Interleukin-6: Molecule in the Intersection of Cancer, Ageing and COVID-19
Abstract
:1. Introduction
2. Physiological Functions of IL-6
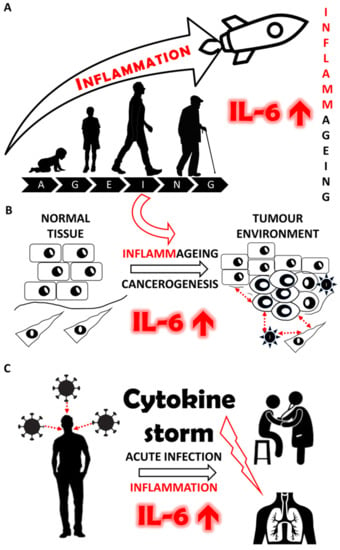
3. IL-6 and Ageing
3.1. “Inflammaging” as a Developmentally Controlled Process
3.2. Non-Steroid Anti-Inflammatory Drugs as “Inflammaging” Therapy in Ageing
3.3. Summary of the Role in IL-6 in Ageing
4. IL-6 and Chronic Inflammatory Diseases
5. Cancer and IL-6
5.1. Cancer as a Complex Tissue/Organ
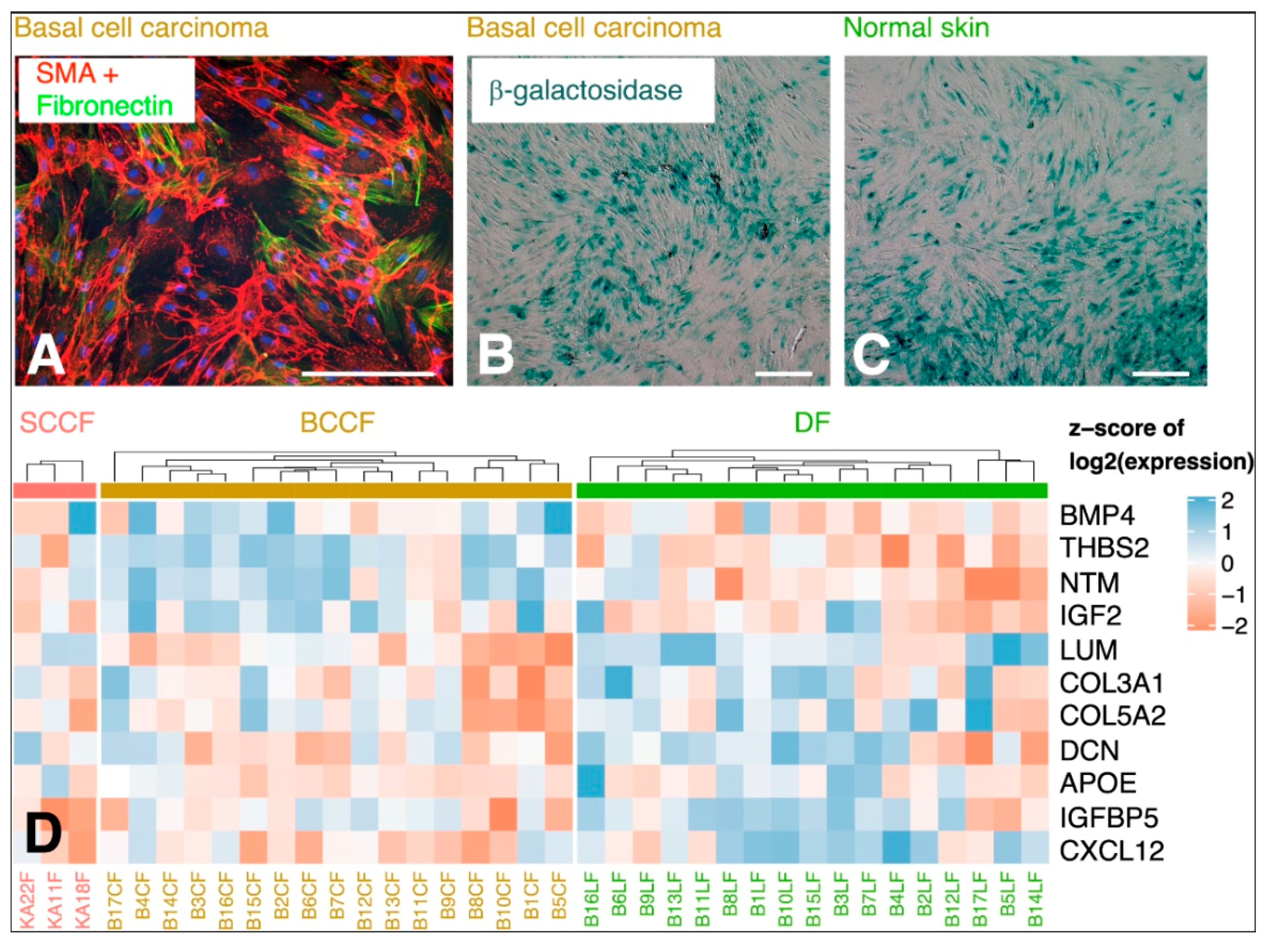
5.2. CAFs as Producers of IL-6
5.3. Local and Systemic Effect of IL-6 in Cancer Progression
5.4. Summary of the Role of IL-6 and Cancer
6. COVID-19
6.1. Covid-19 and IL-6
6.2. Summary of the Role of IL-6 in COVID-19
7. Targeting the IL-6/IL-6R/gp130-Dependent Signalling
7.1. Antibodies
7.2. Natural and Synthetic Small Molecules as IL-6 Receptor Complex Inhibitors
7.2.1. Oestrogen Analogues—Experimental Drugs for Inhibition of IL-6 Signalling
7.2.2. Other Small Molecules—Experimental Drugs
8. Direct Targeting of CAFs
9. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | Acetylsalicylic acid |
| ARDS | Acute respiratory distress syndrome |
| BCC | Basal cell carcinoma |
| BCCF | Basal cell carcinoma-associated fibroblasts |
| BDDF | Brain-derived neurotrophic factor |
| CNTF | Ciliary neurotrophic factor |
| CAFs | Cancer-associated fibroblasts |
| CLC | Cardiotrophin-like cytokine |
| COX | Cyclooxygenase |
| CRP | C-reactive protein |
| CXCR-4 | CXC-chemokine receptor 4 |
| DAPI | 4’,6-Diamidino-2-phenylindole |
| DF | Dermal fibroblast |
| ERBF | 20S,21-Epoxy-resibufogenin-3-formate |
| FAK | Focal adhesion kinase |
| FAP | Fibroblast-activating protein |
| FGF | Fibroblast growth factor |
| FGFR | Fibroblast growth factor receptor |
| GTF | Connective tissue growth factor |
| G-CSF | Granulocyte colony-stimulating factor |
| Gp130 | Glycoprotein 130 |
| IFN | Interferon |
| IGF-1 | Insulin growth factor-1 |
| IL | Interleukin |
| IL-6R | Receptor for IL-6 |
| LIF | Leukaemia inhibitory factor |
| LOXL-2 | Lysyl oxidase-like 2 |
| MFGE8 | Milk fat globule epiderma drowth factor E8 |
| MERS | Middle east respiratory syndrome |
| NFκB | Nuclear factor κ B |
| NK cells | Nature killer cells |
| PDGF | Platelet-derived growth factor |
| ROCK | RHO kinase |
| ROS | Reactive oxygen species |
| SARS | Severe acute respiratory syndrome |
| SASP | Senescence-associated secretory phenotype |
| SCC | Squamous cell carcinoma |
| SCCF | Squamous cell carcinoma-associated fibroblasts |
| sHH | Sonic hedgehog |
| SMA | α-Smooth muscle actin |
| Treg | Regulatory T lymphocytes |
| TGF-β | Transforming growth factor β |
| TNF | Tumour necrosis factor |
| VEGFA | Vascular endothelial growth factor A |
References
- Tissue Expression of IL6—Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000136244-IL6/tissue (accessed on 21 September 2020).
