.M.; Evangelou, E.; Kerkhof, H.J.; Tamm, A.; Doherty, S.A.; Kisand, K.; Tamm, A.; Kerna, I.; Uitterlinden, A.; Hofman, A.; et al. The GDF5 rs143383 polymorphism is associated with osteoarthritis of the knee with genome-wide statistical significance. Ann. Rheum. Dis. 2011, 70, 873–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, C.M.; Tuan, R.S. Functional role of growth/differentiation factor 5 in chondrogenesis of limb mesenchymal cells. Mech. Dev. 2003, 120, 823–836. [Google Scholar] [CrossRef]
- Murphy, M.K.; Huey, D.J.; Hu, J.C.; Athanasiou, K.A. TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2015, 33, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, H. Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models. Mol. Cells 2008, 25, 1–6. [Google Scholar]
- Amano, K.; Densmore, M.; Nishimura, R.; Lanske, B. Indian hedgehog signaling regulates transcription and expression of collagen type X via Runx2/Smads interactions. J. Biol. Chem. 2014, 289, 24898–24910. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, R.; Wakabayashi, M.; Hata, K.; Matsubara, T.; Honma, S.; Wakisaka, S.; Kiyonari, H.; Shioi, G.; Yamaguchi, A.; Tsumaki, N.; et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J. Biol. Chem. 2012, 287, 33179–33190. [Google Scholar] [CrossRef] [Green Version]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef] [Green Version]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Van der Eerden, B.C.; Karperien, M.; Wit, J.M. Systemic and local regulation of the growth plate. Endocr. Rev. 2003, 24, 782–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone-cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsenty, G.; Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef] [Green Version]
- Yagami, K.; Suh, J.Y.; Enomoto-Iwamoto, M.; Koyama, E.; Abrams, W.R.; Shapiro, I.M.; Pacifici, M.; Iwamoto, M. Matrix GLA protein is a developmental regulator of chondrocyte mineralization and, when constitutively expressed, blocks endochondral and intramembranous ossification in the limb. J. Cell Biol. 1999, 147, 1097–1108. [Google Scholar] [CrossRef]
- Newman, B.; Gigout, L.I.; Sudre, L.; Grant, M.E.; Wallis, G.A. Coordinated expression of matrix Gla protein is required during endochondral ossification for chondrocyte survival. J. Cell Biol. 2001, 154, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Ma, X.; Tong, W.; Doyran, B.; Sun, Z.; Wang, L.; Zhang, X.; Zhou, Y.; Badar, F.; Chandra, A.; et al. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc. Natl. Acad. Sci. USA 2016, 113, 14360–14365. [Google Scholar] [CrossRef] [Green Version]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Quarto, N.; Amalric, F. Heparan sulfate proteoglycans as transducers of FGF-2 signalling. J. Cell Sci. 1994, 107 Pt 11, 3201–3212. [Google Scholar]
- Brewer, J.R.; Mazot, P.; Soriano, P. Genetic insights into the mechanisms of Fgf signaling. Genes Dev. 2016, 30, 751–771. [Google Scholar] [CrossRef] [Green Version]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, T.L. Targeting mechanotransduction pathways in osteoarthritis: A focus on the pericellular matrix. Curr. Opin. Pharmacol. 2013, 13, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018, 71, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannitti, T.; Lodi, D.; Palmieri, B. Intra-articular injections for the treatment of osteoarthritis: Focus on the clinical use of hyaluronic acid. Drugs R D 2011, 11, 13–27. [Google Scholar] [CrossRef]
- Simanek, V.; Kren, V.; Ulrichova, J.; Gallo, J. The efficacy of glucosamine and chondroitin sulfate in the treatment of osteoarthritis: Are these saccharides drugs or nutraceuticals? Biomed. Pap. 2005, 149, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Kakizaki, I.; Tatara, Y.; Majima, M.; Kato, Y.; Endo, M. Identification of proteoglycan from salmon nasal cartilage. Arch. Biochem. Biophys. 2011, 506, 58–65. [Google Scholar] [CrossRef]
- Sano, M.; Shang, Y.; Nakane, A.; Saito, T. Salmon nasal cartilage proteoglycan enhances growth of normal human dermal fibroblast through Erk1/2 phosphorylation. Biosci. Biotechnol. Biochem. 2017, 81, 1379–1385. [Google Scholar] [CrossRef] [Green Version]
- Shukunami, C.; Shigeno, C.; Atsumi, T.; Ishizeki, K.; Suzuki, F.; Hiraki, Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: Differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J. Cell Biol. 1996, 133, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Shukunami, C.; Ishizeki, K.; Atsumi, T.; Ohta, Y.; Suzuki, F.; Hiraki, Y. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J. Bone Min. Res. 1997, 12, 1174–1188. [Google Scholar] [CrossRef]
- Kim, J.R.; Yoo, J.J.; Kim, H.A. Therapeutics in Osteoarthritis Based on an Understanding of Its Molecular Pathogenesis. Int. J. Mol. Sci. 2018, 19, 674. [Google Scholar] [CrossRef] [Green Version]
