Genome-Wide Differential DNA Methylation and miRNA Expression Profiling Reveals Epigenetic Regulatory Mechanisms Underlying Nitrogen-Limitation-Triggered Adaptation and Use Efficiency Enhancement in Allotetraploid Rapeseed
Abstract
1. Introduction
2. Results
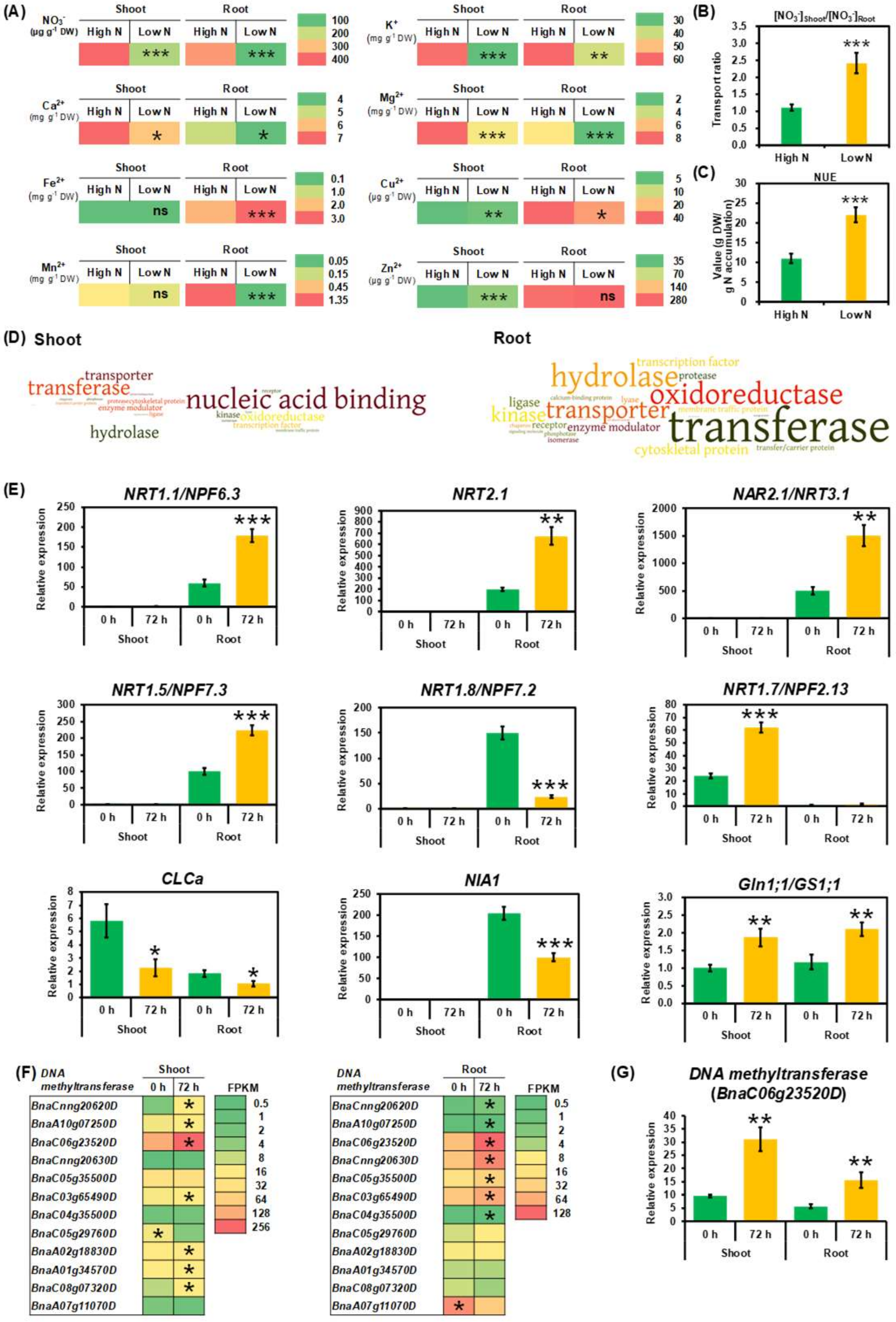
2.1. Physiological Adaptive Responses of Rapeseed Plants to N Limitation
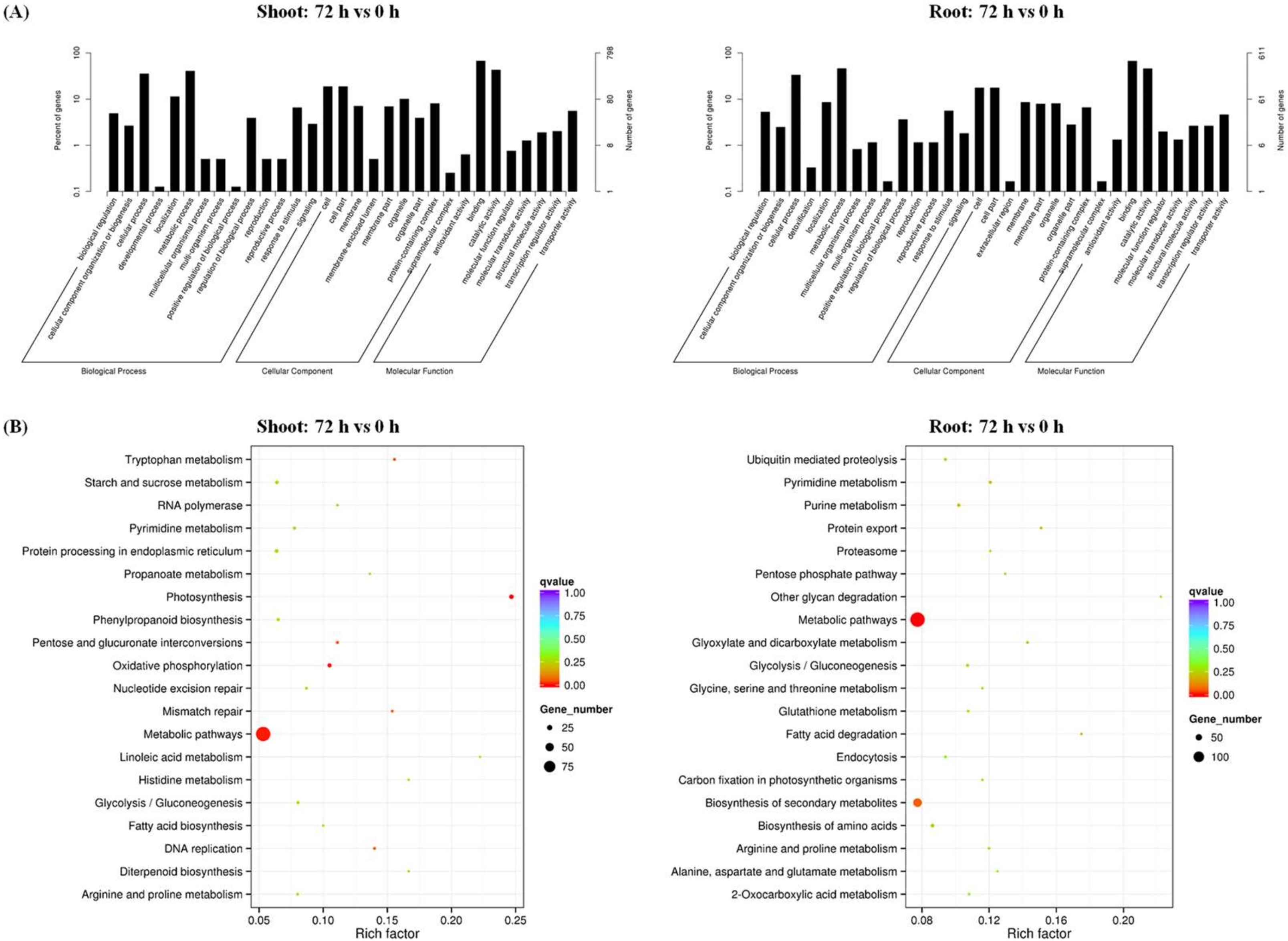
2.2. Transcriptional Adaptive Responses of Rapeseed Plants to N Limitation
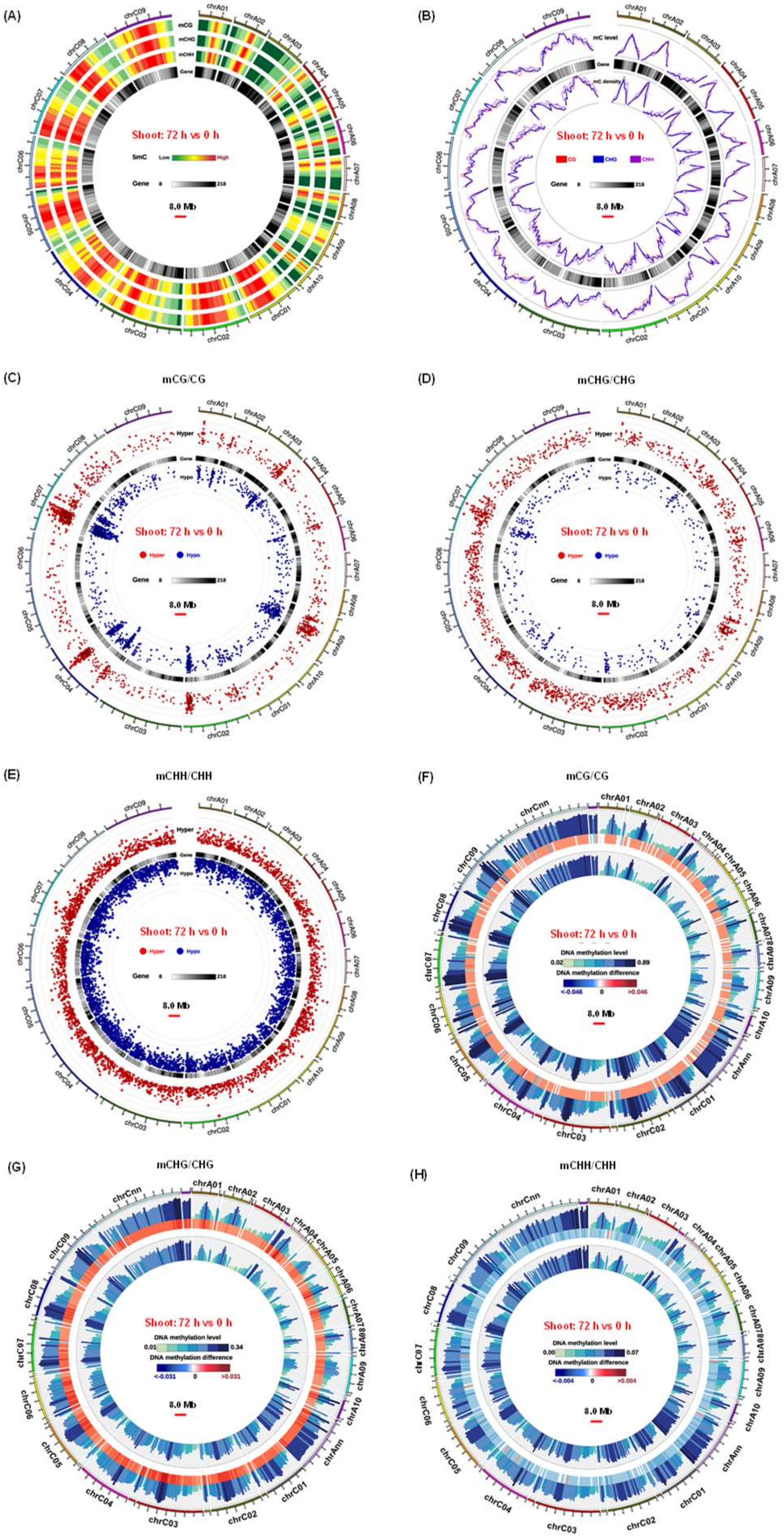
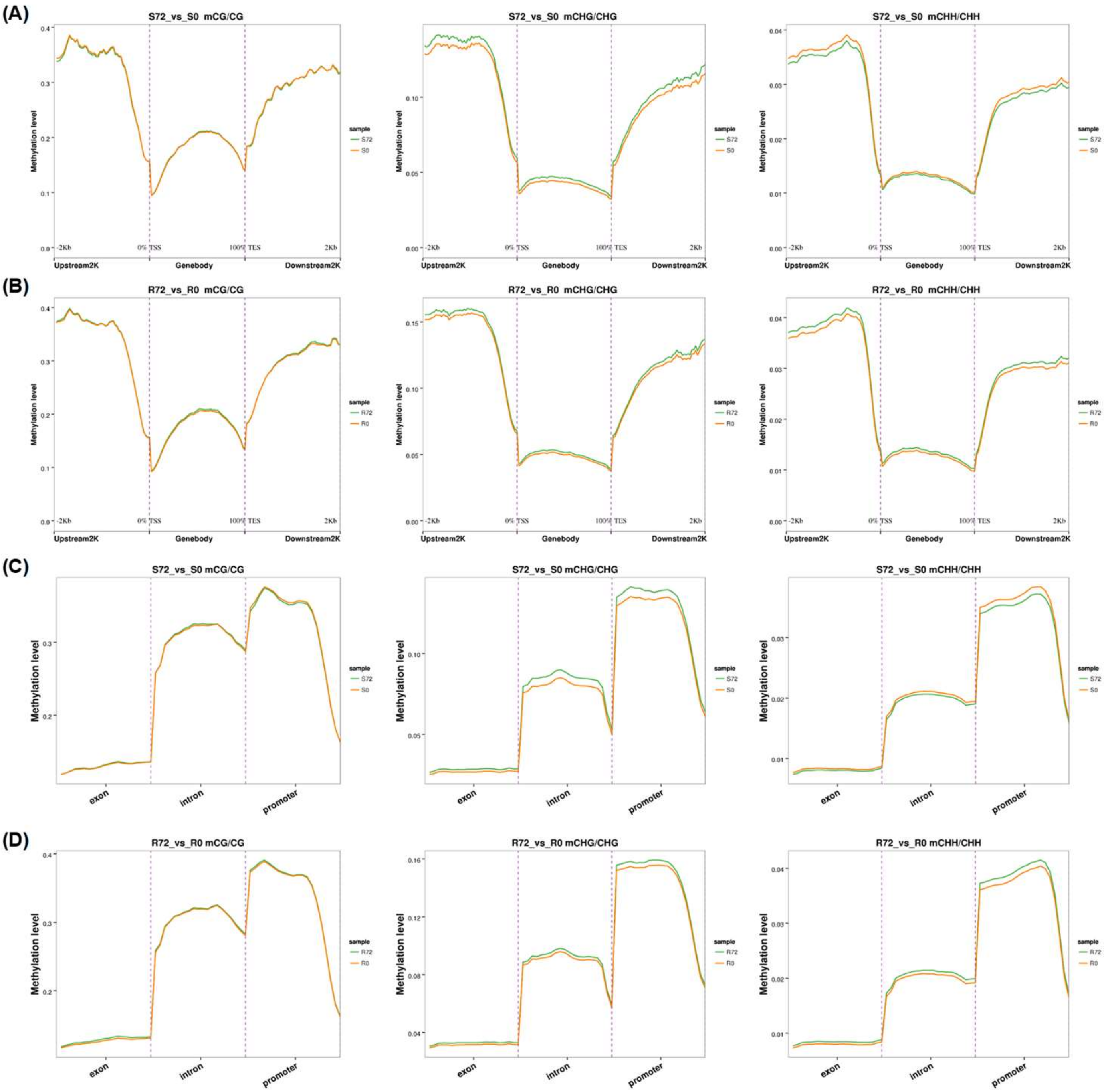
2.3. Genome-Wide High-Resolution DNA Methylation Fingerprints in Response to N Limitation
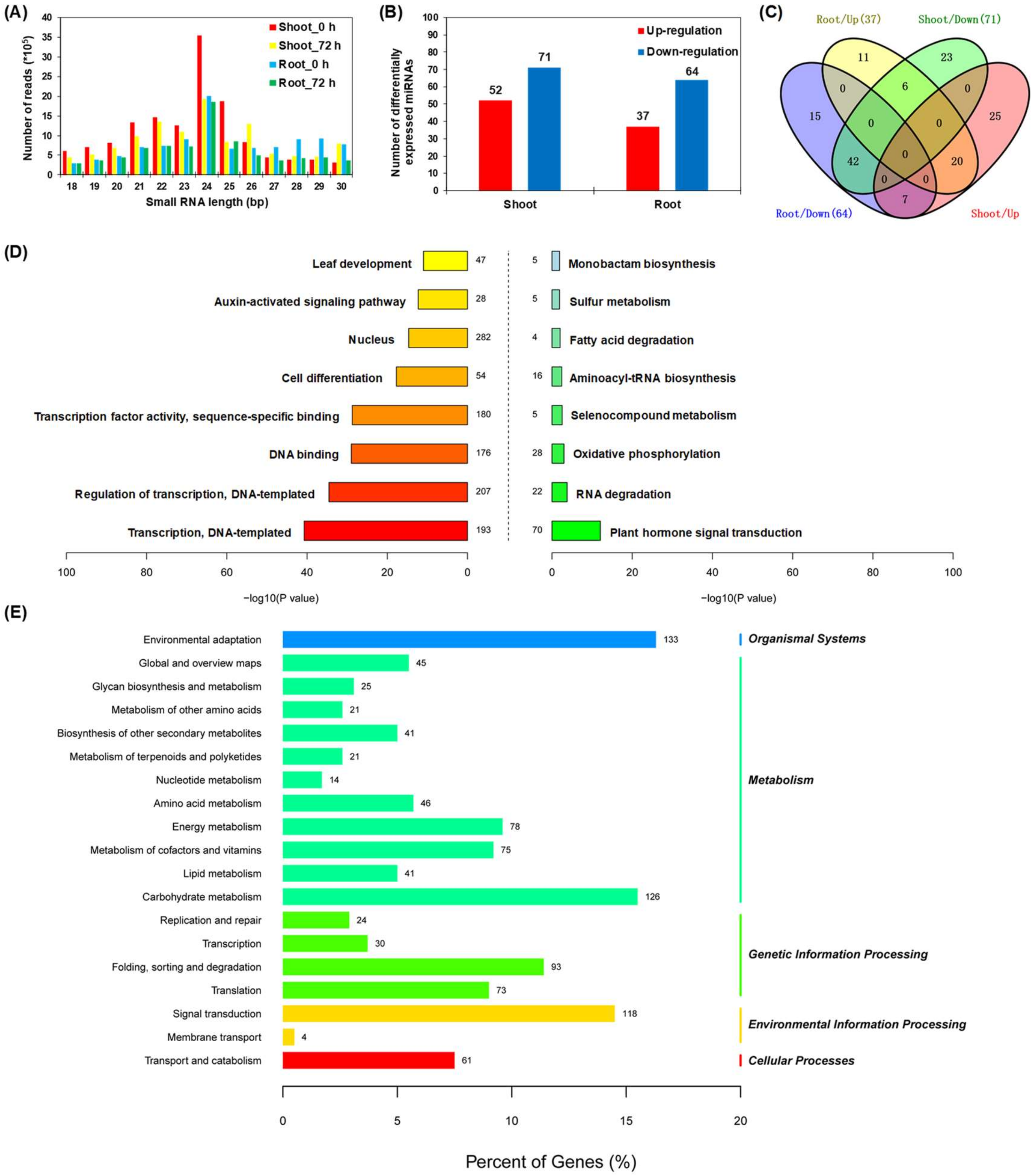
2.4. Transcriptional Regulation of Rapeseed miRNAs in Response to N Limitation
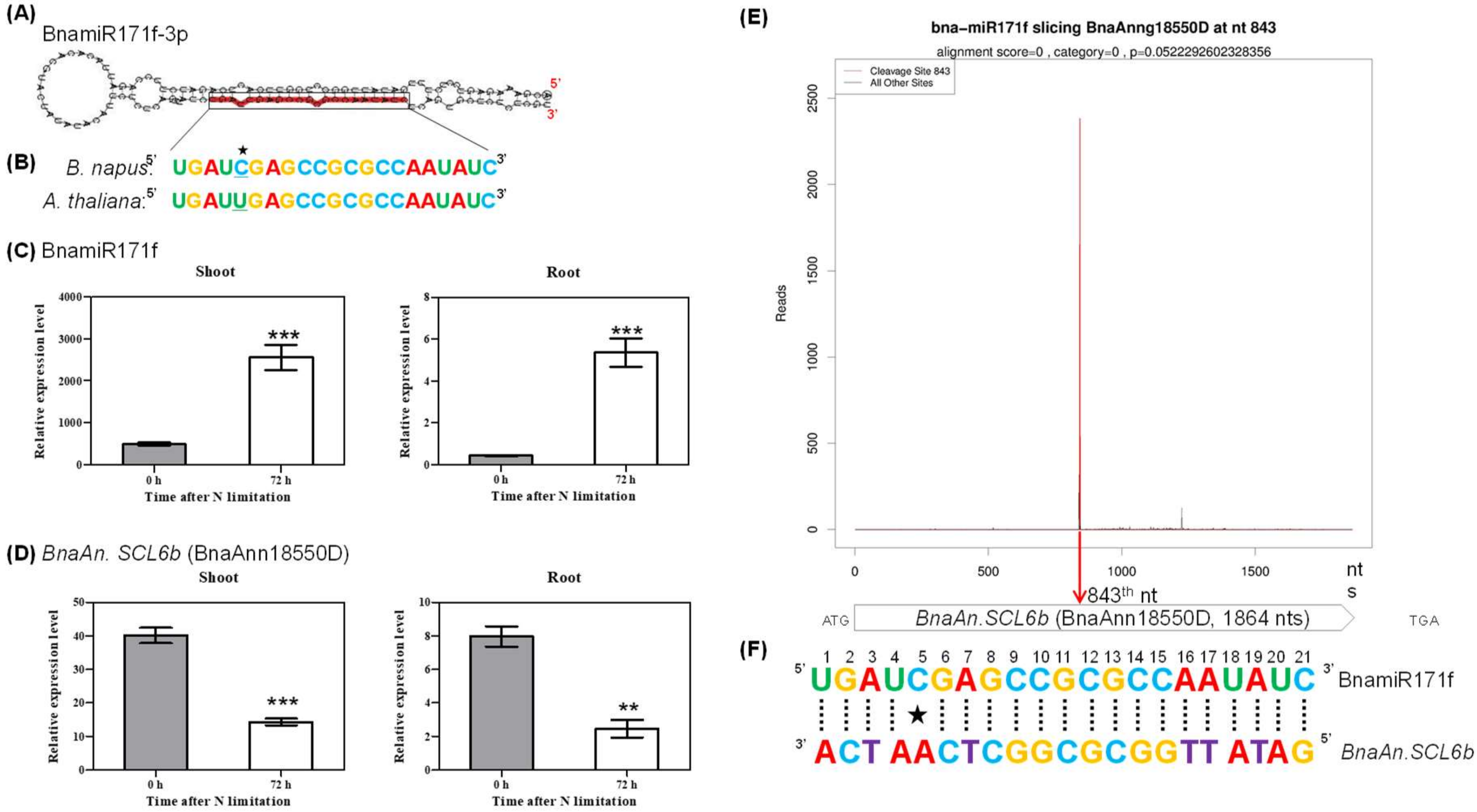
2.5. Degradome Sequencing-Assisted Identification of Genes Targeted by Differentially Expressed miRNAs in Response to N Limitation
3. Discussion
3.1. N Limitation Triggered Adaptation and NUE Enhancement
3.2. Genome-Wide Differential DNA Methylation Reveals Pivotal Roles of Epigenetic Regulation in Adaptive Responses of Rapeseed to N Limitation
3.3. Global miRNA and Degradome Sequencing-Assisted Identification of Differentially Expressed miRNAs Uncover Epigenetic Regulatory Mechanisms Underlying N-Limitation-Induced Morphogenesis
4. Material and Methods
4.1. Plant Materials and Growth Conditions
4.2. Quantification of Morpho-Physiological Characteristics
4.3. DNA and RNA Isolation, Quantification, and Qualification
4.4. Library Preparation for Whole Genome Bisulfite Sequencing (WGBS) and miRNA Sequencing
4.5. Clustering and Sequencing
4.6. Data Analysis
4.6.1. Quality Control
4.6.2. Reads Mapping to the Reference Sequences
4.6.3. miRNA Alignment and Target Genes Prediction
4.7. Differential Analysis of DNA Methylation and miRNA Expression
4.8. Gene Ontology and KEGG Enrichment Analysis
4.9. Degradome Analysis
4.10. Reverse Transcription Quantitative PCR (RT-qPCR) Assays
4.11. Statistical Tests
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLC | chloride channel |
| DEG | differentially expressed gene |
| DMG | differentially methylated gene |
| DMR | differentially methylated region |
| gDNA | genomic DNA |