- Zilberstein, A.; Ruggieri, R.; Korn, J.H.; Revel, M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986, 5, 2529–2537. [Google Scholar] [CrossRef]
- Haegeman, G.; Content, J.; Volckaert, G.; Derynck, R.; Tavernier, J.; Fiers, W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur. J. Biochem. 1986, 159, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Taga, T.; Nakano, N.; Yasukawa, K.; Kashiwamura, S.; Shimizu, K.; Nakajima, K.; Pyun, K.H.; Kishimoto, T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc. Natl. Acad. Sci. USA 1985, 82, 5490–5494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakenhoff, J.P.; de Groot, E.R.; Evers, R.F.; Pannekoek, H.; Aarden, L.A. Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J. Immunol. 1987, 139, 4116–4121. [Google Scholar] [PubMed]
- Nordan, R.P.; Pumphrey, J.G.; Rudikoff, S. Purification and NH2-terminal sequence of a plasmacytoma growth factor derived from the murine macrophage cell line P388D1. J. Immunol. 1987, 139, 813–817. [Google Scholar] [PubMed]
- Gauldie, J.; Richards, C.; Harnish, D.; Lansdorp, P.; Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. USA 1987, 84, 7251–7255. [Google Scholar] [CrossRef] [Green Version]
- Ikebuchi, K.; Wong, G.G.; Clark, S.C.; Ihle, J.N.; Hirai, Y.; Ogawa, M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc. Natl. Acad. Sci. USA 1987, 84, 9035–9039. [Google Scholar] [CrossRef] [Green Version]
- Takai, Y.; Wong, G.G.; Clark, S.C.; Burakoff, S.J.; Herrmann, S.H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J. Immunol. 1988, 140, 140. [Google Scholar]
- Groeger, S.; Meyle, J. Oral mucosal epithelial cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [Green Version]
- Pritts, T.; Hungness, E.; Wang, Q.; Robb, B.; Hershko, D.; Hasselgren, P.O. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia—Role of transcription factors and regulation by the stress response. Am. J. Surg. 2002, 183, 372–383. [Google Scholar] [CrossRef]
- Uehling, D.T.; Brooke Johnson, D.; Hopkins, W.J. The urinary tract response to entry of pathogens. World J. Urol. 1999, 17, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.Y.; Poon, L.L.M.; Ng, I.H.Y.; Luk, W.; Sia, S.-F.; Wu, M.H.S.; Chan, K.-H.; Yuen, K.-Y.; Gordon, S.; Guan, Y.; et al. Cytokine Responses in Severe Acute Respiratory Syndrome Coronavirus-Infected Macrophages In Vitro: Possible Relevance to Pathogenesis. J. Virol. 2005, 79, 7819–7826. [Google Scholar] [CrossRef] [Green Version]
- Kyotani, Y.; Takasawa, S.; Yoshizumi, M. Proliferative pathways of vascular smooth muscle cells in response to intermittent hypoxia. Int. J. Mol. Sci. 2019, 20, 2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbalho, S.; Vieira Prado Neto, E.; de Alvares Goulart, R.; Bechara, M.; Federighi Baisi Chagas, E.; Audi, M.; Guissoni Campos, L.; Landgraf Guiger, E.; Leoni Buchain, R.; Buchain, D.; et al. Myokines: A descriptive review. J. Sports Med. Phys. Fit. 2020. [Google Scholar] [CrossRef]
- Kovács, B.; Vajda, E.; Nagy, E.E. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Chen, Q. Adipokines: New Therapeutic Target for Osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Lacina, L.; Brábek, J.; Král, V.; Kodet, O.; Smetana, K. Interleukin-6: A molecule with complex biological impact in cancer. Histol. Histopathol. 2019, 34, 125–136. [Google Scholar]
- Unver, N.; McAllister, F. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018, 41, 10–17. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopf, M.; Baumann, H.; Freer, G.; Freudenberg, M.; Lamers, M.; Kishimoto, T.; Zinkernagel, R.; Bluethmann, H.; Köhler, G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 1994, 368, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, A.J.; Kopf, M. IL-6 Gene Knockout Mice. In Cytokine Knockouts. Contemporary Immunology; Durum, S.K., Muegge, K., Eds.; Humana Press: Totowa, NJ, USA, 1998; pp. 227–236. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, Immunity, And disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, F.; Okano, A.; Suzuki, C.; Chieda, R.; Takahara, Y.; Hirano, T.; Kishimoto, T.; Hamuro, J.; Akiyama, Y. Human recombinant IL-6/B cell stimulatory factor 2 augments murine antigen-specific antibody responses in vitro and in vivo. J. Immunol. 1988, 141, 3072–3077. [Google Scholar]
- Luger, T.A.; Krutmann, J.; Kirnbauer, R.; Urbanski, A.; Schwarz, T.; Klappacher, G.; Köck, A.; Micksche, M.; Malejczyk, J.; Schauer, E. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J. Immunol. 1989, 143, 1206–1209. [Google Scholar]
- Mendel, I.; Katz, A.; Kozak, N.; Ben-Nun, A.; Revel, M. Interleukin-6 functions in autoimmune encephalomyelitis: A study in gene-targeted mice. Eur. J. Immunol. 1998, 28, 1727–1737. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Ezquerro, S.; Méndez-Giménez, L.; Frühbeck, G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol. 2017, 219, 362–381. [Google Scholar] [CrossRef]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002, 8, 75–79. [Google Scholar] [CrossRef]
- Tamura, T.; Udagawa, N.; Takahashi, N.; Miyaura, C.; Tanaka, S.; Yamada, Y.; Koishihara, Y.; Ohsugi, Y.; Kumaki, K.; Taga, T.; et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 11924–11928. [Google Scholar] [CrossRef] [Green Version]
- Poli, V.; Balena, R.; Fattori, E.; Markatos, A.; Yamamoto, M.; Tanaka, H.; Ciliberto, G.; Rodan, G.A.; Costantini, F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994, 13, 1189–1196. [Google Scholar] [CrossRef]
- Ahmad, S.I. Aging: Exploring a Complex Phenomenon; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1315283875. [Google Scholar]
- World Health Organization. World Report on Ageing And Health; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Feltes, B.C.; De Faria Poloni, J.; Bonatto, D. The developmental aging and origins of health and disease hypotheses explained by different protein networks. Biogerontology 2011, 12, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, R.; Ashman, M.; Asthana, D. Effects of Ageing on the Immune System: Infants to Elderly. Scand. J. Immunol. 2016, 83, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun. Ageing 2016, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, K.C.L.; de Rezende, V.B.; Lima-Silva, M.L.; de Santos, L.J.S.; Costa, C.G.; de Mambrini, J.V.M.; Peixoto, S.V.; Tarazona-Santos, E.; Martins Filho, O.A.; Lima-Costa, M.F.; et al. Immune senescence and biomarkers profile of Bambuí aged population-based cohort. Exp. Gerontol. 2018, 103, 47–56. [Google Scholar] [CrossRef]
