Coenzyme Q10: Novel Formulations and Medical Trends
Abstract
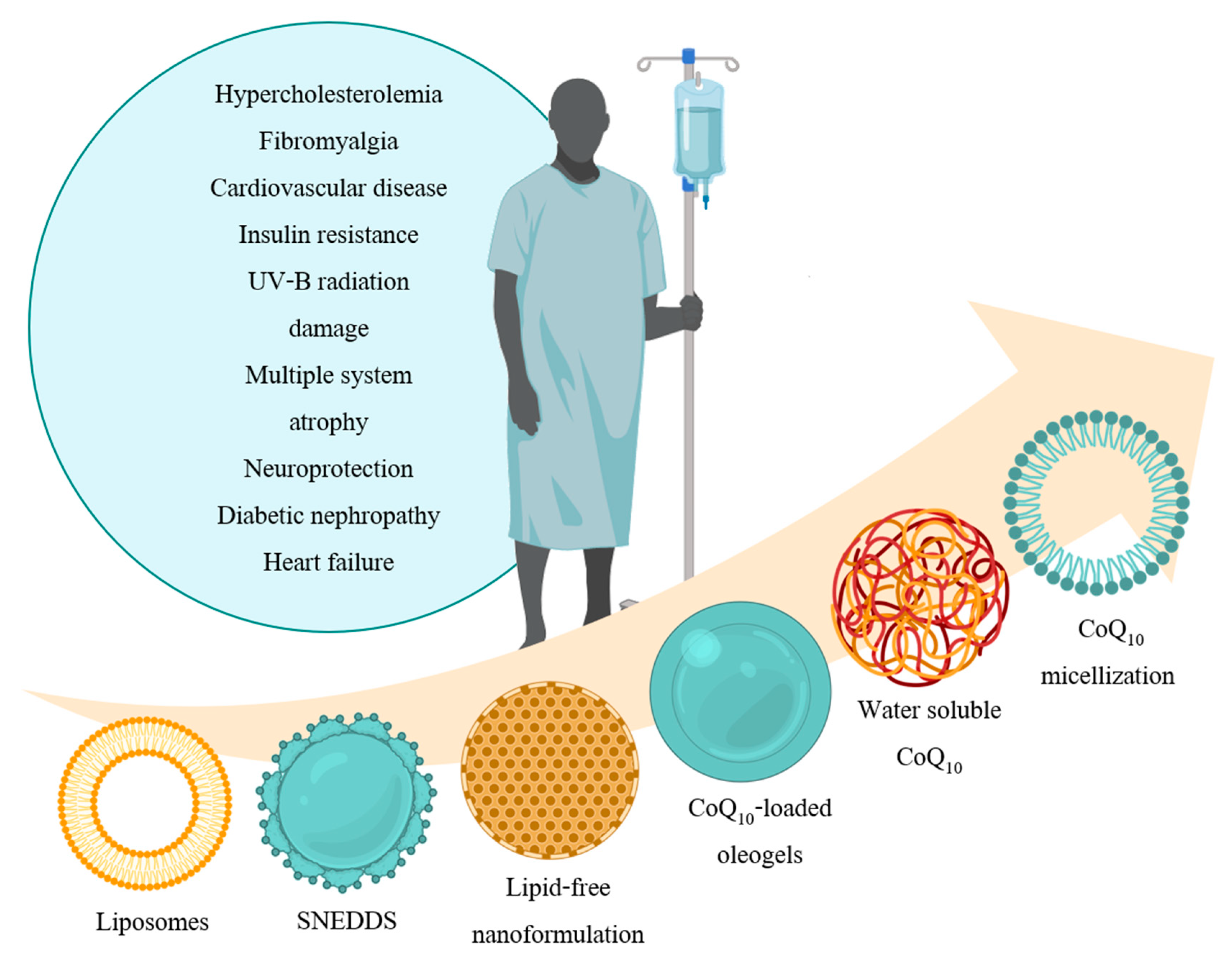
:1. Introduction
2. CoQ10 Applications in the Medical Field
2.1. Diabetic Cardiovascular Diseases and Diabetic Nephropathy
2.2. Multiple System Atrophy
2.3. Immunity and Immunological Disorders
2.4. Neuroprotection
2.5. Against UVB Radiation Damage
2.6. For Heart Failure Treatment
2.7. Barth Syndrome and Membrane Instability-Related Diseases
2.8. Insulin Resistance
2.9. Pain Alleviation in Fibromyalgia
2.10. Familial Hypercholesterolemia and Atherosclerosis
3. Recent Formulations
3.1. Liposomes
3.2. Self-Nanoemulsifying Delivery System
3.3. Novel Lipid-Free Nanoformulation
3.4. CoQ10-Loaded Oleogels
3.5. Novel Water-Soluble CoQ10
3.6. Micellization of CoQ10 by Caspofungin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CoQ10 | Coenzyme Q10 |
| ADI | Acceptable daily intake |
| NOAEL | No-observed-adverse-effect level |
| UVB | Ultraviolet B |
| CVD | Diabetic cardiovascular disease |
| ROS | Reactive oxygen species |
| RAGE | Receptor for advanced glycation end products |
| AGEs | Advanced glycation end products |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| PKC | Protein kinase C |
| DN | Diabetic nephropathy |
| UTMD | Ultrasound-targeted microbubble destruction |
| MSA | Multiple system atrophy |
| CSF | Cerebrospinal fluid |
| COQ2 | Coenzyme Q2 |
| COQ9 | Coenzyme Q9 |
| COQ8A | Coenzyme Q8A |
| ETFDH | Electron transfer flavoprotein dehydrogenase |
| PDSS2 | Decaprenyl diphosphate synthase subunit 2 |
| AIDS | Acquired immune deficiency syndrome |
| CRP | C-reactive protein |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor necrosis factor alpha |
| P-gp | P-glycoprotein |
| TPGS | α-tocopherol polyethylene glycol 1000 succinate |
| RGC | Retinal ganglion cells |
| IOP | Intraocular pressure |
| PD | Parkinson disease |
| NF-β | Nuclear factor beta |
| POPC | Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| IMM | Inner mitochondrial membrane |
| IIF | Flavin site in complex II |
| FM | Fibromyalgia |
| IL-1β | Interleukin 1 beta |
| UV | Ultraviolet |
| IL-18 | Interleukin 18 |
| FH | Familial hypercholesterolemia |
| GIT | Gastrointestinal tract |
| SNEDDS | Self-nanoemulsifying drug delivery systems |
| HPH | High-pressure homogenization |
| PHCO | PEG40 hydrogenated castor oil |
| TPGS | Tocopherol polyethylene glycol succinate |
| EC | Ethyl cellulose |
| MCT | Medium-chain triglyceride |
| SMS | SMS detergent |
| CMC | Critical micelle concentration |
| HMGCR | 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase |
| LDL | Low-density lipoprotein |
| LDL-R | Low-density lipoprotein receptor |
| ACE | Angiotensin-converting enzyme |
| CHF | Chronic heart failure |
References
- Festenstein, G.N.; Heaton, F.W.; Lowe, J.S.; Morton, R.A. A constituent of the unsaponifiable portion of animal tissue lipids (lambda max. 272 m mu). Biochem. J. 1955, 59, 558–566. [Google Scholar] [CrossRef]
- Crane, F.L.; Hatefi, Y.; Lester, R.L.; Widmer, C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta 1957, 25, 220–221. [Google Scholar] [CrossRef]
- Overvad, K.; Diamant, B.; Holm, L.; Hùlmer, G.; Mortensen, S.A.; Stender, S. Review Coenzyme Q 10 in health and disease. Eur. J. Clin. Nutr. 1999, 53, 764–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacchino, F.; Succurro, A.; Ebenhöh, O.; Gerace, D. Optimal efficiency of the Q-cycle mechanism around physiological temperatures from an open quantum systems approach. Sci. Rep. 2019, 9, 16657. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M.; Lehninger, A.L. Lehninger Principles of Biochemistry; W. H. Freeman: New York, NY, USA, 2017. [Google Scholar]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1995, 1271, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.; Saneto, R.; Falk, M.J.; Anselm, I.; Cohen, B.H.; Haas, R.; Medicine Society, T.M. A modern approach to the treatment of mitochondrial disease. Curr. Treat. Options Neurol. 2009, 11, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Groneberg, D.A.; Kindermann, B.; Althammer, M.; Klapper, M.; Vormann, J.; Littarru, G.P.; Döring, F. Coenzyme Q10 affects expression of genes involved in cell signalling, metabolism and transport in human CaCo-2 cells. Int. J. Biochem. Cell Biol. 2005, 37, 1208–1218. [Google Scholar] [CrossRef]
- Crane, F.L. New functions for CoenzymeQ10. Protoplasma 1999, 209, iii. [Google Scholar] [CrossRef]
- Alleva, R.; Tomasetti, M.; Andera, L.; Gellert, N.; Borghi, B.; Weber, C.; Murphy, M.P.; Neuzil, J. Coenzyme Q blocks biochemical but not receptor-mediated apoptosis by increasing mitochondrial antioxidant protection. FEBS Lett. 2001, 503, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef]
