The Role of Age, Neutrophil Infiltration and Antibiotics Timing in the Severity of Streptococcus pneumoniae Pneumonia. Insights from a Multi-Level Mathematical Model Approach
Abstract
1. Introduction
2. Results
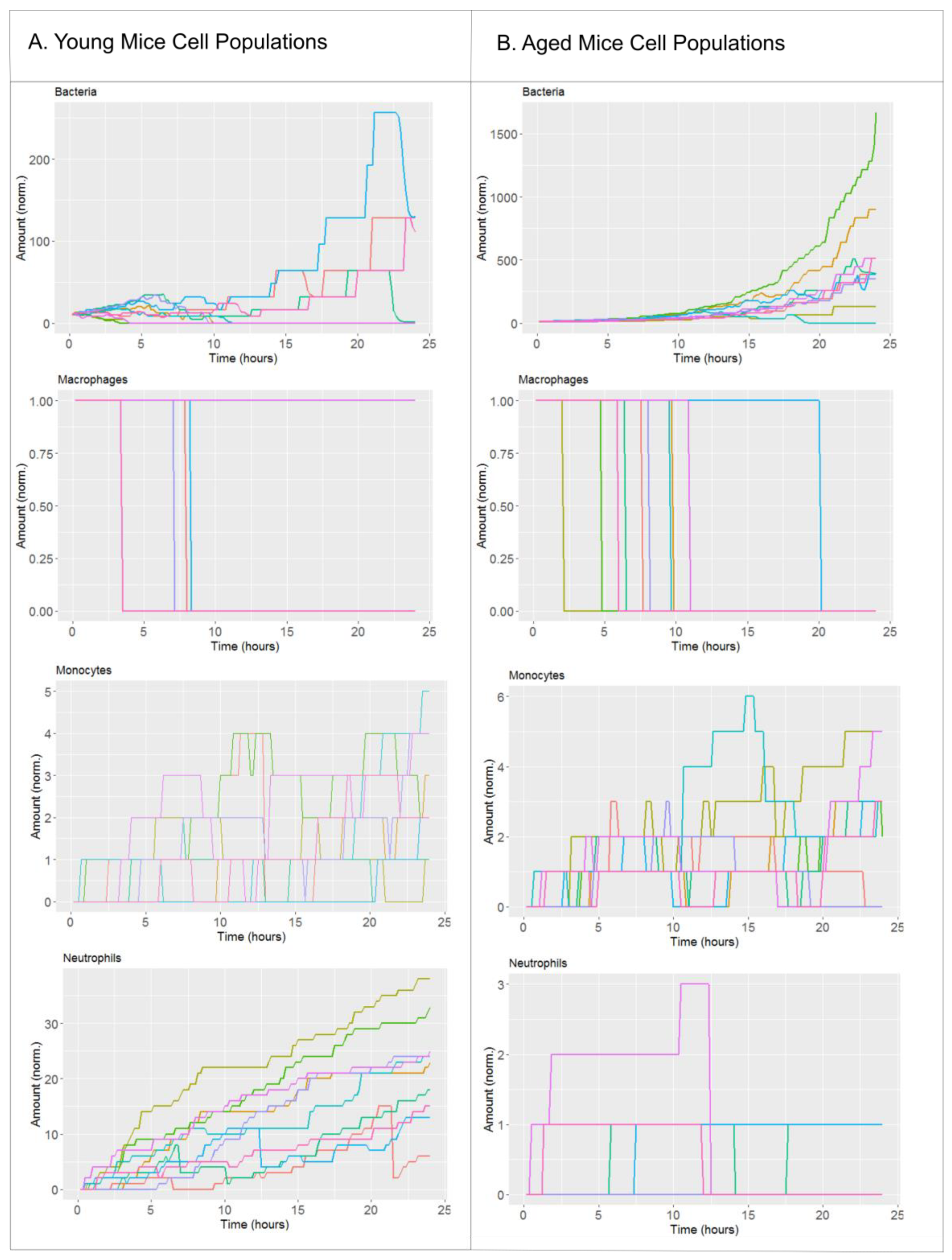
2.1. Biological Scenarios Modelled
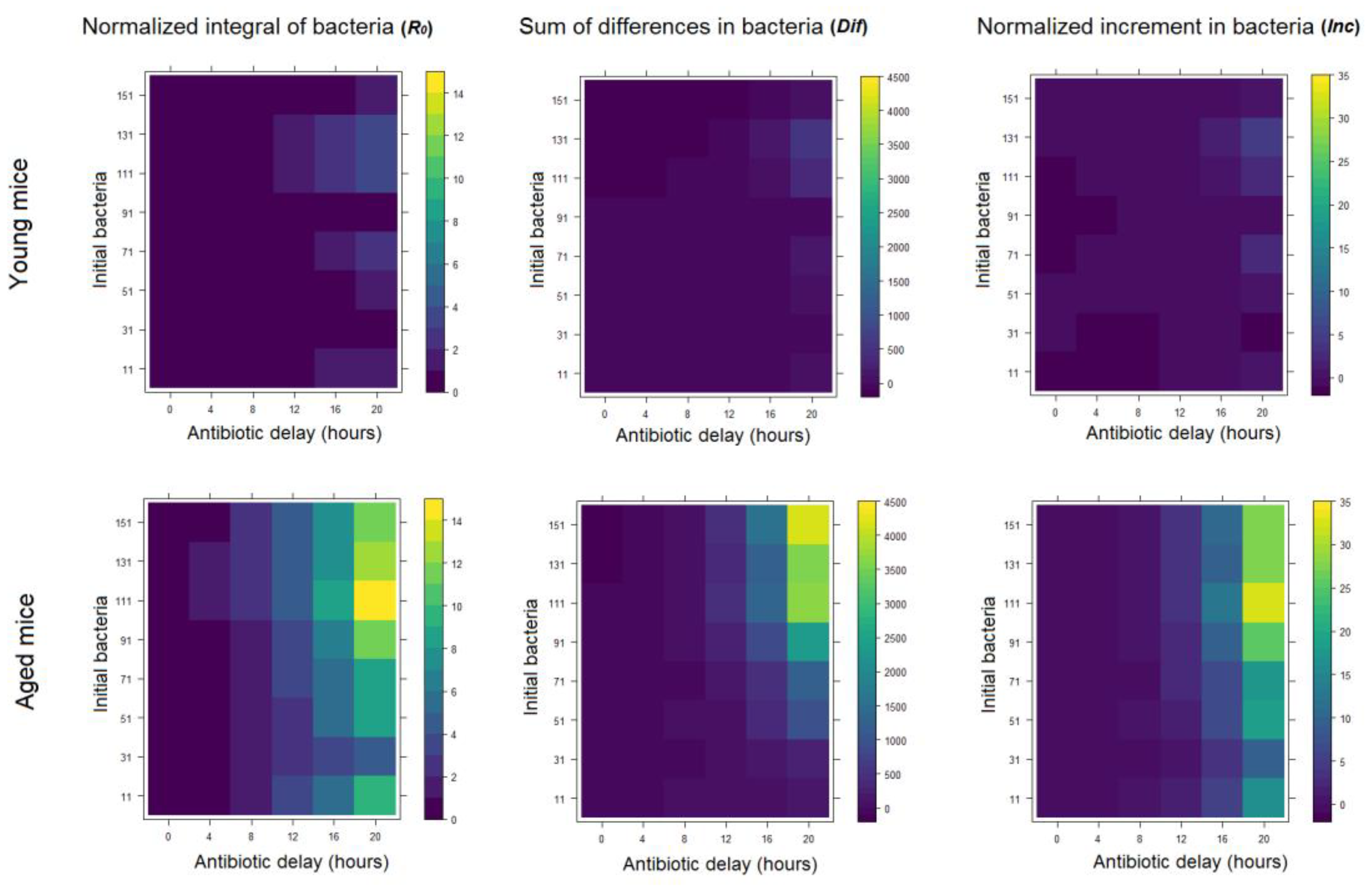
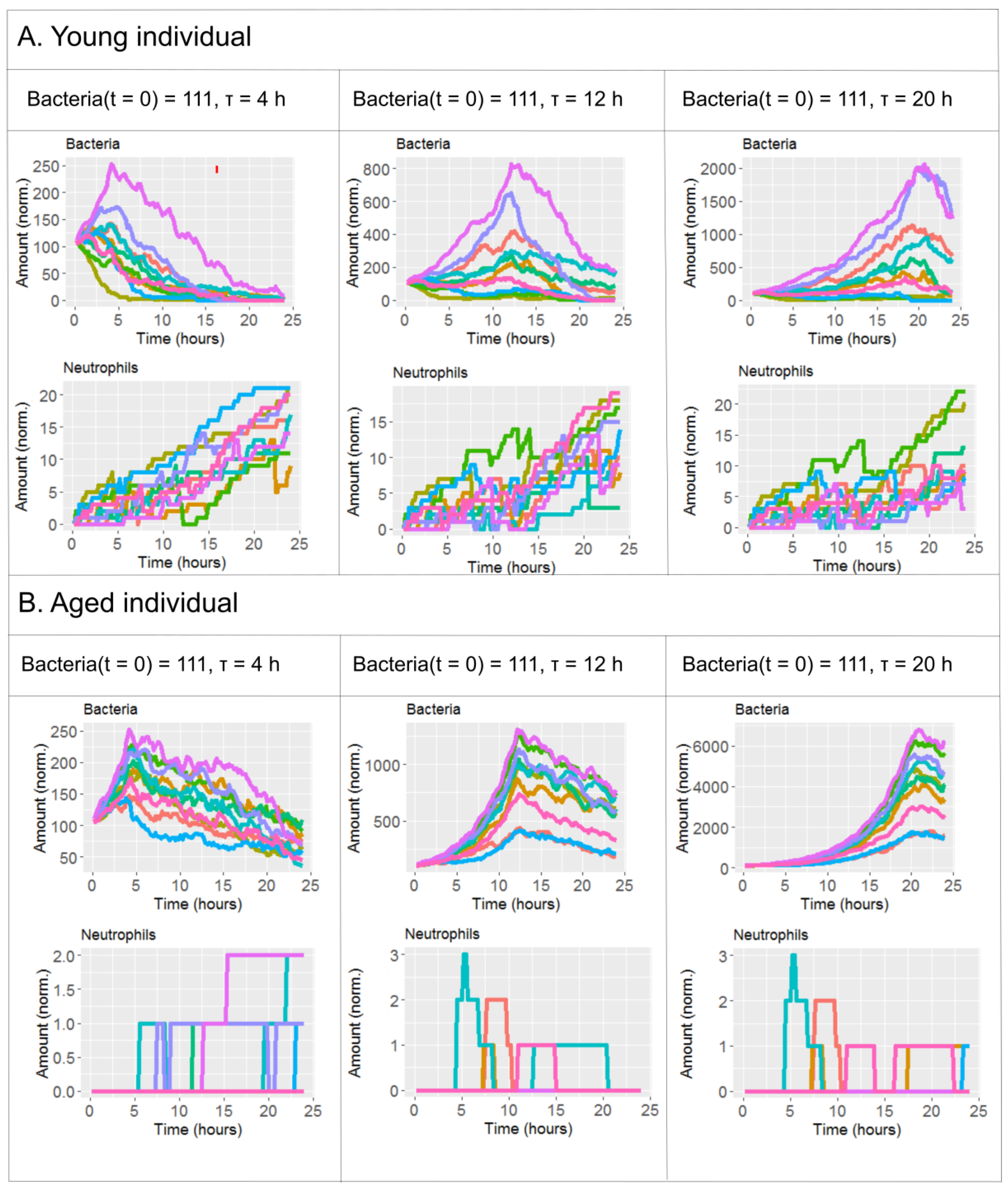
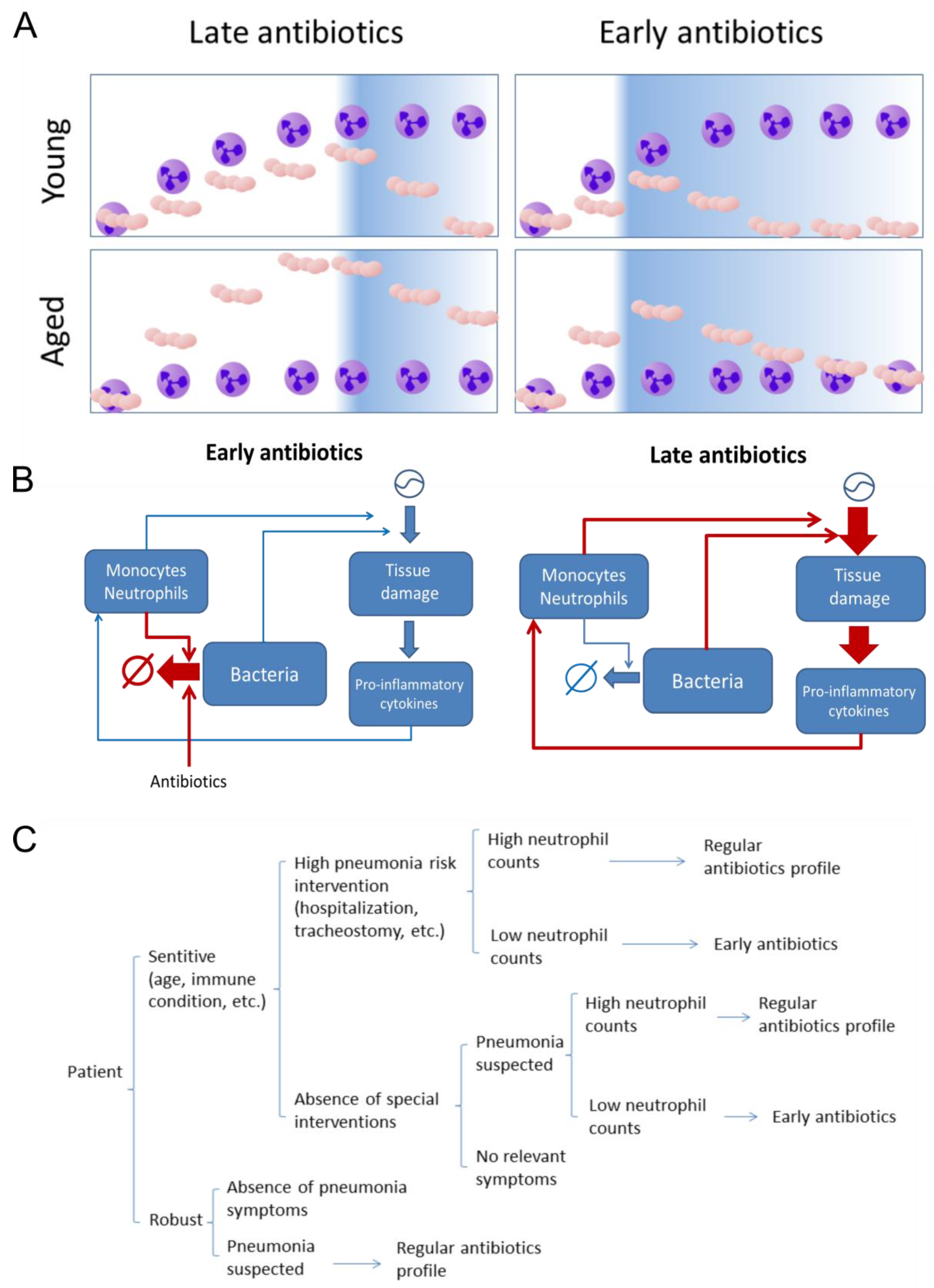
2.2. The Effect of Antibiotics Administration Timing in Early Lung Infection for Aged Individuals with Impaired Neutrophils Recruitment
3. Material and Methods
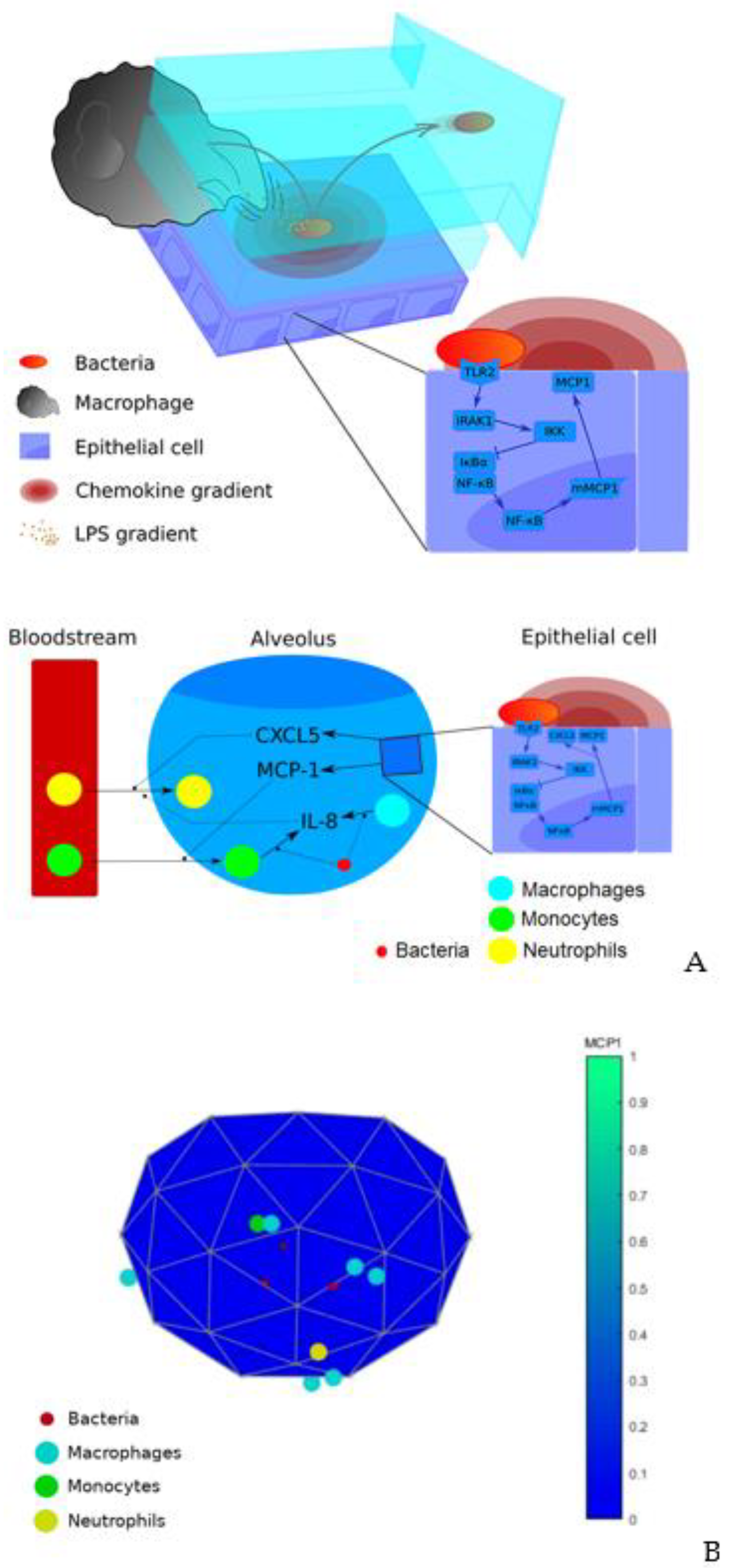
3.1. Model Description
3.2. Simulations
3.2.1. Handling of Stochasticity
3.2.2. Modelling of Antibiotics Administration
3.2.3. Modelling Variability in Bacterial Load
3.2.4. Solutions
3.2.5. Metrics for Infection Progression
3.3. Running Environment for Simulations and Availability of the Source Code
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Sharma, S.; Maycher, B.; Eschun, G. Radiological imaging in pneumonia: Recent innovations. Curr. Opin. Pulm. Med. 2007, 13, 159–169. [Google Scholar] [CrossRef] [PubMed]
- El-Solh, A.A.; Sikka, P.; Ramadan, F.; Davies, J. Etiology of severe pneumonia in the very elderly. Am. J. Respir. Crit. Care Med. 2001, 163, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Evans, S.E. Bacterial Pneumonia in Patients with Cancer: Novel Risk Factors and Management. Clin. Chest Med. 2017, 38, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Mizgerd, J.P. Inflammation and Pneumonia: Why Are Some More Susceptible than Others? Clin. Chest Med. 2018, 39, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Henry, K.M.; Herman, K.D.; Thompson, A.A.; Isles, H.M.; Tulotta, C.; Sammut, D.; Rougeot, J.J.; Khoshaein, N.; Reese, A.E.; et al. Inhibition of ErbB kinase signalling promotes resolution of neutrophilic inflammation. Elife 2019, 8. [Google Scholar] [CrossRef]
- Snelgrove, R.J.; Jackson, P.L.; Hardison, M.T.; Noerager, B.D.; Kinloch, A.; Gaggar, A.; Shastry, S.; Rowe, S.M.; Shim, Y.M.; Hussell, T.; et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 2010, 330, 90–94. [Google Scholar] [CrossRef]
- Nouailles, G.; Dorhoi, A.; Koch, M.; Zerrahn, J.; Weiner, J.; Faé, K.C.; Arrey, F.; Kuhlmann, S.; Bandermann, S.; Loewe, D.; et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J. Clin. Investig. 2014, 124, 1268–1282. [Google Scholar] [CrossRef]
- Yan, C.; Li, B.; Liu, X.; Deng, C.; Cai, R.; Shen, Y.; Tang, H. Involvement of multiple transcription factors in regulation of IL-β-induced MCP-1 expression in alveolar type II epithelial cells. Mol. Immunol. 2019, 111, 95–105. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Nguyen, V.; Mendelsohn, A.; Larrick, J.W. Interleukin-7 and Immunosenescence. J. Immunol. Res. 2017, 2017, 4807853. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, K.; Spindler, C.; Ortqvist, A.; Kalin, M.; Sandgren, A.; Kühlmann-Berenzon, S.; Normark, B.H. Clonal and Capsular Types Decide Whether Pneumococci Will Act as a Primary or Opportunistic Pathogen. Clin. Infect. Dis. 2006, 42, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hospital-acquired pneumonia in adults: Diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am. J. Respir. Crit. Care Med. 1996, 153, 1711–1725. [CrossRef]
- Cantone, M.; Santos, G.; Wentker, P.; Lai, X.; Vera, J. Multiplicity of Mathematical Modeling Strategies to Search for Molecular and Cellular Insights into Bacteria Lung Infection. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Vera, J.; Bachmann, J.; Pfeifer, A.C.; Becker, V.; Hormiga, J.A.; Darias, N.; Timmer, J.; Klingmüller, U.; Wolkenhauer, O. A systems biology approach to analyse amplification in the JAK2-STAT5 signalling pathway. BMC Syst. Biol. 2008, 2, 38. [Google Scholar] [CrossRef]
- Ben-Jacob, E.; Lu, M.; Schultz, D.; Onuchic, J.N. The physics of bacterial decision making. Front. Cell Infect. Microbiol. 2014, 4, 154. [Google Scholar] [CrossRef]
- Shih, V.F.-S.; Davis-Turak, J.; Macal, M.; Huang, J.Q.; Ponomarenko, J.; Kearns, J.D.; Yu, T.; Fagerlund, R.; Asagiri, M.; Zuniga, E.I.; et al. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-κB pathways. Nat. Immunol. 2012, 13, 1162–1170. [Google Scholar] [CrossRef]