| Gln/GS | glutamine synthetase |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| miRNA | microRNA |
| N | nitrogen |
| NIA | nitrate reductase |
| NPF | nitrate/peptide family |
| NRT | nitrate transporter |
| NO3− | nitrate |
| NUE | nitrogen use efficiency |
| NLA | nitrogen limitation adaptation |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| RSA | root system architecture |
| WGBS | whole genome bisulfite sequencing |
References
- Kant, S. Understanding nitrate uptake, signaling and remobilization for improving plant nitrogen use efficiency. Semin. Cell Dev. Biol. 2018, 74, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Bi, Y.-M.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2010, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, S.J. Returning to Our Roots: Making Plant Biology Research Relevant to Future Challenges in Agriculture. Plant Cell 2007, 19, 2695–2699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raun, W.R.; Johnson, G.V. Improving Nitrogen Use Efficiency for Cereal Production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Duan, C.-G.; Zhu, J.-K.; Cao, X. Retrospective and perspective of plant epigenetics in China. J. Genet. Genom. 2018, 45, 621–638. [Google Scholar] [CrossRef]
- Thiebaut, F.; Hemerly, A.S.; Ferreira, P.C.G. A Role for Epigenetic Regulation in the Adaptation and Stress Responses of Non-model Plants. Front. Plant Sci. 2019, 10, 246. [Google Scholar] [CrossRef]
- Takeda, S.; Paszkowski, J. DNA methylation and epigenetic inheritance during plant gametogenesis. Chromosoma 2005, 115, 27–35. [Google Scholar] [CrossRef]
- Kou, H.; Li, Y.; Song, X.; Ou, X.; Xing, S.; Ma, J.; Von Wettstein, D.; Liu, B. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice (Oryza sativa L.). J. Plant Physiol. 2011, 168, 1685–1693. [Google Scholar] [CrossRef]
- Secco, D.; Whelan, J.; Rouached, H.; Lister, R. Nutrient stress-induced chromatin changes in plants. Curr. Opin. Plant Biol. 2017, 39, 1–7. [Google Scholar] [CrossRef]
- Yong-Villalobos, L.; González-Morales, S.I.; Wrobel, K.; Gutiérrez-Alanis, D.; Cervantes-Peréz, S.A.; Hayano-Kanashiro, C.; Oropeza-Aburto, A.; Cruz-Ramírez, A.; Martínez, O.; Herrera-Estrella, L. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proc. Natl. Acad. Sci. USA 2015, 112, E7293–E7302. [Google Scholar] [CrossRef]
