Determinants of Increased Serum Calprotectin in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Inflammatory Parameters
2.3. Independent Determinants of Serum Calprotectin
2.4. Serum Calprotectin Related to CVD
2.5. Sensitivity Analyses
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patients
4.3. Dietary Assessment
4.4. Outcome Measurement
4.5. Covariates
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 Diabetes Mellitus |
| CVD | Cardiovascular diseases |
| CRP | C-reactive protein |
References
- García-Arias, M.; Pascual-Salcedo, D.; Ramiro, S.; Ueberschlag, M.E.; Jermann, T.M.; Cara, C.; Martín-Mola, E.; Balsa, A. Calprotectin in rheumatoid arthritis: Association with disease activity in a cross-sectional and longitudinal cohort. Mol. Diagn. Ther. 2013, 17, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Abildtrup, M.; Kingsley, G.H.; Scott, D.L. Calprotectin as a biomarker in rheumatoid arthritis: A systematic review. J. Rheumatol. 2015, 42, 760–770. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Van Rheenen, P.F.; van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. Br. Med. J. 2010, 341, c3369. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Flores-Guerrero, J.L.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Sundrehagen, E.; Seidu, S.; Dullaart, R.P.; Bakker, S.J. Plasma calprotectin and risk of cardiovascular disease: Findings from the PREVEND prospective cohort study. Atherosclerosis 2018, 275, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Sabater, M.; Moreno-Navarrete, J.M.; Pueyo, N.; Botas, P.; Delgado, E.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur. J. Endocrinol. 2012, 167, 569–578. [Google Scholar] [CrossRef]
- Mortensen, O.H.; Nielsen, A.R.; Erikstrup, C.; Plomgaard, P.; Fischer, C.P.; Krogh-Madsen, R.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; Pedersen, B.K. Calprotectin, a novel marker of obesity. PLoS ONE 2009, 4, e7419. [Google Scholar] [CrossRef]