- Hidaka, T.; Fujii, K.; Funahashi, I.; Fukutomi, N.; Hosoe, K. Safety assessment of coenzyme Q 10 (CoQ 10). BioFactors 2008, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, Á.; Cordero, M.D.; Salviati, L.; Artuch, R.; Pineda, M.; Briones, P.; Izquierdo, L.G.; Cotán, D.; Navas, P.; Sánchez-Alcázar, J.A. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 2009, 5, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farough, S.; Karaa, A.; Walker, M.A.; Slate, N.; Dasu, T.; Verbsky, J.; Fusunyan, R.; Canapari, C.; Kinane, T.B.; Van Cleave, J.; et al. Coenzyme Q10 and immunity: A case report and new implications for treatment of recurrent infections in metabolic diseases. Clin. Immunol. 2014, 155, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Coady, S.; Sorlie, P.D.; D’Agostino, R.B.; Pencina, M.J.; Vasan, R.S.; Meigs, J.B.; Levy, D.; Savage, P.J. Increasing cardiovascular disease burden due to diabetes mellitus: The Framingham Heart Study. Circulation 2007, 115, 1544–1550. [Google Scholar] [CrossRef]
- Faria, A.; Persaud, S.J. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Yue, T.; Xu, H.L.; Chen, P.P.; Zheng, L.; Huang, Q.; Sheng, W.S.; Zhuang, Y.D.; Jiao, L.Z.; Chi, T.T.; ZhuGe, D.L.; et al. Combination of coenzyme Q10-loaded liposomes with ultrasound targeted microbubbles destruction (UTMD) for early theranostics of diabetic nephropathy. Int. J. Pharm. 2017, 528, 664–674. [Google Scholar] [CrossRef]
- Mima, A. Inflammation and oxidative stress in diabetic nephropathy: New insights on its inhibition as new therapeutic targets. J. Diabetes Res. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Biochemicstry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- ElBayoumi, T.A.; Torchilin, V.P. Current Trends in Liposome Research BT-Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers; Weissig, V., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 1–27. [Google Scholar]
- Zhang, Y.; Ye, C.; Xu, Y.; Dong, X.; Li, J.; Liu, R.; Gao, Y. Ultrasound-mediated microbubble destruction increases renal interstitial capillary permeability in early diabetic nephropathy rats. Ultrasound Med. Biol. 2014, 40, 1273–1281. [Google Scholar] [CrossRef]
- Fanciulli, A.; Wenning, G.K. Multiple-System Atrophy. N. Engl. J. Med. 2015, 372, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Ben-Shlomo, Y.; Lees, A.J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002, 125, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, S.; Aoki, N.; Uitti, R.J.; Van Gerpen, J.A.; Cheshire, W.P.; Josephs, K.A.; Wszolek, Z.K.; Langston, J.W.; Dickson, D.W. When DLB, PD, and PSP masquerade as MSA. Neurology 2015, 85, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, T.; Tokuda, T.; Ohmichi, T.; Ishii, R.; Tatebe, H.; Nakagawa, M.; Mizuno, T. Serum Levels of Coenzyme Q10 in Patients with Multiple System Atrophy. PLoS ONE 2016, 11, e0147574. [Google Scholar] [CrossRef]
- Mitsui, J.; Matsukawa, T.; Yasuda, T.; Ishiura, H.; Tsuji, S. Plasma coenzyme q10 levels in patients with multiple system atrophy. JAMA Neurol. 2016, 73, 977–980. [Google Scholar] [CrossRef] [Green Version]
- Compta, Y.; Giraldo, D.M.; Muñoz, E.; Antonelli, F.; Fernández, M.; Bravo, P.; Soto, M.; Cámara, A.; Torres, F.; Martí, M.J.; et al. Cerebrospinal fluid levels of coenzyme Q10 are reduced in multiple system atrophy. Parkinsonism Relat. Disord. 2018, 46, 16–23. [Google Scholar] [CrossRef]
- American, N. Mutations in COQ2 in Familial and Sporadic Multiple-System Atrophy. N. Engl. J. Med. 2013, 369, 233–244. [Google Scholar] [CrossRef]
- Mitsui, J.; Koguchi, K.; Momose, T.; Takahashi, M.; Matsukawa, T.; Yasuda, T.; Tokushige, S.I.; Ishiura, H.; Goto, J.; Nakazaki, S.; et al. Three-Year Follow-Up of High-Dose Ubiquinol Supplementation in a Case of Familial Multiple System Atrophy with Compound Heterozygous COQ2 Mutations. Cerebellum 2017, 16, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Rötig, A.; Appelkvist, E.-L.; Geromel, V.; Chretien, D.; Kadhom, N.; Edery, P.; Lebideau, M.; Dallner, G.; Munnich, A.; Ernster, L.; et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 2018, 356, 391–395. [Google Scholar] [CrossRef]
- Bird, L. Getting enough energy for immunity. Nat. Rev. Immunol. 2019, 19, 269. [Google Scholar] [CrossRef]
- Arias, A.; García-Villoria, J.; Rojo, A.; Buján, N.; Briones, P.; Ribes, A. Analysis of coenzyme Q10 in lymphocytes by HPLC–MS/MS. J. Chromatogr. B 2012, 908, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Hanioka, T.; Xia, L.-J.; McRee, J.T.; Langsjoen, P. Coenzyme Q10 increases T4/T8 ratios of lymphocytes in ordinary subjects and relevance to patients having the aids related complex. Biochem. Biophys. Res. Commun. 1991, 176, 786–791. [Google Scholar] [CrossRef]