- Khan, F.M.; Marquardt, S.; Gupta, S.K.; Knoll, S.; Schmitz, U.; Spitschak, A.; Engelmann, D.; Vera, J.; Wolkenhauer, O.; Pützer, B.M. Unraveling a tumor type-specific regulatory core underlying E2F1-mediated epithelial-mesenchymal transition to predict receptor protein signatures. Nat. Commun. 2017, 8, 198. [Google Scholar] [CrossRef]
- Passante, E.; Würstle, M.L.; Hellwig, C.T.; Leverkus, M.; Rehm, M. Systems analysis of apoptosis protein expression allows the case-specific prediction of cell death responsiveness of melanoma cells. Cell Death Differ. 2013, 20, 1521–1531. [Google Scholar] [CrossRef]
- Schoeberl, B.; Pace, E.A.; Fitzgerald, J.B.; Harms, B.D.; Xu, L.; Nie, L.; Linggi, B.; Kalra, A.; Paragas, V.; Bukhalid, R.; et al. Therapeutically targeting ErbB3: A key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci. Signal. 2009, 2, ra31. [Google Scholar] [CrossRef]
- Lysenko, E.S.; Lijek, R.S.; Brown, S.P.; Weiser, J.N. Within-host competition drives selection for the capsule virulence determinant of Streptococcus pneumoniae. Curr. Biol. 2010, 20, 1222–1226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Margolis, E.; Yates, A.; Levin, B.R. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: The role of competition and interactions with host’s immune response. BMC Microbiol. 2010, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Weimer, K.E.; Seok, S.-C.; Ray, W.C.; Jayaprakash, C.; Vieland, V.J.; Swords, W.E.; Das, J. Host-to-host variation of ecological interactions in polymicrobial infections. Phys. Biol. 2014, 12, 016003. [Google Scholar] [CrossRef] [PubMed]