- Adriaensen, W.; Matheï, C.; Van Pottelbergh, G.; Vaes, B.; Legrand, D.; Wallemacq, P.; Degryse, J.M. Significance of serum immune markers in identification of global functional impairment in the oldest old: Cross-sectional results from the BELFRAIL study. Age (Omaha) 2014, 36, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Adriaensen, W.; Matheï, C.; Vaes, B.; van Pottelbergh, G.; Wallemacq, P.; Degryse, J.M. Interleukin-6 as a first-rated serum inflammatory marker to predict mortality and hospitalization in the oldest old: A regression and CART approach in the BELFRAIL study. Exp. Gerontol. 2015, 69, 53–61. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Rattan, S.I.S. Aging is not a disease: Implications for intervention. Aging Dis. 2014, 5, 196–202. [Google Scholar] [CrossRef]
- Strnadova, K.; Sandera, V.; Dvorankova, B.; Kodet, O.; Duskova, M.; Smetana, K.; Lacina, L. Skin aging: The dermal perspective. Clin. Dermatol. 2019, 37, 326–335. [Google Scholar] [CrossRef]
- Schrell, U.M.H.; Koch, U.; Marschalek, R.; Schrauzer, T.; Anders, M.; Adams, E.; Fahlbusch, R. Formation of autocrine loops in human cerebral meningioma tissue by leukemia inhibitor factor, interleukin-6, and oncostatin M: Inhibition of meningioma cell growth in vitro by recombinant oncostatin M. Neurosurg. Focus 2008, 2, E9. [Google Scholar] [CrossRef]
- Chambers, E.S.; Akbar, A.N. Can blocking inflammation enhance immunity during aging? J. Allergy Clin. Immunol. 2020, 145, 1323–1331. [Google Scholar] [CrossRef]
- Win, T.T.; Aye, S.N.; Fern, J.L.C.; Fei, C.O. Aspirin and reducing risk of gastric cancer: Systematic review and meta-analysis of the observational studies. J. Gastrointest. Liver Dis. 2020, 29, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Chen, X.; Zhang, F.; Li, X. Aspirin use and endometrial cancer risk: A meta-analysis and systematic review. Ann. Transl. Med. 2020, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Fiala, C.; Pasic, M.D. Aspirin: Bitter pill or miracle drug? Clin. Biochem. 2020, 85, 1–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, W.; Jiang, J. Prevention and treatment of cancer targeting chronic inflammation: Research progress, potential agents, clinical studies and mechanisms. Sci. China Life Sci. 2017, 60, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E. Melanoma inhibition by cyclooxygenase inhibitors: Role of interleukin-6 suppression, a putative mechanism of action, and clinical implications. Med. Oncol. 2007, 24, 1–6. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Chiu, H.H.; Wang, C.H.; Kuo, C.H. Aspirin modifies inflammatory mediators and metabolomic profiles and contributes to the suppression of obesity-associated breast cancer cell growth. Int. J. Mol. Sci. 2020, 21, 4652. [Google Scholar] [CrossRef]
- Tian, Y.; Ye, Y.; Gao, W.; Chen, H.; Song, T.; Wang, D.; Mao, X.; Ren, C. Aspirin promotes apoptosis in a murine model of colorectal cancer by mechanisms involving downregulation of IL-6-STAT3 signaling pathway. Int. J. Colorectal. Dis. 2011, 26, 13–22. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Aspirin and Cancer. J. Am. Coll. Cardiol. 2016, 68, 967–976. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Gillman, M.; Harper, D.M.; Kemper, A.R.; Krist, A.H.; et al. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2016, 164, 836–845. [Google Scholar] [CrossRef] [Green Version]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; O’Mahony, M.S.; Calver, B.L.; Woodhouse, K.W. Nutrition, inflammation, and leptin levels in aging and frailty. J. Am. Geriatr. Soc. 2008, 56, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Cook, N.R.; Gaziano, J.M.; Price, J.F.; Belch, J.F.F.; Roncaglioni, M.C.; Morimoto, T.; Mehta, Z. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: Analysis of individual patient data from randomised trials. Lancet 2018, 392, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Straub, R.H.; Schradin, C. Chronic inflammatory systemic diseases—An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public Health 2016, 2016, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Sacco, A.; Bruno, A.; Contursi, A.; Dovizio, M.; Tacconelli, S.; Ricciotti, E.; Guillem-Llobat, P.; Salvatore, T.; Di Francesco, L.; Fullone, R.; et al. Platelet-Specific Deletion of Cyclooxygenase-1 Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice s. J. Pharmacol. Exp. Ther. J. Pharmacol. Exp. Ther 2019, 370, 416–426. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in rheumatoid arthritis. Int. J. Mol. Sci. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Smetana, K.; Lacina, L.; Szabo, P.; Dvořánková, B.; Broẑ, P.; Ŝedo, A. Ageing as an important risk factor for cancer. Anticancer Res. 2016, 36, 5009–5017. [Google Scholar] [CrossRef] [Green Version]
- Moraes, M.C.S. DNA repair mechanisms protect our genome from carcinogenesis. Front. Biosci. 2012, 17, 1362. [Google Scholar] [CrossRef] [Green Version]
- Edifizi, D.; Schumacher, B. Genome instability in development and aging: Insights from nucleotide excision repair in humans, mice, and worms. Biomolecules 2015, 5, 1855–1869. [Google Scholar] [CrossRef] [Green Version]
- Kareva, I. What can ecology teach us about cancer? Transl. Oncol. 2011, 4, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Birbrair, A. Stem cell microenvironments and beyond. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2017; Volume 1041, pp. 1–3. [Google Scholar]
- Flier, J.S.; Underhill, L.H.; Dvorak, H.F. Tumors: Wounds That Do Not Heal. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Smetana, K.; Szabo, P.; Gál, P.; André, S.; Gabius, H.J.; Kodet, O.; Dvořánková, B. Emerging role of tissue lectins as microenvironmental effectors in tumors and wounds. Histol. Histopathol. 2015, 30, 293–309. [Google Scholar] [PubMed]