- Tiku, M.L.; Sabaawy, H.E. Cartilage regeneration for treatment of osteoarthritis: A paradigm for nonsurgical intervention. Ther. Adv. Musculoskelet. Dis. 2015, 7, 76–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, I.; Kimura-Yoshida, C. Extracellular distribution of diffusible growth factors controlled by heparan sulfate proteoglycans during mammalian embryogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonherr, E.; Sunderkotter, C.; Iozzo, R.V.; Schaefer, L. Decorin, a novel player in the insulin-like growth factor system. J. Biol. Chem. 2005, 280, 15767–15772. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Kishioka, Y.; Wakamatsu, J.; Nishimura, T. Decorin activates Akt downstream of IGF-IR and promotes myoblast differentiation. Anim. Sci. J. 2013, 84, 669–674. [Google Scholar] [CrossRef]
- Mrosek, E.H.; Lahm, A.; Erggelet, C.; Uhl, M.; Kurz, H.; Eissner, B.; Schagemann, J.C. Subchondral bone trauma causes cartilage matrix degeneration: An immunohistochemical analysis in a canine model. Osteoarthr. Cartil. 2006, 14, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Scrimgeour, J.; McLane, L.T.; Chang, P.S.; Curtis, J.E. Single-Molecule Imaging of Proteoglycans in the Pericellular Matrix. Biophys. J. 2017, 113, 2316–2320. [Google Scholar] [CrossRef] [Green Version]
- Watt, F.E.; Gulati, M. New Drug Treatments for Osteoarthritis: What is on the Horizon? Eur. Med. J. Rheumatol. 2017, 2, 50–58. [Google Scholar]
- Ghouri, A.; Conaghan, P.G. Update on novel pharmacological therapies for osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19864492. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, J.; Ryu, J.H.; Oh, H.; Chun, C.H.; Kim, B.J.; Min, B.H.; Chun, J.S. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef]
- Akiyama, H.; Shukunami, C.; Nakamura, T.; Hiraki, Y. Differential expressions of BMP family genes during chondrogenic differentiation of mouse ATDC5 cells. Cell Struct. Funct. 2000, 25, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Francis-West, P.H.; Abdelfattah, A.; Chen, P.; Allen, C.; Parish, J.; Ladher, R.; Allen, S.; MacPherson, S.; Luyten, F.P.; Archer, C.W. Mechanisms of GDF-5 action during skeletal development. Development 1999, 126, 1305–1315. [Google Scholar] [PubMed]
- Bhumiratana, S.; Eton, R.E.; Oungoulian, S.R.; Wan, L.Q.; Ateshian, G.A.; Vunjak-Novakovic, G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc. Natl. Acad. Sci. USA 2014, 111, 6940–6945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanne, Y.; Tanimoto, K.; Tanaka, N.; Ueki, M.; Lin, Y.Y.; Ohkuma, S.; Kamiya, T.; Tanaka, E.; Tanne, K. Expression and activity of Runx2 mediated by hyaluronan during chondrocyte differentiation. Arch. Oral Biol. 2008, 53, 478–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatabe, T.; Mochizuki, S.; Takizawa, M.; Chijiiwa, M.; Okada, A.; Kimura, T.; Fujita, Y.; Matsumoto, H.; Toyama, Y.; Okada, Y. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann. Rheum. Dis. 2009, 68, 1051–1058. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, Y.; Kimata, K. Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. J. Biochem. 1998, 124, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Yamada, Y. Chondrodysplasia of gene knockout mice for aggrecan and link protein. Glycoconj. J. 2002, 19, 269–273. [Google Scholar] [CrossRef]
- Chang, J.K.; Chang, L.H.; Hung, S.H.; Wu, S.C.; Lee, H.Y.; Lin, Y.S.; Chen, C.H.; Fu, Y.C.; Wang, G.J.; Ho, M.L. Parathyroid hormone 1-34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009, 60, 3049–3060. [Google Scholar] [CrossRef]
- Sampson, E.R.; Hilton, M.J.; Tian, Y.; Chen, D.; Schwarz, E.M.; Mooney, R.A.; Bukata, S.V.; O’Keefe, R.J.; Awad, H.; Puzas, J.E.; et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci. Transl. Med. 2011, 3, 101ra93. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Matsubara, J.; Wakamatsu, K.; Tanaka, Y.T.; Masutani, T.; Yonezuka, M.; Ito, K.; Takayama, K.T.; Tsuboi, M. Ingestion of Salmon Nasal Cartilage-Derived Proteoglycan Improves Skin Condition: A Randomized, Double-Blind, Controlled Study. Immunol. Endocr. Metab. Agents Med. Chem. 2015, 15, 160–167. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masutani, T.; Yamada, S.; Hara, A.; Takahashi, T.; Green, P.G.; Niwa, M. Exogenous Application of Proteoglycan to the Cell Surface Microenvironment Facilitates to Chondrogenic Differentiation and Maintenance. Int. J. Mol. Sci. 2020, 21, 7744. https://doi.org/10.3390/ijms21207744
Masutani T, Yamada S, Hara A, Takahashi T, Green PG, Niwa M. Exogenous Application of Proteoglycan to the Cell Surface Microenvironment Facilitates to Chondrogenic Differentiation and Maintenance. International Journal of Molecular Sciences. 2020; 21(20):7744. https://doi.org/10.3390/ijms21207744
Chicago/Turabian StyleMasutani, Teruaki, Shuhei Yamada, Akira Hara, Tatsuji Takahashi, Paul G Green, and Masayuki Niwa. 2020. "Exogenous Application of Proteoglycan to the Cell Surface Microenvironment Facilitates to Chondrogenic Differentiation and Maintenance" International Journal of Molecular Sciences 21, no. 20: 7744. https://doi.org/10.3390/ijms21207744
APA StyleMasutani, T., Yamada, S., Hara, A., Takahashi, T., Green, P. G., & Niwa, M. (2020). Exogenous Application of Proteoglycan to the Cell Surface Microenvironment Facilitates to Chondrogenic Differentiation and Maintenance. International Journal of Molecular Sciences, 21(20), 7744. https://doi.org/10.3390/ijms21207744