- Pedersen, L.; Nybo, M.; Poulsen, M.K.; Henriksen, J.E.; Dahl, J.; Rasmussen, L.M. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc. Disord. 2014, 14, 196. [Google Scholar] [CrossRef]
- Berezin, A. The Cardiovascular Risk Prognostication in Diabetes Mellitus: The Role of Myeloid-related Protein Complex Calprotectin. Int. J. Pathol. Clin. Res. 2016, 2, 026. [Google Scholar] [CrossRef]
- Kruzliak, P.; Novak, J.; Novak, M.; Fodor, G.J. Role of calprotectin in cardiometabolic diseases. Cytokine Growth Factor Rev. 2014, 25, 67–75. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Pruimboom, L.; Dijck-Brouwer, D.J.; Muskiet, F.A. Lifestyle and nutritional imbalances associated with Western diseases: Causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J. Nutr. Biochem. 2013, 24, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; FitzGerald, G.A. Oxidative stress and smoking-induced vascular injury. Prog. Cardiovasc. Dis. 2003, 46, 79–90. [Google Scholar] [CrossRef]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on biomarkers of subclinical inflammation: A systematic review and meta-analysis of intervention studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Roth, B.; Larsson, E.; Höglund, P. Calprotectin in serum and zonulin in serum and feces are elevated after introduction of a diet with lower carbohydrate content and higher fiber, fat and protein contents. Biomed. Rep. 2017, 6, 411–422. [Google Scholar] [CrossRef]
- Kretowicz, M.; Johnson, R.J.; Ishimoto, T.; Nakagawa, T.; Manitius, J. The impact of fructose on renal function and blood pressure. Int. J. Nephrol. 2011, 2011, 315879. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Fernández-Real, J.M.; Salvador, J.; et al. Increased levels of calprotectin in obesity are related to macrophage content: Impact on inflammation and effect of weight loss. Mol. Med. 2011, 17, 1157–1167. [Google Scholar] [CrossRef]