- Tateda, K.; Takashima, K.; Miyazaki, H.; Matsumoto, T.; Hatori, T.; Yamaguchi, K. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob. Agents Chemother. 1996, 40, 1520–1525. [Google Scholar] [CrossRef]
- Chen, M.M.; Palmer, J.L.; Plackett, T.P.; Deburghgraeve, C.R.; Kovacs, E.J. Age-related differences in the neutrophil response to pulmonary pseudomonas infection. Exp. Gerontol. 2014, 54, 42–46. [Google Scholar] [CrossRef]
- Berger, S.; Goekeri, C.; Gupta, S.K.; Vera, J.; Dietert, K.; Behrendt, U.; Lienau, J.; Wienhold, S.-M.; Gruber, A.D.; Suttorp, N.; et al. Delay in antibiotic therapy results in fatal disease outcome in murine pneumococcal pneumonia. Crit. Care 2018, 22, 287. [Google Scholar] [CrossRef]
- Santos, G.; Lai, X.; Eberhardt, M.; Vera, J. Bacterial Adherence and Dwelling Probability: Two Drivers of Early Alveolar Infection by Streptococcus pneumoniae Identified in Multi-Level Mathematical Modeling. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Feldman, C.; Mitchell, T.J.; Andrew, P.W.; Boulnois, G.J.; Read, R.C.; Todd, H.C.; Cole, P.J.; Wilson, R. The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb. Pathog. 1990, 9, 275–284. [Google Scholar] [CrossRef]
- Lindert, J.; Perlman, C.E.; Parthasarathi, K.; Bhattacharya, J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am. J. Respir. Cell Mol. Biol. 2007, 36, 688–696. [Google Scholar] [CrossRef]