- Folkers, K.; Morita, M.; McRee, J. The Activities of Coenzyme Q10 and Vitamin B6 for Immune Responses. Biochem. Biophys. Res. Commun. 1993, 193, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.l.; Chen, L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharm. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef]
- Herder, C.; Illig, T.; Rathmann, W.; Martin, S.; Haastert, B.; Mller-Scholze, S.; Holle, R.; Thorand, B.; Koenig, W.; Wichmann, H.E.; et al. Inflammation and type 2 diabetes: Results from KORA Augsburg. Gesundheitswesen 2005, 67 (Suppl. S1). [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [Green Version]
- Tudorache, E.; Oancea, C.; Avram, C.; Fira-Mladinescu, O.; Petrescu, L.; Timar, B. Balance impairment and systemic inflammation in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1847. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860. [Google Scholar] [CrossRef]
- Rosner, M.H.; Ronco, C.; Okusa, M.D. The Role of Inflammation in the Cardio-Renal Syndrome: A Focus on Cytokines and Inflammatory Mediators. Semin. Nephrol. 2018, 32, 70–78. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Fernandez-Twinn, D.S.; Hargreaves, I.P.; Neergheen, V.; Aiken, C.E.; Martin-Gronert, M.S.; McConnell, J.M.; Ozanne, S.E. Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth1. Am. J. Clin. Nutr. 2016, 103, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Camacho, J.D.; Bernier, M.; López-Lluch, G.; Navas, P. Coenzyme Q(10) Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Haidari, M.; Javadi, E.; Sadeghi, B.; Hajilooi, M.; Ghanbili, J. Evaluation of C-reactive protein, a sensitive marker of inflammation, as a risk factor for stable coronary artery disease. Clin. Biochem. 2001, 34, 309–315. [Google Scholar] [CrossRef]
- Nakanishi, S.; Yamane, K.; Kamei, N.; Okubo, M.; Kohno, N. Elevated C-Reactive Protein Is a Risk Factor for the Development of Type 2 Diabetes in Japanese Americans. Diabetes Care 2003, 26, 2754–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.J.; Kim, H.R.; Lee, M.K.; Woo, Y.S. Proflie of Human β-Defensins 1,2 and Proinflammatory Cytokines (TNF-α, IL-6) in Patients with Chronic Kidney Disease. Kidney Blood Press. Res. 2013, 37, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Srivastava, N.; Banerjee, M. Association of IL-6, TNF-α and IL-10 gene polymorphisms with type 2 diabetes mellitus. Mol. Biol. Rep. 2013, 40, 6271–6279. [Google Scholar] [CrossRef]
- Liu, Y. IL-6 Haplotypes, Inflammation, and Risk for Cardiovascular Disease in a Multiethnic Dialysis Cohort. J. Am. Soc. Nephrol. 2006, 17, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Ebadi, M.; Sharma, S.K.; Wanpen, S.; Amornpan, A. Coenzyme Q10 inhibits mitochondrial complex-1 down-regulation and nuclear factor-kappa B activation. J. Cell. Mol. Med. 2004, 8, 213–222. [Google Scholar] [CrossRef]
- Schmelzer, C.; Lindner, I.; Rimbach, G.; Niklowitz, P.; Menke, T.; Döring, F. Functions of coenzyme Q10 in inflammation and gene expression. BioFactors 2008, 32, 179–183. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Yacoubian, T.A.; Standaert, D.G. Targets for neuroprotection in Parkinson’s disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2009, 1792, 676–687. [Google Scholar] [CrossRef]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Hastings, T.G.; Lewis, D.A.; Zigmond, M.J. Reactive Dopamine Metabolites and Neurotoxicity BT-Biological Reactive Intermediates V: Basic Mechanistic Research in Toxicology and Human Risk Assessment; Snyder, R., Sipes, I.G., Jollow, D.J., Monks, T.J., Kocsis, J.J., Kalf, G.F., Greim, H., Witmer, C.M., Eds.; Springer US: Boston, MA, USA, 1996; pp. 97–106. [Google Scholar]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Jenner, P.; Clark, J.B.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. Lancet 2018, 333, 1269. [Google Scholar] [CrossRef]
- Glickman, R.D. Ultraviolet phototoxicity to the retina. Eye Contact Lens 2011, 37, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tohari, A.M.; Marcheggiani, F.; Zhou, X.; Reilly, J.; Tiano, L.; Shu, X. Therapeutic Potential of Co-enzyme Q10 in Retinal Diseases. Curr. Med. Chem. 2017, 24, 4329–4339. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Kaufman, Y.; Washington, I. Coenzyme Q10 in the Human Retina. Investig. Opthalmol. Vis. Sci. 2009, 50, 1814. [Google Scholar] [CrossRef] [Green Version]
- Martucci, A.; Nucci, C. Evidence on neuroprotective properties of coenzyme Q10 in the treatment of glaucoma. Neural Regen Res. 2019, 14, 197–200. [Google Scholar] [CrossRef]