- Schmaderer, C.; Kemmner, S.; Burkhardt, K.; Heemann, U.; Baumann, M. Serum myeloid-related protein 8/14 complex is associated with microalbuminuria in patients with type 2 diabetes. Ther. Adv. Cardiovasc. Dis. 2014, 8, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Festa, A.; D’agostino, R., Jr.; Howard, G.; Mykkänen, L.; Tracy, R.P.; Haffner, S.M. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000, 58, 1703–1710. [Google Scholar] [CrossRef]
- Poullis, A.; Foster, R.; Mendall, M.A.; Fagerhol, M.K. Emerging role of calprotectin in gastroenterology. J. Gastroenterol. Hepatol. 2003, 18, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Fagerhol, M.K.; Dale, I.; Andersson, T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull. Eur. Physiopathol. Respir. 1980, 16, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Marinopoulos, S.; Berkenblit, G.; Rami, T.; Brancati, F.L.; Powe, N.R.; Golden, S.H. Meta-Analysis: Glycosylated Hemoglobin and Cardiovascular Disease in Diabetes Mellitus. Ann. Intern. Med. 2004, 141, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, J.; Rensen, S.S.; Slaats, Y.; Van Dielen, F.M.; Buurman, W.A.; Greve, J.W.M. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity 2009, 17, 2014–2018. [Google Scholar] [CrossRef]
- Gant, C.M.; Binnenmars, S.H.; Berg EV, D.; Bakker, S.J.; Navis, G.; Laverman, G.D. Integrated Assessment of Pharmacological and Nutritional Cardiovascular Risk Management: Blood Pressure Control in the DIAbetes and LIfEstyle Cohort Twente (DIALECT). Nutrients 2017, 9, 709. [Google Scholar] [CrossRef]
- Feunekes, G.I.; Van Staveren, W.A.; De Vries, J.H.; Burema, J.; Hautvast, J.G. Relative and Biomarker-Based Validity of a Food-Frequency Questionnaire Estimating Intake of Fats and Cholesterol. Am. J. Clin. Nutr. 1993, 58, 489–496. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment (RIVM). Dutch Food Composition Database–NEVO Online Version 2013/4.0; RIVM: Bilthoven, The Netherlands, 2013. [Google Scholar]
- Wendel-Vos, G.W.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Willet, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