- Chen, X.; Schonberger, B.; Menz, J.; Ludewig, U. Plasticity of DNA methylation and gene expression under zinc deficiency in Arabidopsis roots. Plant Cell Physiol. 2018, 59, 1790–1802. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Li, M.; Luo, R.; Zhao, F.J.; Salt, D.E. Epigenetic regulation of sulphur homeostasis in plants. J. Exp. Bot. 2019, 70, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.-J. The role of microRNAs in sensing nutrient stress. Plant Cell Environ. 2007, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.N.; Rothstein, S.J.; Spangenberg, G.C.; Kant, S. Role of microRNAs involved in plant response to nitrogen and phosphorous limiting conditions. Front. Plant Sci. 2015, 6, 629. [Google Scholar] [CrossRef]
- Zhao, M.; Ding, H.; Zhu, J.-K.; Zhang, F.; Li, W.-X. Involvement of miR169 in the nitrogen-starvation responses in Arabidopsis. N. Phytol. 2011, 190, 906–915. [Google Scholar] [CrossRef]
- Gifford, M.L.; Dean, A.; Gutierrez, R.A.; Coruzzi, G.M.; Birnbaum, K.D. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA 2008, 105, 803–808. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Q.; Wang, K.; Du, Q.; Liu, S. Nitrogen Limitation Adaptation (NLA) is involved in source-to-sink remobilization of nitrate by mediating the degradation of NRT1.7 in Arabidopsis. N. Phytol. 2016, 214, 734–744. [Google Scholar] [CrossRef]
- Park, B.S.; Yao, T.; Seo, J.S.; Wong, E.C.C.; Mitsuda, N.; Huang, C.-H.; Chua, N.-H. Arabidopsis nitrogen limitation adaptation regulates ORE1 homeostasis during senescence induced by nitrogen deficiency. Nat. Plants 2018, 4, 898–903. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.P.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Rathke, G.-W.; Christen, O.; Diepenbrock, W. Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crop. Res. 2005, 94, 103–113. [Google Scholar] [CrossRef]
- Peng, M.; Hannam, C.; Gu, H.; Bi, Y.-M.; Rothstein, S.J. A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J. 2007, 50, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Zhou, T.; Liao, Q.; Yao, J.-Y.; Liang, G.-H.; Song, H.-X.; Guan, C.-Y.; Hua, Y.-P. Integrated physiologic, genomic and transcriptomic strategies involving the adaptation of allotetraploid rapeseed to nitrogen limitation. BMC Plant Biol. 2018, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Huang, C.