- Lulli, M.; Witort, E.; Papucci, L.; Torre, E.; Schipani, C.; Bergamini, C.; Dal Monte, M.; Capaccioli, S. Coenzyme Q10 Instilled as Eye Drops on the Cornea Reaches the Retina and Protects Retinal Layers from Apoptosis in a Mouse Model of Kainate-Induced Retinal Damage. Investig. Ophthalmol. Visual Sci. 2012, 53, 8295–8302. [Google Scholar] [CrossRef]
- Davis, B.M.; Tian, K.; Pahlitzsch, M.; Brenton, J.; Ravindran, N.; Butt, G.; Malaguarnera, G.; Normando, E.M.; Guo, L.; Cordeiro, M.F. Topical Coenzyme Q10 demonstrates mitochondrial-mediated neuroprotection in a rodent model of ocular hypertension. Mitochondrion 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Itagaki, S.; Ochiai, A.; Kobayashi, M.; Sugawara, M.; Hirano, T.; Iseki, K. Interaction of Coenzyme Q10 with the Intestinal Drug Transporter P-Glycoprotein. J. Agric. Food Chem. 2008, 56, 6923–6927. [Google Scholar] [CrossRef]
- Fato, R.; Bergamini, C.; Leoni, S.; Pinna, A.; Carta, F.; Cardascia, N.; Ferrari, T.M.; Sborgia, C.; Lenaz, G. Coenzyme Q10 vitreous levels after administration of coenzyme Q10 eyedrops in patients undergoing vitrectomy. Acta Ophthalmol. 2010, 88, 150–151. [Google Scholar] [CrossRef]
- Nakajima, Y.; Inokuchi, Y.; Nishi, M.; Shimazawa, M.; Otsubo, K.; Hara, H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008, 1226, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Therapeutic Effects of Coenzyme Q10 in Neurodegenerative Diseases. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2004; Volume 382, pp. 473–487. [Google Scholar]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1660, 171–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogeski, I.; Gulaboski, R.; Kappl, R.; Mirceski, V.; Stefova, M.; Petreska, J.; Hoth, M. Calcium Binding and Transport by Coenzyme Q. J. Am. Chem. Soc. 2011, 133, 9293–9303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharm. 2013, 13, 12–15. [Google Scholar] [CrossRef]
- Osborne, N.N.; del Olmo-Aguado, S. Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 16–22. [Google Scholar] [CrossRef]
- Nucci, C.; Tartaglione, R.; Cerulli, A.; Mancino, R.; Spanò, A.; Cavaliere, F.; Rombolà, L.; Bagetta, G.; Corasaniti, M.T.; Morrone, L.A. Retinal Damage Caused by High Intraocular Pressure–Induced Transient Ischemia is Prevented by Coenzyme Q10 in Rat. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2007; Volume 82, pp. 397–406. [Google Scholar]
- Russo, R.; Cavaliere, F.; Rombolà, L.; Gliozzi, M.; Cerulli, A.; Nucci, C.; Fazzi, E.; Bagetta, G.; Corasaniti, M.T.; Morrone, L.A. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. In Progress in Brain Research; Nucci, C., Cerulli, L., Osborne, N.N., Bagetta, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 173, pp. 575–582. [Google Scholar]
- Lee, D.; Shim, M.S.; Kim, K.-Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.-K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Investig. Ophthalmol. Visual Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Vega, B.; Nicieza, J.; Álvarez-Barrios, A.; Álvarez, L.; García, M.; Fernández-Vega, C.; Vega, J.A.; González-Iglesias, H. The Use of Vitamins and Coenzyme Q10 for the Treatment of Vascular Occlusion Diseases Affecting the Retina. Nutrients 2020, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, M.; Kumar, A. Neuroprotective mechanism of Coenzyme Q10 (CoQ10) against PTZ induced kindling and associated cognitive dysfunction: Possible role of microglia inhibition. Pharmacol. Rep. 2016, 68, 1301–1311. [Google Scholar] [CrossRef]
- Lucas, S.-M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharm. 2009, 147, S232–S240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papucci, L.; Schiavone, N.; Witort, E.; Donnini, M.; Lapucci, A.; Tempestini, A.; Formigli, L.; Zecchi-Orlandini, S.; Orlandini, G.; Carella, G.; et al. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J. Biol. Chem. 2003, 278, 28220–28228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, M.; Orsucci, D.; Calsolaro, V.; Choub, A.; Siciliano, G. Coenzyme Q10 and Neurological Diseases. Pharmaceuticals 2009, 2, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Al Shaal, L.; Shegokar, R.; Müller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Pegoraro, N.S.; Barbieri, A.V.; Camponogara, C.; Mattiazzi, J.; Brum, E.S.; Marchiori, M.C.L.; Oliveira, S.M.; Cruz, L. Nanoencapsulation of coenzyme Q10 and vitamin E acetate protects against UVB radiation-induced skin injury in mice. Colloids Surf. B Biointerfaces 2017, 150, 32–40. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, S.; Jindal, N.; Jindal, A.; Bansal, R. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H.; Devi, P.; Mohan, V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharm. Ther. 2009, 124, 259–268. [Google Scholar] [CrossRef]