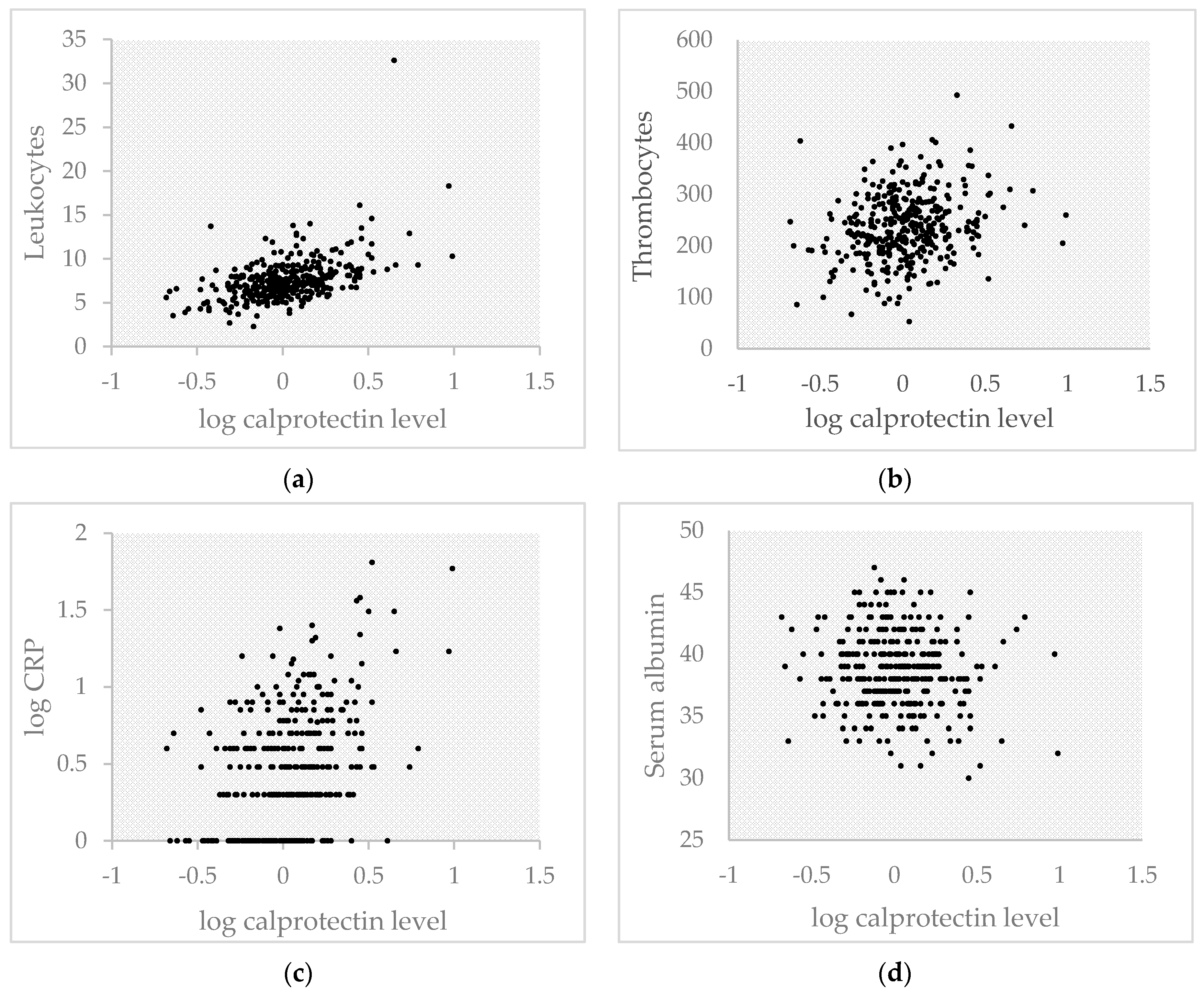
| Variable | Total Population | R | p-Value |
|---|---|---|---|
| N | 362 | ||
| Serum calprotectin (mg/L) | 1.04 [0.73–1.46] | ||
| Age (years) | 63 ± 9 | −0.035 | 0.51 |
| Body Mass Index (kg/m2) | 32.7 ± 5.8 | −0.025 | 0.65 |
| Normal weight (BMI < 25 kg/m2) | 18 (5) | Ref | |
| Overweight (BMI 25–30 kg/m2) | 110 (30) | 0.060 | 0.61 |
| Obese (BMI > 30 kg/m2) | 234 (65) | 0.061 | 0.60 |
| Urinary creatinine excretion (mmol/24 h) | 13.8 ± 4.9 | −0.031 | 0.62 |
| Waist circumference (cm) 1 | 112 ± 13 | 0.030 | 0.57 |
| Smoking status | |||
| Never smoker | 110 (30) | Ref | |
| Former smoker | 190 (53) | 0.135 | 0.022 |
| Current smoker | 62 (17) | 0.266 | <0.001 |
| Alcohol consumption | |||
| No alcohol consumption | 130 (36) | Ref | |
| <1 consumption per day | 140 (39) | −0.031 | 0.61 |
| >1 consumption per day | 92 (25) | 0.076 | 0.23 |
| Physical activity 1 | 205 (57) | 0.066 | 0.21 |
| Systolic blood pressure (mmHg) | 139 ± 16 | −0.070 | 0.18 |
| Diastolic blood pressure (mmHg) | 76 ± 10 | −0.091 | 0.08 |
| HbA1c | 57 ± 12 | −0.063 | 0.23 |
| Insulin use (n, %) | 227 (63) | −0.016 | 0.77 |
| Diabetes duration (years) | 11 [7–18] | −0.015 | 0.78 |
| eGFR (mL/min/1.73 m2) | 78 ± 24 | −0.087 | 0.10 |
| Albuminuria (n, %) | 110 (30) | 0.135 | 0.011 |
| Nutrition | |||
| Energy (kcal/day) | 1993 ± 640 | 0.048 | 0.40 |
| Protein (g/day) | 78 ± 12 | −0.022 | 0.68 |
| Vegetable protein (g/day) | 27 ± 6 | −0.084 | 0.11 |
| Animal protein (g/day) | 51 ± 13 | 0.015 | 0.78 |
| Fatty acids (g/day) | 88 ± 14 | 0.015 | 0.78 |
| Carbohydrates (g/day) | 205 ± 34 | 0.056 | 0.29 |
| Mono- and disaccharides (g/day) | 91 ± 28 | 0.127 | 0.015 |
| Polysaccharides (g/day) | 111 ± 26 | −0.063 | 0.23 |
| Fibre (g/day) | 20 ± 5 | −0.011 | 0.83 |
| Estimated sodium intake (mg/day) 2 | 4284 ± 1838 | −0.055 | 0.32 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| B | p-Value | B | p-Value | B | p-Value | |