-Y.; Tsay, Y.-F. CHL1 Is a Dual-Affinity Nitrate Transporter of Arabidopsis Involved in Multiple Phases of Nitrate Uptake. Plant Cell 1999, 11, 865. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Kotur, Z.; Glass, A.D.M. Characterization of an intact two-component high-affinity nitrate transporter from Arabidopsis roots. Plant J. 2010, 63, 739–748. [Google Scholar] [CrossRef]
- Lin, S.-H.; Kuo, H.-F.; Canivenc, G.; Lin, C.-S.; Lepetit, M.; Hsu, P.-K.; Tillard, P.; Lin, H.-L.; Wang, Y.-Y.; Tsai, C.-B.; et al. Mutation of the Arabidopsis NRT1.5 Nitrate Transporter Causes Defective Root-to-Shoot Nitrate Transport. Plant Cell 2008, 20, 2514–2528. [Google Scholar] [CrossRef]
- Li, J.-Y.; Fu, Y.-L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.-Z.; Zhang, Y.; Li, H.-M.; Huang, J.; et al. The Arabidopsis Nitrate Transporter NRT1.8 Functions in Nitrate Removal from the Xylem Sap and Mediates Cadmium Tolerance. Plant Cell 2010, 22, 1633–1646. [Google Scholar] [CrossRef]
- Fan, S.-C.; Lin, C.-S.; Hsu, P.-K.; Lin, S.-H.; Tsay, Y.-F. The Arabidopsis Nitrate Transporter NRT1.7, Expressed in Phloem, Is Responsible for Source-to-Sink Remobilization of Nitrate. Plant Cell 2009, 21, 2750–2761. [Google Scholar] [CrossRef]
- Hua, Y.-P.; Zhou, T.; Song, H.-X.; Guan, C.-Y.; Zhang, Z.-H. Integrated genomic and transcriptomic insights into the two-component high-affinity nitrate transporters in allotetraploid rapeseed. Plant Soil 2018, 427, 245–268. [Google Scholar] [CrossRef]
- De Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 2006, 442, 939–942. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Wang, M.; Fu, S.-X.; Yang, W.-C.; Qi, C.-K.; Wang, X.-J. Small RNA Profiling in Two Brassica napus Cultivars Identifies MicroRNAs with Oil Production and Development-Correlated Expression and New Small RNA Classes. Plant Physiol. 2012, 158, 813–823. [Google Scholar] [CrossRef]