- Linnane, A.W.; Kopsidas, G.; Zhang, C.; Yarovaya, N.; Kovalenko, S.; Papakostopoulos, P.; Eastwood, H.; Graves, S.; Richardson, M. Cellular Redox Activity of Coenzyme Q 10: Effect of CoQ 10 Supplementation on Human Skeletal Muscle. Free Radic. Res. 2002, 36, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Folkers, K.; Vadhanavikit, S.; Mortensen, S.A. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc. Natl. Acad. Sci. USA 1985, 82, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belardinelli, R.; Muçaj, A.; Lacalaprice, F.; Solenghi, M.; Seddaiu, G.; Principi, F.; Tiano, L.; Littarru, G.P. Coenzyme Q10 and exercise training in chronic heart failure. Eur. Heart J. 2006, 27, 2675–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lorenzo, A.; Iannuzzo, G.; Parlato, A.; Cuomo, G.; Testa, C.; Coppola, M.; D’Ambrosio, G.; Oliviero, D.A.; Sarullo, S.; Vitale, G.; et al. Clinical Evidence for Q10 Coenzyme Supplementation in Heart Failure: From Energetics to Functional Improvement. J. Clin. Med. 2020, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Korzeniowska, K.; Cieślewicz, A.; Jabłecka, A. Coenzyme Q10–A new player in the treatment of heart failure? Pharmacol. Rep. 2016, 68, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, S.L.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An Independent Predictor of Mortality in Chronic Heart Failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gvozdjáková, A.; Kucharská, J.; Mizera, S.; Braunová, Z.; Schreinerová, Z.; Schrameková, E.; Pecháň, I.; Fabián, J. Coenzyme Q10 depletion and mitochondrial energy disturbances in rejection development in patients after heart transplantation. BioFactors 1999, 9, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.G.; Scholte, H.R.; Berden, J.A.; Van Der Klei-Van Moorsel, J.M.; Luyt-Houwen, I.E.M.; Van’T Veer-Korthof, E.T.; Van Der Harten, J.J.; Sobotka-Plojhar, M.A. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 1983, 62, 327–355. [Google Scholar] [CrossRef]
- Schlame, M.; Ren, M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006, 580, 5450–5455. [Google Scholar] [CrossRef] [Green Version]
- Schlame, M.; Rua, D.; Greenberg, M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000, 39, 257–288. [Google Scholar] [CrossRef]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradies, G.; Ruggiero, F.M. Effect of hyperthyroidism on the transport of pyruvate in rat-heart mitochondria. Biochim. Biophys. Acta (BBA) Bioenerg. 1988, 935, 79–86. [Google Scholar] [CrossRef]
- Paradies, G.; Ruggiero, F.M.; Dinoi, P. The influence of hypothyroidism on the transport of phosphate and on the lipid composition in rat-liver mitochondria. Biochim. Biophys. Acta (BBA) Biomembr. 1991, 1070, 180–186. [Google Scholar] [CrossRef]
- Lee, H.-J.; Mayette, J.; Rapoport, S.I.; Bazinet, R.P. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids Health Dis. 2006, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparagna, G.C.; Johnson, C.A.; McCune, S.A.; Moore, R.L.; Murphy, R.C. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J. Lipid Res. 2005, 46, 1196–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradies, G.; Petrosillo, G.; Gadaleta, M.N.; Ruggiero, F.M. The effect of aging and acetyl-l-carnitine on the pyruvate transport and oxidation in rat heart mitochondria. FEBS Lett. 1999, 454, 207–209. [Google Scholar] [CrossRef] [Green Version]
- Petrosillo, G.; Francesca, M.R.; Di Venosa, N.; Paradies, A.G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: Role of reactive oxygen species and cardiolipin. FASEB J. 2003, 17, 714–716. [Google Scholar] [CrossRef]
- Yan, N.; Liu, Y.; Liu, L.; Du, Y.; Liu, X.; Zhang, H.; Zhang, Z. Bioactivities and Medicinal Value of Solanesol and Its Accumulation, Extraction Technology, and Determination Methods. Biomolecules 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Kaurola, P.; Sharma, V.; Vonk, A.; Vattulainen, I.; Róg, T. Distribution and dynamics of quinones in the lipid bilayer mimicking the inner membrane of mitochondria. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 2116–2122. [Google Scholar] [CrossRef]
- Agmo Hernández, V.; Eriksson, E.K.; Edwards, K. Ubiquinone-10 alters mechanical properties and increases stability of phospholipid membranes. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2233–2243. [Google Scholar] [CrossRef]