- Srivastava, A.; Henneke, P.; Visintin, A.; Morse, S.C.; Martin, V.; Watkins, C.; Paton, J.C.; Wessels, M.R.; Golenbock, D.T.; Malley, R. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 2005, 73, 6479–6487. [Google Scholar] [CrossRef]
- Mahbub, S.; Brubaker, A.L.; Kovacs, E.J. Aging of the Innate Immune System: An Update. Curr. Immunol. Rev. 2011, 7, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Pawelec, G.; Tarazona, R. Aging and innate immunity. Immunity 2006, 24, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Carrie, A.G.; Marrie, T.J. Use of intravenous antibiotics for the treatment of community-acquired pneumonia in the emergency department. Ther. Clin. Risk Manag. 2005, 1, 49–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulz, C.; Lai, X.; Bertrams, W.; Jung, A.L.; Sittka-Stark, A.; Herkt, C.E.; Janga, H.; Zscheppang, K.; Stielow, C.; Schulte, L.; et al. THP-1-derived macrophages render lung epithelial cells hypo-responsive to Legionella pneumophila - a systems biology study. Sci. Rep. 2017, 7, 11988. [Google Scholar] [CrossRef] [PubMed]
- Schmeck, B.; Moog, K.; Zahlten, J.; van Laak, V.; N’Guessan, P.D.; Opitz, B.; Rosseau, S.; Suttorp, N.; Hippenstiel, S. Streptococcus pneumoniae induced c-Jun-N-terminal kinase- and AP-1 -dependent IL-8 release by lung epithelial BEAS-2B cells. Respir. Res. 2006, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Carroad, P.A.; Bell, R.L. Estimation of diffusion coefficients of proteins. Biotechnol. Bioeng. 1980, 22, 947–955. [Google Scholar] [CrossRef]