- Huang, D.; Koh, C.; Feurtado, J.A.; Tsang, E.W.; Cutler, A.J. MicroRNAs and their putative targets in Brassica napus seed maturation. BMC Genom. 2013, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Dong, Y.; Zhang, Q.X.; Zhang, L.; Luo, Y.Z.; Sun, J.; Fan, Y.L.; Wang, L. Identification of miRNAs and their targets from Brassica napus by high-throughput sequencing and degradome analysis. BMC Genom. 2012, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.C.; Bartel, D.P.; Bartel, B. MicroRNA-Directed Regulation of Arabidopsis Auxin Response Factor17 Is Essential for Proper Development and Modulates Expression of Early Auxin Response Genes. Plant Cell 2005, 17, 1360–1375. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-F.; Tian, Q.; Reed, J.W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 2006, 133, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Yang, B.; Zhang, A.; Ma, J.; Ding, Y.; Chen, Z.; Li, J.; Xu, X.; Liu, L. Genome-Wide Identification of MicroRNAs in Response to Cadmium Stress in Oilseed Rape (Brassica napus L.) Using High-Throughput Sequencing. Int. J. Mol. Sci. 2018, 19, 1431. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.-Y.; Felippes, F.F.; Liu, C.-J.; Weigel, D.; Wang, J.-W. Negative Regulation of Anthocyanin Biosynthesis in Arabidopsis by a miR156-Targeted SPL Transcription Factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Yu, D. Identification of Nitrogen Starvation-Responsive MicroRNAs in Arabidopsis thaliana. PLoS ONE 2012, 7, e48951. [Google Scholar] [CrossRef]
- Guo, H.-S.; Xie, Q.; Fei, J.-F.; Chua, N.-H. MicroRNA Directs mRNA Cleavage of the Transcription Factor NAC1 to Downregulate Auxin Signals for Arabidopsis Lateral Root Development. Plant Cell 2005, 17, 1376–1386. [Google Scholar] [CrossRef]
- Wang, L.; Mai, Y.-X.; Zhang, Y.-C.; Luo, Q.; Yang, H.-Q. MicroRNA171c-Targeted SCL6-II, SCL6-III, and SCL6-IV Genes Regulate Shoot Branching in Arabidopsis. Mol. Plant 2010, 3, 794–806. [Google Scholar] [CrossRef]
- Khan, G.A.; Declerck, M.; Sorin, C.; Hartmann, C.; Crespi, M.; Lelandais-Brière, C. MicroRNAs as regulators of root development and architecture. Plant Mol. Biol. 2011, 77, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Fredes, I.; Moreno, S.; Díaz, F.P.; A Gutiérrez, R. Nitrate signaling and the control of Arabidopsis growth and development. Curr. Opin. Plant Biol. 2019, 47, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y.; et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Konishi, M.; Kiba, T.; Sakuraba, Y.; Sawaki, N.; Kurai, T.; Ueda, Y.; Sakakibara, H.; Yanagisawa, S. A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Park, S.; Jeong, J.S.; Seo, J.S.; Park, B.S.; Chua, N.