- Lacina, L.; Kodet, O.; Dvořánková, B.; Szabo, P.; Smetana, K. Ecology of melanoma cell. Histol. Histopathol. 2018, 33, 247–254. [Google Scholar] [PubMed]
- Lacina, L.; Smetana, K.; Dvořánková, B.; Pytlík, R.; Kideryová, L.; Kučerová, L.; Plzáková, Z.; Štork, J.; Gabius, H.J.; André, S. Stromal fibroblasts from basal cell carcinoma affect phenotype of normal keratinocytes. Br. J. Dermatol. 2007, 156, 819–829. [Google Scholar] [CrossRef]
- Lacina, L.; Dvořánkova, B.; Smetana Jr., K.; Chovanec, M.; Plzǎk, J.; Tachezy, R.; Kideryovǎ, L.; Kučerová, L.; Čada, Z.; Bouček, J.; et al. Marker profiling of normal keratinocytes identifies the stroma from squamous cell carcinoma of the oral cavity as a modulatory microenvironment in co-culture. Int. J. Radiat. Biol. 2007, 83, 837–848. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, R.; Pietras, K. Heterogeneity of cancer-associated fibroblasts: Opportunities for precision medicine. Cancer Sci. 2020, 111, 2708–2717. [Google Scholar] [CrossRef]
- Dvořánková, B.; Smetana, K.; Říhová, B.; Kučera, J.; Mateu, R.; Szabo, P. Cancer-associated fibroblasts are not formed from cancer cells by epithelial-to-mesenchymal transition in nu/nu mice. Histochem. Cell Biol. 2015, 143, 463–469. [Google Scholar] [CrossRef]
- Hill, B.S.; Pelagalli, A.; Passaro, N.; Zannetti, A. Tumor-Educated mesenchymal stem cells promote Pro-Metastatic phenotype. Oncotarget 2017, 8, 73296–73311. [Google Scholar] [CrossRef] [Green Version]
- Barcellos-Hoff, M.H.; Ravani, S.A. Irradiated Mammary Gland Stroma Promotes the Expression of Tumorigenic Potential by Unirradiated Epithelial Cells 1. Cancer Res. 2000, 60, 1254–1260. [Google Scholar]
- Dvořánková, B.; Szabo, P.; Lacina, L.; Gal, P.; Uhrova, J.; Zima, T.; Kaltner, H.; André, S.; Gabius, H.-J.; Sykova, E.; et al. Human galectins induce conversion of dermal fibroblasts into myofibroblasts and production of extracellular matrix: Potential application in tissue engineering and wound repair. Cells Tissues Organs 2011, 194, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Travers, J.B.; Machado, C.; Somani, A.K.; Spandau, D.F. Reversing the aging stromal phenotype prevents carcinoma initiation. Aging (Albany. NY) 2011, 3, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; Demaria, M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr. Biol. 2017, 27, 2652–2660. [Google Scholar] [CrossRef] [Green Version]
- Dvořánková, B.; Szabo, P.; Lacina, L.; Kodet, O.; Matouškové, E.; Smetana, K. Fibroblasts prepared from different types of malignant tumors stimulate expression of luminal marker keratin 8 in the EM-G3 breast cancer cell line. Histochem. Cell Biol. 2012, 137, 679–685. [Google Scholar] [CrossRef]
- Trylcova, J.; Busek, P.; Smetana, K.; Balaziova, E.; Dvorankova, B.; Mifkova, A.; Sedo, A. Effect of cancer-associated fibroblasts on the migration of glioma cells in vitro. Tumor Biol. 2015, 36, 5873–5879. [Google Scholar] [CrossRef]
- Szabó, P.; Kolář, M.; Dvořánková, B.; Lacina, L.; Štork, J.; Vlček, Č.; Strnad, H.; Tvrdek, M.; Smetana, K. Mouse 3T3 fibroblasts under the influence of fibroblasts isolated from stroma of human basal cell carcinoma acquire properties of multipotent stem cells. Biol. Cell 2011, 103, 233–248. [Google Scholar] [CrossRef]
- Plzák, J.; Bouček, J.; Bandúrová, V.; Kolář, M.; Hradilová, M.; Szabo, P.; Lacina, L.; Chovanec, M.; Smetana, K. The head and neck squamous cell carcinoma microenvironment as a potential target for cancer therapy. Cancers 2019, 11, 440. [Google Scholar] [CrossRef] [Green Version]
- Heneberg, P. Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit. Rev. Oncol. Hematol. 2016, 97, 303–311. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Xiong, S.; Wang, X.; Zhao, Z.; Bai, S.; Wang, Y.; Zhao, Y.; Cheng, B. Cancer-associated fibroblasts promote the stemness of CD24 + liver cells via paracrine signaling. J. Mol. Med. 2019, 97, 243–255. [Google Scholar] [CrossRef]
- Nagasaki, T.; Hara, M.; Nakanishi, H.; Takahashi, H.; Sato, M.; Takeyama, H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: Anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br. J. Cancer 2014, 110, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omland, S.H.; Wettergren, E.E.; Mourier, T.; Hansen, A.J.; Asplund, M.; Mollerup, S.; Robert, R. Cancer associated fibroblasts (CAFs) are activated in cutaneous basal cell carcinoma and in the peritumoural skin. BMC Cancer 2017, 17, 675. [Google Scholar] [CrossRef]
- Depner, S.; Lederle, W.; Gutschalk, C.; Linde, N.; Zajonz, A.; Mueller, M.M. Cell type specific interleukin-6 induced responses in tumor keratinocytes and stromal fibroblasts are essential for invasive growth. Int. J. Cancer 2014, 135, 551–562. [Google Scholar] [CrossRef]
- Jobe, N.P.; Živicová, V.; Mifková, A.; Rösel, D.; Dvořánková, B.; Kodet, O.; Strnad, H.; Kolář, M.; Šedo, A.; Smetana, K.; et al. Fibroblasts potentiate melanoma cells in vitro invasiveness induced by UV-irradiated keratinocytes. Histochem. Cell Biol. 2018, 149, 503–516. [Google Scholar] [CrossRef]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gyamfi, J.; Eom, M.; Koo, J.S.; Choi, J. Multifaceted Roles of Interleukin-6 in Adipocyte—Breast Cancer Cell Interaction. Transl. Oncol. 2018, 11, 275–285. [Google Scholar] [CrossRef]
- Jobe, N.P.; Rösel, D.; Dvořánková, B.; Kodet, O.; Lacina, L.; Mateu, R.; Smetana, K.; Brábek, J. Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem. Cell Biol. 2016, 146, 205–217. [Google Scholar] [CrossRef]
- Jayatilaka, H.; Tyle, P.; Chen, J.J.; Kwak, M.; Ju, J.; Kim, H.J.; Lee, J.S.H.; Wu, P.H.; Gilkes, D.M.; Fan, R.; et al. Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat. Commun. 2017, 8, 15584. [Google Scholar] [CrossRef] [Green Version]