- Hoehn, K.L.; Salmon, A.B.; Hohnen-Behrens, C.; Turner, N.; Hoy, A.J.; Maghzal, G.J.; Stocker, R.; Van Remmen, H.; Kraegen, E.W.; Cooney, G.J.; et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 17787–17792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.-T.; Price Iii, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Chaudhuri, R.; Yang, P.; Maghzal, G.J.; Thomas, K.C.; Krycer, J.R.; Humphrey, S.J.; Parker, B.L.; Fisher-Wellman, K.H.; Meoli, C.C.; et al. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. eLife 2018, 7, e32111. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Åberg, F.; Appelkvist, E.-L.; Dallner, G.; Ernster, L. Uptake of Dietary Coenzyme Q Supplement is Limited in Rats. J. Nutr. 1995, 125, 446–453. [Google Scholar]
- Brand, M.D.; Goncalves, R.L.S.; Orr, A.L.; Vargas, L.; Gerencser, A.A.; Borch Jensen, M.; Wang, Y.T.; Melov, S.; Turk, C.N.; Matzen, J.T.; et al. Suppressors of Superoxide-H(2)O(2) Production at Site I(Q) of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metab. 2016, 24, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Orr, A.L.; Vargas, L.; Turk, C.N.; Baaten, J.E.; Matzen, J.T.; Dardov, V.J.; Attle, S.J.; Li, J.; Quackenbush, D.C.; Goncalves, R.L.S.; et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Biol. 2015, 11, 834–836. [Google Scholar] [CrossRef] [Green Version]
- Cederberg, H.; Stančáková, A.; Yaluri, N.; Modi, S.; Kuusisto, J.; Laakso, M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: A 6 year follow-up study of the METSIM cohort. Diabetologia 2015, 58, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Preiss, D.; Seshasai, S.; Welsh, P.; Murphy, S.A.; Ho, J.E.; Waters, D.D.; Demicco, D.A.; Barter, P.; Cannon, C.P.; Sabatine, M.S.; et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011, 305, 2556–2564. [Google Scholar] [CrossRef] [Green Version]
- Cordero, M.D.; Moreno-Fernández, A.M.; de Miguel, M.; Bonal, P.; Campa, F.; Jiménez-Jiménez, L.M.; Ruiz-Losada, A.; Sánchez-Domínguez, B.; Sánchez Alcázar, J.A.; Salviati, L.; et al. Coenzyme Q10 distribution in blood is altered in patients with Fibromyalgia. Clin. Biochem. 2009, 42, 732–735. [Google Scholar] [CrossRef]
- Cordero, M.D.; De Miguel, M.; Moreno Fernández, A.M.; Carmona López, I.M.; Garrido Maraver, J.; Cotán, D.; Gómez Izquierdo, L.; Bonal, P.; Campa, F.; Bullon, P.; et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010, 12, R17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagis, S.; Tamer, L.; Sahin, G.; Bilgin, R.; Guler, H.; Ercan, B.; Erdogan, C. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005, 25, 188–190. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; Culic, O.; Carrión, A.M.; de Miguel, M.; Díaz-Parrado, E.; Pérez-Villegas, E.M.; Bullón, P.; Battino, M.; Sánchez-Alcazar, J.A. NLRP3 Inflammasome Is Activated in Fibromyalgia: The Effect of Coenzyme Q10. Antioxid. Redox Signal. 2014, 20, 1169–1180. [Google Scholar] [CrossRef] [Green Version]
- Menu, P.; Vince, J.E. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cordero, M.D.; Díaz-Parrado, E.; Carrión, A.M.; Alfonsi, S.; Sánchez-Alcazar, J.A.; Bullón, P.; Battino, M.; de Miguel, M. Is Inflammation a Mitochondrial Dysfunction-Dependent Event in Fibromyalgia? Antioxid. Redox Signal. 2013, 18, 800–807. [Google Scholar] [CrossRef] [Green Version]
- Avogaro, P.; Cazzolato, G. Familial hyper-HDL-(α)-cholesterolemia. Atherosclerosis 1975, 22, 63–77. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; de la Mata, M.; Pavón, A.D.; Villanueva-Paz, M.; Povea-Cabello, S.; Cotán, D.; Álvarez-Córdoba, M.; Villalón-García, I.; Ybot-González, P.; Salas, J.J.; et al. Intracellular cholesterol accumulation and coenzyme Q10 deficiency in Familial Hypercholesterolemia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 3697–3713. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.; Preta, G.; Sheldon, I.M. Inhibiting mevalonate pathway enzymes increases stromal cell resilience to a cholesterol-dependent cytolysin. Sci. Rep. 2017, 7, 17050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzetti, E.; Csiszar, A.; Dutta, D.; Balagopal, G.; Calvani, R.; Leeuwenburgh, C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: From mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H459–H476. [Google Scholar] [CrossRef] [Green Version]
- Vercesi, A.E.; Castilho, R.F.; Kowaltowski, A.J.; Oliveira, H.C. Mitochondrial energy metabolism and redox state in dyslipidemias. IUBMB Life 2007, 59, 263–268. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Najibi, A.; Amirzadegan, N.; Gharibi, R.; Dashti, A.; Omidi, M.; Saeedi, A.; Ghafarian-Bahreman, A.; Niknahad, H. Coenzyme Q10 remarkably improves the bio-energetic function of rat liver mitochondria treated with statins. Eur. J. Pharmacol. 2015, 762, 270–274. [Google Scholar] [CrossRef]