-H. Arabidopsis ubiquitin-specific proteases UBP12 and UBP13 shape ORE1 levels during leaf senescence induced by nitrogen deficiency. N. Phytol. 2019, 223, 1447–1460. [Google Scholar] [CrossRef]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty Years from Transport to Signaling Networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef]
- Wang, Q.; Nian, J.; Xie, X.; Yu, H.; Zhang, J.; Bai, J.; Dong, G.; Hu, J.; Bai, B.; Chen, L.; et al. Genetic variations in ARE1 mediate grain yield by modulating nitrogen utilization in rice. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth–metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the Nitrate Transporter Gene OsNRT1.1A/OsNPF6.3 Confers High Yield and Early Maturation in Rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef]
- Parker, J.L.; Newstead, S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 2014, 507, 68–72. [Google Scholar] [CrossRef]
- Widiez, T.; El Kafafi, E.S.; Girin, T.; Berr, A.; Ruffel, S.; Krouk, G.; Vayssières, A.; Shen, W.-H.; Coruzzi, G.M.; Gojon, A.; et al. High Nitrogen Insensitive 9 (HNI9)-mediated systemic repression of root NO3−uptake is associated with changes in histone methylation. Proc. Natl. Acad. Sci. USA 2011, 108, 13329–13334. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J.; et al. Adaption of Roots to Nitrogen Deficiency Revealed by 3D Quantification and Proteomic Analysis. Plant Physiol. 2019, 179, 329–347. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-K.; Tsay, Y.-F. Two Phloem Nitrate Transporters, NRT1.11 and NRT1.12, Are Important for Redistributing Xylem-Borne Nitrate to Enhance Plant Growth. Plant Physiol. 2013, 163, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, Y.; Walch-Liu, P.; Neumann, G.; Römheld, V.; Von Wirén, N.; Bangerth, F. Root-derived cytokinins as long-distance signals for NO3--induced stimulation of leaf growth. J. Exp. Bot. 2005, 56, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jennings, A.; Barlow, P.W.; Forde, B.G. Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. USA 1999, 96, 6529–6534. [Google Scholar] [CrossRef]