- Von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, K.; Jones, J.; Lwin, Z.; Coward, J.I.G. Interleukin-6: An angiogenic target in solid tumours. Crit. Rev. Oncol. Hematol. 2014, 89, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kučera, J.; Strnadová, K.; Dvořánková, B.; Lacina, L.; Krajsová, I.; Štork, J.; Kovářová, H.; Skalníková, H.K.H.K.; Vodička, P.; Motlík, J.; et al. Serum proteomic analysis of melanoma patients with immunohistochemical profiling of primary melanomas and cultured cells: Pilot study. Oncol. Rep. 2019, 42, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Kodet, O.; Dvořánková, B.; Bendlová, B.; Sýkorová, V.; Krajsová, I.; Štork, J.; Kučera, J.; Szabo, P.; Strnad, H.; Kolář, M.; et al. Microenvironment-driven resistance to B-Raf inhibition in a melanoma patient is accompanied by broad changes of gene methylation and expression in distal fibroblasts. Int. J. Mol. Med. 2018, 41, 2687–2703. [Google Scholar] [CrossRef] [Green Version]
- Kolb, A.D.; Shupp, A.B.; Mukhopadhyay, D.; Marini, F.C.; Bussard, K.M. Osteoblasts are “educated” by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment. Breast Cancer Res. 2019, 21, 31. [Google Scholar] [CrossRef] [Green Version]
- Stoll, J.R.; Vaidya, T.S.; Mori, S.; Dusza, S.W.; Lacouture, M.E.; Markova, A. Association of interleukin-6 and tumor necrosis factor-α with mortality in hospitalized patients with cancer. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef]
- White, J.P. IL-6, cancer and cachexia: Metabolic dysfunction creates the perfect storm. Transl. Cancer Res. 2017, 6, S280–S285. [Google Scholar] [CrossRef]
- Shinsyu, A.; Bamba, S.; Kurihara, M.; Matsumoto, H.; Sonoda, A.; Inatomi, O.; Andoh, A.; Takebayashi, K.; Kojima, M.; Iida, H.; et al. Inflammatory cytokines, appetite-regulating hormones, and energy metabolism in patients with gastrointestinal cancer. Oncol. Lett. 2020, 20, 1469–1479. [Google Scholar] [CrossRef]
- Kays, J.K.; Koniaris, L.G.; Cooper, C.A.; Pili, R.; Jiang, G.; Liu, Y.; Zimmers, T.A. The combination of low skeletal muscle mass and high tumor interleukin-6 associates with decreased survival in clear cell renal cell carcinoma. Cancers 2020, 12, 1605. [Google Scholar] [CrossRef]
- Dwarkasing, J.T.; Witkamp, R.F.; Boekschoten, M.V.; Ter Laak, M.C.; Heins, M.S.; van Norren, K. Increased hypothalamic serotonin turnover in inflammation-induced anorexia. BMC Neurosci. 2016, 17, 26. [Google Scholar] [CrossRef] [Green Version]
- Shimura, Y.; Kurosawa, H.; Tsuchiya, M.; Sawa, M.; Kaneko, H.; Liu, L.; Makino, Y.; Nojiri, H.; Iwase, Y.; Kaneko, K.; et al. Serum interleukin 6 levels are associated with depressive state of the patients with knee osteoarthritis irrespective of disease severity. Clin. Rheumatol. 2017, 36, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Keaton, S.A.; Madaj, Z.B.; Heilman, P.; Smart, L.A.; Grit, J.; Gibbons, R.; Postolache, T.T.; Roaten, K.; Achtyes, E.D.; Brundin, L. An inflammatory profile linked to increased suicide risk. J. Affect. Disord. 2019, 247, 57–65. [Google Scholar] [CrossRef]
- Pormohammad, A.; Ghorbani, S.; Baradaran, B.; Khatami, A.J.; Turner, R.; Mansournia, M.A.; Kyriacou, D.N.; Idrovo, J.P.; Bahr, N.C. Clinical characteristics, laboratory findings, radiographic signs and outcomes of 61,742 patients with confirmed COVID-19 infection: A systematic review and meta-analysis. Microb. Pathog. 2020, 147, 104390. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guo, Y.; Mao, R.; Zhang, J. Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta-analysis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Bonam, S.R.; Kaveri, S.V.; Sakuntabhai, A.; Gilardin, L.; Bayry, J. Adjunct Immunotherapies for the Management of Severely Ill COVID-19 Patients. Cell Reports Med. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Paces, J.; Strizova, Z.; Smrz, D.; Cerny, J. COVID-19 and the immune system. Physiol. Res. 2020, 69, 379–388. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Hellmuth, J.C.; Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Level of IL-6 Predicts Respiratory Failure in Hospitalized Symptomatic COVID-19 Patients; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2020. [Google Scholar]
- Liu, W.-J.; Wang, X.-D.; Wu, W.; Huang, X. Relationship between depression and blood cytokine levels in lung cancer patients. Médecine/Sciences 2018, 34, 113–115. [Google Scholar] [CrossRef] [Green Version]
- Ulhaq, Z.S.; Soraya, G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020, 50, 382–383. [Google Scholar] [CrossRef]
- Polidoro, R.B.; Hagan, R.S.; de Santis Santiago, R.; Schmidt, N.W. Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19. Front. Immunol. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-metastatic and Anti-invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosel, D.; Fernandes, M.; Sanz-Moreno, V.; Brábek, J. Migrastatics: Redirecting R&D in Solid Cancer Towards Metastasis? Trends Cancer 2019, 5, 755–756. [Google Scholar] [PubMed]
- Fulciniti, M.; Hideshima, T.; Vermot-Desroches, C.; Pozzi, S.; Nanjappa, P.; Shen, Z.; Patel, N.; Smith, E.S.; Wang, W.; Prabhala, R.; et al. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin. Cancer Res. 2009, 15, 7144–7152. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Vaidya, G.; Czer, L.S.C.; Kobashigawa, J.; Kittleson, M.; Patel, J.; Chang, D.; Kransdorf, E.; Shikhare, A.; Tran, H.; Vo, A.; et al. Successful Treatment of Severe COVID-19 Pneumonia With Clazakizumab in a Heart Transplant Recipient: A Case Report. Transplant. Proc. 2020. [Google Scholar] [CrossRef]
- Moreno-Pérez, O.; Andres, M.; Leon-Ramirez, J.M.; Sánchez-Payá, J.; Rodríguez, J.C.; Sánchez, R.; García-Sevila, R.; Boix, V.; Gil, J.; Merino, E. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study. J. Autoimmun. 2020, 114, 102523. [Google Scholar] [CrossRef] [PubMed]
- Palanques-Pastor, T.; López-Briz, E.; Poveda Andrés, J.L. Involvement of interleukin 6 in SARS-CoV-2 infection: Siltuximab as a therapeutic option against COVID-19. Eur. J. Hosp. Pharm. 2020, 27, 297–298. [Google Scholar] [CrossRef]
- Tomasiewicz, K.; Piekarska, A.; Stempkowska-Rejek, J.; Serafińska, S.; Gawkowska, A.; Parczewski, M.; Niścigorska-Olsen, J.; Łapiński, T.W.; Zarębska-Michaluk, D.; Kowalska, J.D.; et al. Tocilizumab for patients with severe COVID-19: A retrospective, multi-centre study. Expert Rev. Anti Infect. Ther. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 25 September 2020).