| Age | −0.035 | 0.51 | −0.019 | 0.72 | −0.036 | 0.48 |
| Former smokers | 0.137 | 0.021 | 0.133 | 0.023 | ||
| Current smokers | 0.264 | <0.001 | 0.269 | <0.001 | ||
| Mono- and disaccharides (g/day) | 0.143 | 0.005 | ||||
| Albuminuria | 0.132 | 0.011 |
| Variable | Standardized Beta | p-Value | Percentage Difference (95% CI) |
|---|---|---|---|
| Sex | 0.155 | 0.003 | 20 (6 to 35) |
| Former smokers | 0.133 | 0.023 | 16 (2 to 33) |
| Current smokers | 0.269 | <0.001 | 51 (27 to 79) |
| Intake of mono- and disaccharides | 0.143 | 0.005 | 0.2 (0 to 0.5) |
| Albuminuria | 0.132 | 0.011 | 18 (4 to 34) |
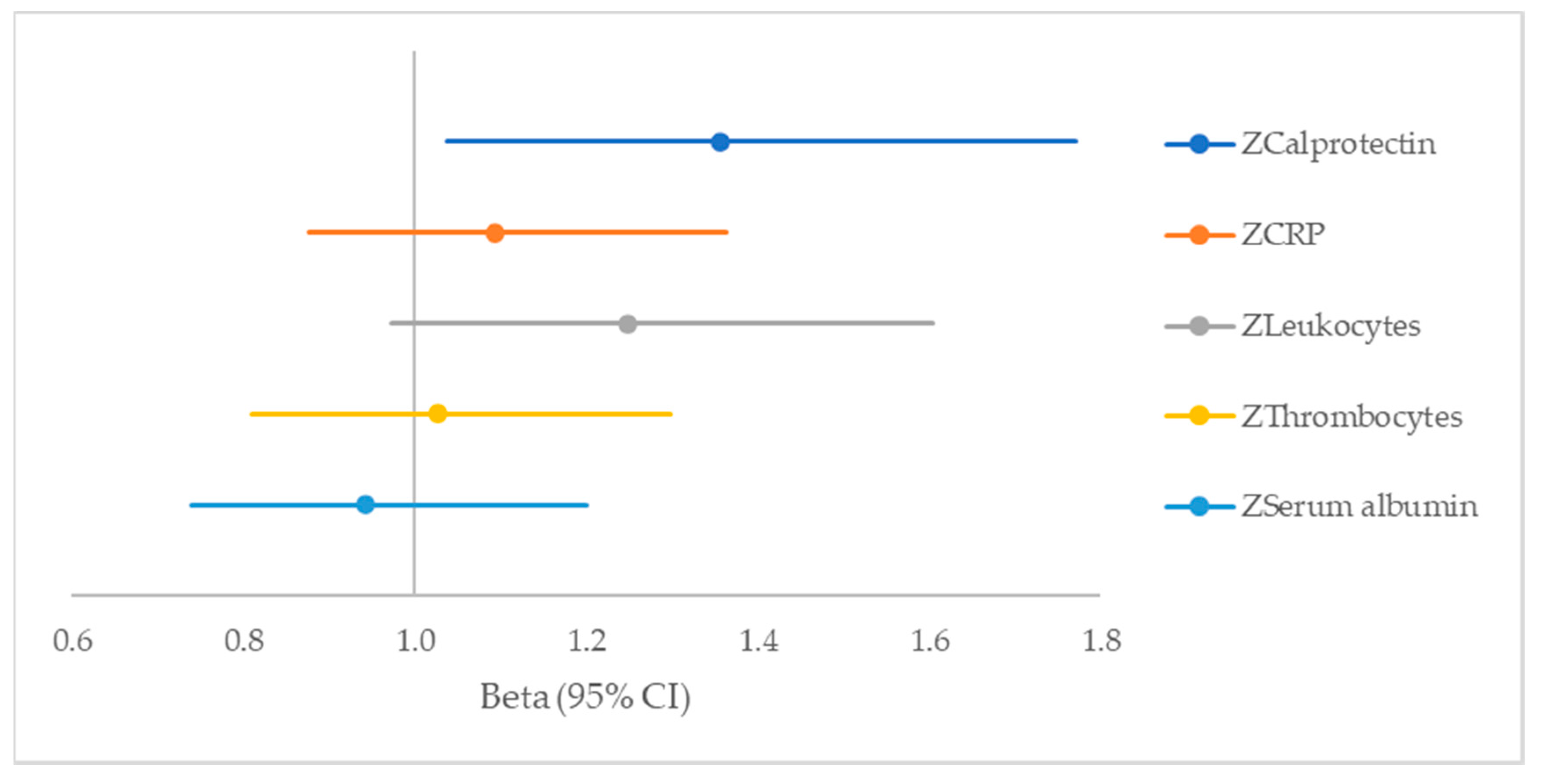
| Variable | Beta (95% CI) | p-Value |
|---|---|---|
| ZCalprotectin | 1.356 (1.037–1.772) | 0.026 |
| ZCRP | 1.093 (0.877–1.363) | 0.43 |
| ZLeukocytes | 1.248 (0.972–1.604) | 0.08 |
| ZThrombocytes | 1.026 (0.811–1.299) | 0.83 |
| ZSerum albumin | 0.942 (0.740–1.200) | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oosterwijk, M.M.; Bakker, S.J.L.; Nilsen, T.; Navis, G.; Laverman, G.D. Determinants of Increased Serum Calprotectin in Patients with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 8075. https://doi.org/10.3390/ijms21218075
Oosterwijk MM, Bakker SJL, Nilsen T, Navis G, Laverman GD. Determinants of Increased Serum Calprotectin in Patients with Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2020; 21(21):8075. https://doi.org/10.3390/ijms21218075
Chicago/Turabian StyleOosterwijk, Milou M., Stephan J.L. Bakker, Tom Nilsen, Gerjan Navis, and Gozewijn D. Laverman. 2020. "Determinants of Increased Serum Calprotectin in Patients with Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 21, no. 21: 8075. https://doi.org/10.3390/ijms21218075
APA StyleOosterwijk, M. M., Bakker, S. J. L., Nilsen, T., Navis, G., & Laverman, G. D. (2020). Determinants of Increased Serum Calprotectin in Patients with Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 21(21), 8075. https://doi.org/10.3390/ijms21218075