- Graham, R.M.A.; Paton, J.C. Differential role of CbpA and PspA in modulation of in vitro CXC chemokine responses of respiratory epithelial cells to infection with Streptococcus pneumoniae. Infect. Immun. 2006, 74, 6739–6749. [Google Scholar] [CrossRef]
- Küng, E.; Coward, W.R.; Neill, D.R.; Malak, H.A.; Mühlemann, K.; Kadioglu, A.; Hilty, M.; Hathaway, L.J. The pneumococcal polysaccharide capsule and pneumolysin differentially affect CXCL8 and IL-6 release from cells of the upper and lower respiratory tract. PLoS ONE 2014, 9, e92355. [Google Scholar] [CrossRef]
- Cao, J.; Gong, Y.; Yin, Y.; Wang, L.; Ying, B.; Chen, T.; Zhang, X. Pneumococcal proteins PspA and PspC induce CXCL8 production in human neutrophils: Implications in pneumococcal infections. Microbes Infect. 2010, 12, 1051–1060. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lee, C.-H.; Hong, M.-Y.; Tang, H.-J.; Ko, W.-C. Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit. Care 2017, 21, 119. [Google Scholar] [CrossRef]
- Clec’h, C.; Timsit, J.-F.; De Lassence, A.; Azoulay, E.; Alberti, C.; Garrouste-Orgeas, M.; Mourvilier, B.; Troche, G.; Tafflet, M.; Tuil, O.; et al. Efficacy of adequate early antibiotic therapy in ventilator-associated pneumonia: Influence of disease severity. Intensive Care Med. 2004, 30, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Atamna, A.; Shiber, S.; Yassin, M.; Drescher, M.J.; Bishara, J. The accuracy of a diagnosis of pneumonia in the emergency department. Int. J. Infect. Dis. 2019, 89, 62–65. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, G.; Vera, J. The Role of Age, Neutrophil Infiltration and Antibiotics Timing in the Severity of Streptococcus pneumoniae Pneumonia. Insights from a Multi-Level Mathematical Model Approach. Int. J. Mol. Sci. 2020, 21, 8428. https://doi.org/10.3390/ijms21228428
Santos G, Vera J. The Role of Age, Neutrophil Infiltration and Antibiotics Timing in the Severity of Streptococcus pneumoniae Pneumonia. Insights from a Multi-Level Mathematical Model Approach. International Journal of Molecular Sciences. 2020; 21(22):8428. https://doi.org/10.3390/ijms21228428
Chicago/Turabian StyleSantos, Guido, and Julio Vera. 2020. "The Role of Age, Neutrophil Infiltration and Antibiotics Timing in the Severity of Streptococcus pneumoniae Pneumonia. Insights from a Multi-Level Mathematical Model Approach" International Journal of Molecular Sciences 21, no. 22: 8428. https://doi.org/10.3390/ijms21228428
APA StyleSantos, G., & Vera, J. (2020). The Role of Age, Neutrophil Infiltration and Antibiotics Timing in the Severity of Streptococcus pneumoniae Pneumonia. Insights from a Multi-Level Mathematical Model Approach. International Journal of Molecular Sciences, 21(22), 8428. https://doi.org/10.3390/ijms21228428