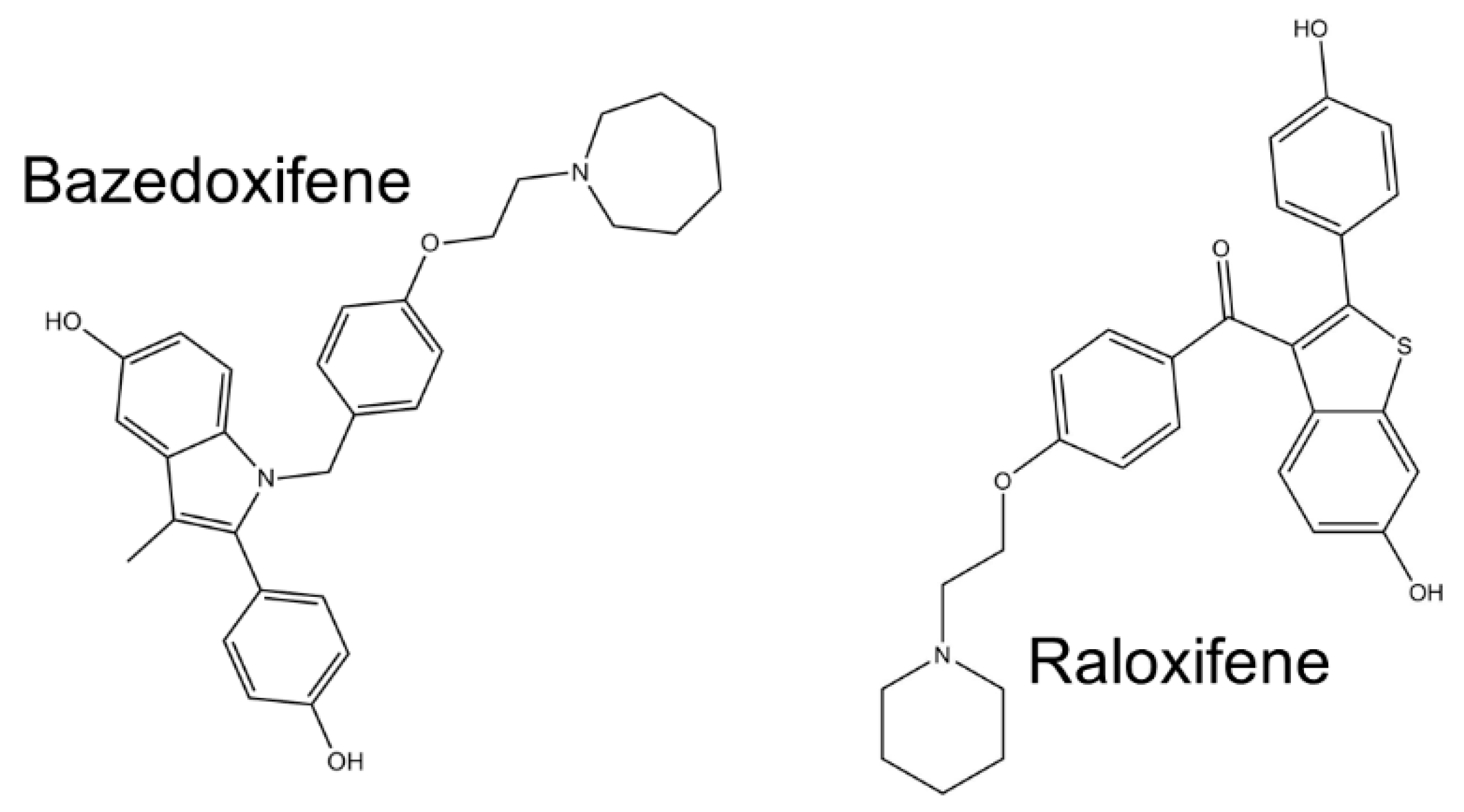
- Gennari, L.; Merlotti, D.; De Paola, V.; Martini, G.; Nuti, R. Bazedoxifene for the prevention of postmenopausal osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 1229–1242. [Google Scholar] [CrossRef] [Green Version]
- Quintanilla Rodriguez, B.S.; Correa, R. Raloxifene; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Xiao, H.; Bid, H.K.; Chen, X.; Wu, X.; Wei, J.; Bian, Y.; Zhao, C.; Li, H.; Li, C.; Lin, J. Repositioning Bazedoxifene as a novel IL-6/GP130 signaling antagonist for human rhabdomyosarcoma therapy. PLoS ONE 2017, 12, e0180297. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Yu, W.; Ren, Y.; Zhu, J.; Wan, C.; Cai, G.; Guo, J.; Zhang, W.; Kong, L. Discovery of bazedoxifene analogues targeting glycoprotein 130. Eur. J. Med. Chem. 2020, 199, 112375. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, B.; Teknos, T.N.; Kumar, P. Bazedoxifene enhances the anti-tumor effects of cisplatin and radiation treatment by blocking IL-6 signaling in head and neck cancer. Oncotarget 2017, 8, 66912–66924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Cao, Y.; Xiao, H.; Li, C.; Lin, J. Bazedoxifene as a novel GP130 inhibitor for pancreatic cancer therapy. Mol. Cancer Ther. 2016, 15, 2609–2619. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tian, J.; Su, G.H.; Lin, J. Blocking IL-6/GP130 Signaling Inhibits Cell Viability/Proliferation, Glycolysis, and Colony Forming Activity in Human Pancreatic Cancer Cells. Curr. Cancer Drug Targets 2018, 19, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, L.; Lai, Y.H.; Zhang, R.; Li, H.; Li, C.; Lin, J. Bazedoxifene as a novel GP130 inhibitor for Colon Cancer therapy. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yan, D.; Wang, Y.; Shi, W.; Liu, T.; Zhao, C.; Huo, S.; Duan, J.; Tao, J.; Zhai, M.; et al. Bazedoxifene exhibits growth suppressive activity by targeting interleukin-6/glycoprotein 130/signal transducer and activator of transcription 3 signaling in hepatocellular carcinoma. Cancer Sci. 2019, 110, 950–961. [Google Scholar] [CrossRef] [Green Version]
- Existing Osteoporosis Drug Shows Potential for Treating COVID-19|News|CORDIS|European Commission. Available online: https://cordis.europa.eu/article/id/421499-existing-osteoporosis-drug-shows-potential-for-treating-covid-19 (accessed on 20 September 2020).