- Little, D.Y.; Rao, H.; Oliva, S.; Daniel-Vedele, F.; Krapp, A.; Malamy, J. The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc. Natl. Acad. Sci. USA 2005, 102, 13693–13698. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. A Central Role for the Nitrate Transporter NRT2.1 in the Integrated Morphological and Physiological Responses of the Root System to Nitrogen Limitation in Arabidopsis. Plant Physiol. 2006, 140, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Bouguyon, E.; Perrine-Walker, F.; Pervent, M.; Rochette, J.; Cuesta, C.; Benkova, E.; Martinière, A.; Bach, L.; Krouk, G.; Gojon, A.; et al. Nitrate controls root development through posttranscriptional regulation of the NRT1.1/NPF6.3 transporter/sensor. Plant Physiol. 2006, 172, 1237–1248. [Google Scholar]
- Sun, C.-H.; Yu, J.-Q.; Hu, D.-G. Nitrate: A Crucial Signal during Lateral Roots Development. Front. Plant Sci. 2017, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; He, J. Protective role of anthocyanins in plants under low nitrogen stress. Biochem. Biophys. Res. Commun. 2018, 498, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Giehl, R.F.; Meyer, R.C.; Altmann, T.; Von Wirén, N. Natural variation of BSK3 tunes brassinosteroid signaling to regulate root foraging under low nitrogen. Nat. Commun. 2019, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dun, X.; Shi, J.; Wang, X.; Liu, G.; Wang, H. Genetic Dissection of Root Morphological Traits Related to Nitrogen Use Efficiency in Brassica napus L. under Two Contrasting Nitrogen Conditions. Front. Plant Sci. 2017, 8, 1709. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhuang, Z.; Cai, H.; Cheng, S.; Soomro, A.A.; Liu, Z.; Gu, R.; Emi, G.; Yuan, L.; Chen, F. Use of genotype-environment interactions to elucidate the pattern of maize root plasticity to nitrogen deficiency. J. Integr. Plant Biol. 2015, 58, 242–253. [Google Scholar] [CrossRef]
- Mager, S.; Ludewig, U. Massive Loss of DNA Methylation in Nitrogen, but Not in Phosphorus-Deficient Zea mays Roots Is Poorly Correlated with Gene Expression Differences. Front. Plant Sci. 2018, 9, 497. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.-H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Bell, A.C.; Felsenfeld, G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 2000, 405, 482–485. [Google Scholar] [CrossRef]