- Villalba, J.M.; Parrado, C.; Santos-Gonzalez, M.; Alcain, F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin. Investig. Drugs 2010, 19, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura, K.; Abe, J.; Hyodo, M.; Haga, S.; Ozaki, M.; Harashima, H. Mitochondrial delivery of Coenzyme Q10 via systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J. Control. Release 2015, 213, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onoue, S.; Uchida, A.; Kuriyama, K.; Nakamura, T.; Seto, Y.; Kato, M.; Hatanaka, J.; Tanaka, T.; Miyoshi, H.; Yamada, S. Novel solid self-emulsifying drug delivery system of coenzyme Q10 with improved photochemical and pharmacokinetic behaviors. Eur. J. Pharm. Sci. 2012, 46, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, G.; Zhang, J.; Sun, N.; Duan, M.; Yan, Z.; Xia, Q. Novel lipid-free nanoformulation for improving oral bioavailability of coenzyme Q10. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Beg, S.; Javed, S.; Kohli, K. Bioavailability enhancement of coenzyme Q10: An extensive review of patents. Recent Patents Drug Deliv. Formul. 2010, 4, 245–255. [Google Scholar] [CrossRef]
- Li, H.; Chen, F. Preparation and quality evaluation of coenzyme Q10 long-circulating liposomes. Saudi J. Biol. Sci. 2017, 24, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, J.; Zhao, D.; Han, D.; Chen, X. Comparative study on preparative methods of DC-Chol/DOPE liposomes and formulation optimization by determining encapsulation efficiency. Int. J. Pharm. 2012, 434, 155–160. [Google Scholar] [CrossRef]
- Pund, S.; Shete, Y.; Jagadale, S. Multivariate analysis of physicochemical characteristics of lipid based nanoemulsifying cilostazol—Quality by design. Colloids Surf. B Biointerfaces 2014, 115, 29–36. [Google Scholar] [CrossRef]
- Khattab, A.; Hassanin, L.; Zaki, N. Self-Nanoemulsifying Drug Delivery System of Coenzyme (Q10) with Improved Dissolution, Bioavailability, and Protective Efficiency on Liver Fibrosis. AAPS PharmSciTech 2017, 18, 1657–1672. [Google Scholar] [CrossRef]
- Singh, A.; Singh, V.; Juyal, D.; Rawat, G. Self emulsifying systems: A review. Asian J. Pharm. 2015, 9, 13–18. [Google Scholar] [CrossRef]
- Ullmann, U.; Metzner, J.; Schulz, C.; Perkins, J.; Leuenberger, B. A new coenzyme Q10 tablet-grade formulation (all-Q (R)) is bioequivalent to Q-Gel (R) and both have better bioavailability properties than Q-SorB (R). J. Med. Food 2005, 8, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Thanatuksorn, P.; Kawai, K.; Hayakawa, M.; Hayashi, M.; Kajiwara, K. Improvement of the oral bioavailability of coenzyme Q10 by emulsification with fats and emulsifiers used in the food industry. LWT-Food Sci. Technol. 2009, 42, 385–390. [Google Scholar] [CrossRef]
- Nepal, P.R.; Han, H.-K.; Choi, H.-K. Enhancement of solubility and dissolution of Coenzyme Q10 using solid dispersion formulation. Int. J. Pharm. 2010, 383, 147–153. [Google Scholar] [CrossRef]
- Belhaj, N.; Dupuis, F.; Arab-Tehrany, E.; Denis, F.M.; Paris, C.; Lartaud, I.; Linder, M. Formulation, characterization and pharmacokinetic studies of coenzyme Q10 PUFA’s nanoemulsions. Eur. J. Pharm. Sci. 2012, 47, 305–312. [Google Scholar] [CrossRef]
- Porter, C.; Trevaskis, N.; Charman, W. Lipids and Lipid-Based Formulations: Optimizing the Oral Delivery of Lipophilic Drugs. Nature Reviews Drug Discovery 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Trevaskis, N.L.; Charman, W.N.; Porter, C.J.H. Lipid-based delivery systems and intestinal lymphatic drug transport: A mechanistic update. Adv. Drug Deliv. Rev. 2008, 60, 702–716. [Google Scholar] [CrossRef]
- Cornaire, G.; Woodley, J.; Hermann, P.; Cloarec, A.; Arellano, C.; Houin, G. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int. J. Pharm. 2004, 278, 119–131. [Google Scholar] [CrossRef]
- Zaki, N.M. Strategies for oral delivery and mitochondrial targeting of CoQ10. Drug Deliv. 2016, 23, 1868–1881. [Google Scholar] [CrossRef] [Green Version]
- Read, J.L.; Whittaker, R.G.; Miller, N.; Clark, S.; Taylor, R.; McFarland, R.; Turnbull, D. Prevalence and severity of voice and swallowing difficulties in mitochondrial disease. Int. J. Lang. Commun. Disord. 2012, 47, 106–111. [Google Scholar] [CrossRef]
- Re, G.L.; Terranova, M.C.; Vernuccio, F.; Calafiore, C.; Picone, D.; Tudisca, C.; Salerno, S.; Lagalla, R. Swallowing impairment in neurologic disorders: The role of videofluorographic swallowing study. Pol. J. Radiol. 2018, 83, e394–e400. [Google Scholar] [CrossRef] [PubMed]