- Smetana, K.; Rosel, D.; BrÁbek, J. Raloxifene and Bazedoxifene Could Be Promising Candidates for Preventing the COVID-19 Related Cytokine Storm, ARDS and Mortality. In Vivo 2020, 34, 3027–3028. [Google Scholar] [CrossRef]
- Smetana, K.; Smetana, K.; Brábek, J.; Brábek, J. Role of interleukin-6 in lung complications in patients with COVID-19: Therapeutic implications. In Vivo (Brooklyn) 2020, 34, 1589–1592. [Google Scholar] [CrossRef]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020, 64, 64. [Google Scholar] [CrossRef]
- Protein Scaffolds—BioProcess InternationalBioProcess International. Available online: https://bioprocessintl.com/upstream-processing/expression-platforms/protein-scaffolds-339588/ (accessed on 21 September 2020).
- Hayashi, M.; Kim, Y.P.; Takamatsu, S.; Enomoto, A.; Shinose, M.; Takahashi, Y.; Tanaka, H.; Komiyama, K.; Omura, S. Madindoline, a novel inhibitor of IL-6 activity from Streptomyces sp. K93-0711. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. (Tokyo) 1996, 49, 1091–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, M.; Rho, M.C.; Enomoto, A.; Fukami, A.; Kim, Y.P.; Kikuchi, Y.; Sunazuka, T.; Hirose, T.; Komiyama, K.; Omura, S. Suppression of bone resorption by madindoline a, a novel nonpeptide antagonist to gp130. Proc. Natl. Acad. Sci. USA 2002, 99, 14728–14733. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, A.; Rho, M.-C.; Fukami, A.; Hiraku, O.; Komiyama, K.; Hayashi, M. Suppression of cancer cachexia by 20S,21-epoxy-resibufogenin-3-acetate—A novel nonpeptide IL-6 receptor antagonist. Biochem. Biophys. Res. Commun. 2004, 323, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.Z.M.; Kevin, L.G.; Billings, S.; van Vranken, D.L.; Krolewski, J.J. Binding of Madindoline A to the Extracellular Domain of gp130†. Biochemistry 2005, 44, 10822–10827. [Google Scholar] [CrossRef] [PubMed]
- Kino, T.; Boos, T.L.; Sulima, A.; Siegel, E.M.; Gold, P.W.; Rice, K.C.; Chrousos, G.P. 3-O-Formyl-20R,21-epoxyresibufogenin suppresses IL-6–type cytokine actions by targeting the glycoprotein 130 subunit: Potential clinical implications. J. Allergy Clin. Immunol. 2007, 120, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Sunazuka, T.; Hirose, T.; Kojima, N.; Kaji, E.; Omura, S. Design, synthesis, and biological activities of madindoline analogues. Bioorganic Med. Chem. Lett. 2006, 16, 2807–2811. [Google Scholar] [CrossRef] [PubMed]
- Aqel, S.I.; Kraus, E.E.; Jena, N.; Kumari, V.; Granitto, M.C.; Mao, L.; Farinas, M.F.; Zhao, E.Y.; Perottino, G.; Pei, W.; et al. Novel small molecule IL-6 inhibitor suppresses autoreactive Th17 development and promotes T reg development. Clin. Exp. Immunol. 2019, 196, 215–225. [Google Scholar] [CrossRef]
- Hong, S.-S.; Choi, J.H.; Lee, S.Y.; Park, Y.-H.; Park, K.-Y.; Lee, J.Y.; Kim, J.; Gajulapati, V.; Goo, J.-I.; Singh, S.; et al. A Novel Small-Molecule Inhibitor Targeting the IL-6 Receptor β Subunit, Glycoprotein 130. J. Immunol. 2015, 195, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Qiao, C.; Xiao, H.; Lin, Z.; Li, Y.; Zhang, J.; Shen, B.; Fu, T.; Feng, J. Structure-based virtual screening and characterization of a novel IL-6 antagonistic compound from synthetic compound database. Drug Des. Dev. Ther. 2016, 10, 4091–4100. [Google Scholar] [CrossRef] [Green Version]
- Kamano, Y.; Nogawa, T.; Yamashita, A.; Hayashi, M.; Inoue, M.; Drašar, P.; Pettit, G.R. Isolation and structure of a 20,21-epoxybufenolide series from “Ch’an Su. ” J. Nat. Prod. 2002, 65, 1001–1005. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorganic Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Davis, M. Clinically relevant concentrations of anticancer drugs: A guide for nonclinical studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, C.; Theillet, C.; Portier, M.; Bataille, R.; Klein, B. Molecular analysis of the IL-6 receptor in human multiple myeloma, an IL-6-related disease. FEBS Lett. 1994, 341, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Stephens, O.W.; Zhang, Q.; Qu, P.; Zhou, Y.; Chavan, S.; Tian, E.; Williams, D.R.; Epstein, J.; Barlogie, B.; Shaughnessy, J.D. An intermediate-risk multiple myeloma subgroup is defined by sIL-6r: Levels synergistically increase with incidence of SNP rs2228145 and 1q21 amplification. Blood 2012, 119, 503–512. [Google Scholar] [CrossRef]
- Buchwald, P.; Bodor, N. Brain-Targeting Chemical Delivery Systems and Their Cyclodextrin-Based Formulations in Light of the Contributions of Marcus E. Brewster. J. Pharm. Sci. 2016, 105, 2589–2600. [Google Scholar] [CrossRef] [Green Version]
- Nigro, A.; Pellegrino, M.; Greco, M.; Comandè, A.; Sisci, D.; Pasqua, L.; Leggio, A.; Morelli, C. Dealing with skin and blood-brain barriers: The unconventional challenges of mesoporous silica nanoparticles. Pharmaceutics 2018, 10, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, E.Y.; Chen, Y.S.; Li, Y.S.; Chen, S.R.; Lee, C.H.; Huang, M.H.; Chuang, H.M.; Harn, H.J.; Yang, H.H.; Lin, S.Z.; et al. Liposome Consolidated with Cyclodextrin Provides Prolonged Drug Retention Resulting in Increased Drug Bioavailability in Brain. Int. J. Mol. Sci. 2020, 21, 4408. [Google Scholar] [CrossRef]
- Dvořáková, P.; Bušek, P.; Knedlík, T.; Schimer, J.; Etrych, T.; Kostka, L.; Stollinová Šromová, L.; Šubr, V.; Šácha, P.; Šedo, A.; et al. Inhibitor-Decorated Polymer Conjugates Targeting Fibroblast Activation Protein. J. Med. Chem. 2017, 60, 8385–8393. [Google Scholar] [CrossRef]
- Šimková, A.; Bušek, P.; Šedo, A.; Konvalinka, J. Molecular recognition of fibroblast activation protein for diagnostic and therapeutic applications. Biochim. Biophys. Acta-Proteins Proteom. 2020, 1868, 140409. [Google Scholar] [CrossRef]








| Name | Author |
|---|---|
| Interferon β-2 | Zilberstein et al., 1986 [2] |
| 26K factor | Haegeman et al., 1986 [3] |
| B-cell stimulatory factor | Hirano et al., 1985 [4] |
| Hybridoma growth factor | Brakenhoff et al., 1987 [5] |
| Plasmacytoma growth factor | Nordan et al., 1987 [6] |
| Hepatocyte stimulatory factor | Gauldie et al., 1987 [7] |
| Haematopoietic factor | Ikebuchi et al., 1987 [8] |
| Cytotoxic T-cell differentiation factor | Takai et al., 1988 [9] |
| Type of cell | Author |
|---|---|
| Keratinocyte | Groeger and Meyle, 2019 [10] |
| Enterocyte | Pritts et al., 2002 [11] |
| Urothelium | Uehling et al., 1999 [12] |
| Hepatocyte | Schmidt-Arras and Rose-John, 2016 [13] |
| Pneumocyte and bronchial epithelial cell | Cheung, 2005 [14] |
| Smooth muscle | Kyotani et al., 2019 [15] |
| Skeletal muscle | Barbalho et al., 2020 [16] |
| Osteoblast | Kovács et al., 2019 [17] |
| Adipocyte | Xie et al., 2019 [18] |
| Macrophage | Shapouri-Moghaddam et al., 2018 [19] |
| Neuron | Shapouri-Moghaddam et al., 2018 [19] |
| Type of Cell | Effect on Tumour Growth and Spreading | Author |
|---|---|---|
| Prostate | + | Heneberg, 2016 [84] |
| Adenocarcinoma of pancreas | + | Heneberg, 2016 [84] |
| Liver | + | Li et al., 2019 [85] |
| Colorectal | + | Nagasaki et al., 2014 [86] |
| Stomach | + | Wu et al., 2017 [87] |
| Lung | + | Wang et al., 2017 [88] |
| Head and neck squamous cell carcinoma | + | Plzák et al., 2019 [83] |
| Basal cell carcinoma of skin | + | Omland et al., 2017 [89] |
| Squamous cell carcinoma of skin | + | Depner et al., 2014 [90] |
| Cutaneous malignant melanoma | + | Jobe et al., 2018 [91] |
| Urinary bladder | + | Goulet et al., 2019 [92] |
| Antibody | Target | Main Application | Producer |
|---|---|---|---|
| Siltuximab * | IL-6 | Renal + prostate cancer Castleman’s disease COVID-19 | EUSA Pharma |
| Sirukumab + | IL-6 | Rheumatoid arthritis COVID-19 | Janssen Biotech |
| Olokizumab + | IL-6 | Rheumatoid arthritis | R-Pharm Group |
| Clazakizumab + | IL-6 | Psoriatic arthritis COVID-19 | Bristol Myers Squibb and Alder Biopharmaceuticals |
| Elsilimomab + | IL-6 | Lymphoma Myeloma | Diaclone |
| Tocilizumab * | IL-6R | Rheumatoid arthritis Multiple myeloma Prostate cancer COVID-19 | Hoffmann-La Roche and Chugai |
| Sarilumab * | Gp130 | Rheumatoid arthritis | Regeneron Pharmaceuticals and Sanofi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brábek, J.; Jakubek, M.; Vellieux, F.; Novotný, J.; Kolář, M.; Lacina, L.; Szabo, P.; Strnadová, K.; Rösel, D.; Dvořánková, B.; et al. Interleukin-6: Molecule in the Intersection of Cancer, Ageing and COVID-19. Int. J. Mol. Sci. 2020, 21, 7937. https://doi.org/10.3390/ijms21217937
Brábek J, Jakubek M, Vellieux F, Novotný J, Kolář M, Lacina L, Szabo P, Strnadová K, Rösel D, Dvořánková B, et al. Interleukin-6: Molecule in the Intersection of Cancer, Ageing and COVID-19. International Journal of Molecular Sciences. 2020; 21(21):7937. https://doi.org/10.3390/ijms21217937
Chicago/Turabian StyleBrábek, Jan, Milan Jakubek, Fréderic Vellieux, Jiří Novotný, Michal Kolář, Lukáš Lacina, Pavol Szabo, Karolína Strnadová, Daniel Rösel, Barbora Dvořánková, and et al. 2020. "Interleukin-6: Molecule in the Intersection of Cancer, Ageing and COVID-19" International Journal of Molecular Sciences 21, no. 21: 7937. https://doi.org/10.3390/ijms21217937
APA StyleBrábek, J., Jakubek, M., Vellieux, F., Novotný, J., Kolář, M., Lacina, L., Szabo, P., Strnadová, K., Rösel, D., Dvořánková, B., & Smetana, K., Jr. (2020). Interleukin-6: Molecule in the Intersection of Cancer, Ageing and COVID-19. International Journal of Molecular Sciences, 21(21), 7937. https://doi.org/10.3390/ijms21217937