- Masotta, N.E.; Martinefski, M.R.; Lucangioli, S.; Rojas, A.M.; Tripodi, V.P. High-dose coenzyme Q10-loaded oleogels for oral therapeutic supplementation. Int. J. Pharm. 2019, 556, 9–20. [Google Scholar] [CrossRef]
- You, Y.-Q.N.; Ling, P.-R.; Qu, J.Z.; Bistrian, B.R. Effects of medium-chain triglycerides, long-chain triglycerides, or 2-monododecanoin on fatty acid composition in the portal vein, intestinal lymph, and systemic circulation in rats. JPEN J. Parenter. Enter. Nutr. 2008, 32, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashwell, M. Concepts of Functional Foods; ILSI: Washington, DC, USA, 2002; pp. 1–35. [Google Scholar]
- Yang, H.; Song, J. [Inclusion of coenzyme Q10 with beta-cyclodextrin studied by polarography]. Yao Xue Xue Bao 2006, 41, 671–674. [Google Scholar] [PubMed]
- Žmitek, J.; Šmidovnik, A.; Fir, M.; Prošek, M.; Žmitek, K.; Walczak, J.; Pravst, I. Relative bioavailability of two forms of a novel water-soluble coenzyme Q10. Ann. Nutr. Metab. 2008, 52, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hekimi, S. Micellization of coenzyme Q by the fungicide caspofungin allows for safe intravenous administration to reach extreme supraphysiological concentrations. Redox. Biol. 2020, 36, 101680. [Google Scholar] [CrossRef] [PubMed]
- Pepić, I.; Lovrić, J.; Hafner, A.; Filipović-Grčić, J. Powder form and stability of Pluronic mixed micelle dispersions for drug delivery applications. Drug Dev. Ind. Pharm. 2014, 40, 944–951. [Google Scholar] [CrossRef]
- Rabanel, J.-M.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-Loaded Nanocarriers: Passive Targeting and Crossing of Biological Barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa, A.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–165. [Google Scholar]
- Nir, Y.; Paz, A.; Sabo, E.; Potasman, I. Fear of injections in young adults: Prevalence and associations. Am. J. Trop. Med. Hyg. 2003, 68, 341–344. [Google Scholar] [CrossRef]

| Disease | CoQ10 Application | References |
|---|---|---|
| Diabetic cardiovascular diseases and diabetic nephropathy | Significant reduction in adverse cardiovascular events | [11,15,16,17,18,19,20,21,22] |
| Multiple system atrophy | Accurate biomarker for MSA when found in low levels in cerebrospinal fluid | [20,23,24,25,26,27,28,29,30] |
| Immunological disorders | Significant improvement of the T cell function Reduction of circulating inflammatory markers | [14,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] |
| Neuropathies | Neuroprotection via Ca2+ buffering and antioxidant activity | [35,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79] |
| UVB radiation damage | Treatment of damaged skin | [66,80,81,82,83,84,85] |
| Heart failure | Supplementation required for improving patient condition | [82,86,87,88,89,90,91,92,93] |
| Barth syndrome and membrane instability-related diseases | CoQ10-based treatment for Barth syndrome patients Development of cholesterol-free liposome-based drug delivery systems | [94,95,96,97,98,99,100,101,102,103,104,105,106] |
| Insulin resistance | Increase in insulin sensitivity by scavenging mitochondrial oxidants | [107,108,109,110,111,112,113,114,115] |
| Fibromyalgia | Pain alleviation | [116,117,118,119,120,121] |
| Familial hypercholesterolemia and atherosclerosis | Supplementation required for improving patient condition | [122,123,124,125,126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor-Maldonado, C.J.; Suárez-Rivero, J.M.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10: Novel Formulations and Medical Trends. Int. J. Mol. Sci. 2020, 21, 8432. https://doi.org/10.3390/ijms21228432
Pastor-Maldonado CJ, Suárez-Rivero JM, Povea-Cabello S, Álvarez-Córdoba M, Villalón-García I, Munuera-Cabeza M, Suárez-Carrillo A, Talaverón-Rey M, Sánchez-Alcázar JA. Coenzyme Q10: Novel Formulations and Medical Trends. International Journal of Molecular Sciences. 2020; 21(22):8432. https://doi.org/10.3390/ijms21228432
Chicago/Turabian StylePastor-Maldonado, Carmen J., Juan M. Suárez-Rivero, Suleva Povea-Cabello, Mónica Álvarez-Córdoba, Irene Villalón-García, Manuel Munuera-Cabeza, Alejandra Suárez-Carrillo, Marta Talaverón-Rey, and José A. Sánchez-Alcázar. 2020. "Coenzyme Q10: Novel Formulations and Medical Trends" International Journal of Molecular Sciences 21, no. 22: 8432. https://doi.org/10.3390/ijms21228432
APA StylePastor-Maldonado, C. J., Suárez-Rivero, J. M., Povea-Cabello, S., Álvarez-Córdoba, M., Villalón-García, I., Munuera-Cabeza, M., Suárez-Carrillo, A., Talaverón-Rey, M., & Sánchez-Alcázar, J. A. (2020). Coenzyme Q10: Novel Formulations and Medical Trends. International Journal of Molecular Sciences, 21(22), 8432. https://doi.org/10.3390/ijms21228432