- Bewick, A.J.; Schmitz, R.J. Gene body DNA methylation in plants. Curr. Opin. Plant Biol. 2017, 36, 103–110. [Google Scholar] [CrossRef]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.-L.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-wide High-Resolution Mapping and Functional Analysis of DNA Methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef]
- Lu, X.; Wang, W.; Ren, W.; Chai, Z.; Guo, W.; Chen, R.; Wang, L.; Zhao, J.; Lang, Z.; Fan, Y.; et al. Genome-Wide Epigenetic Regulation of Gene Transcription in Maize Seeds. PLoS ONE 2015, 10, e0139582. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Q.; Wang, F.L.; Li, H.; Jing, S.; Yu, M.; Li, J.; Wu, W.H.; Kudla, J.; Wang, Y. The transcription factor MYB59 regulates K+/NO3- translocation in the Arabidopsis response to low K+ stress. Plant Cell 2019, 31, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Mounier, E.; Pervent, M.; Ljung, K.; Gojon, A.; Nacry, P. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsisroot architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 2013, 37, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.; Luo, B.; Guo, X.; Li, B.; Chen, J.; Fan, X. Overexpression of Nitrate Transporter OsNRT2.1 Enhances Nitrate-Dependent Root Elongation. Genes 2019, 10, 290. [Google Scholar] [CrossRef]
- Hua, Y.; Zhou, T.; Ding, G.; Yang, Q.; Shi, L.; Xu, F. Physiological, genomic and transcriptional diversity in responses to boron deficiency in rapeseed genotypes. J. Exp. Bot. 2016, 67, 5769–5784. [Google Scholar] [CrossRef]
- Liao, Q.; Zhou, T.; Yao, J.-Y.; Han, Q.-F.; Song, H.-X.; Guan, C.-Y.; Hua, Y.-P.; Zhang, Z. Genome-scale characterization of the vacuole nitrate transporter Chloride Channel (CLC) genes and their transcriptional responses to diverse nutrient stresses in allotetraploid rapeseed. PLoS ONE 2018, 13, e0208648. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Han, Y.; Hua, Y.; Guan, C.; Zhang, Z. Low Nitrogen Enhances Nitrogen Use Efficiency by Triggering NO3–Uptake and Its Long-Distance Translocation. J. Agric. Food Chem. 2019, 67, 6736–6747. [Google Scholar] [CrossRef]
- Li, R.; Yu, C.; Li, Y.; Lam, T.-W.; Yiu, S.-M.; Kristiansen, K.; Wang, J. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2018, 47, D155–D162. [Google Scholar] [CrossRef]
- Lister, R.; Mukamel, E.A.; Nery, J.R.; Urich, M.; Puddifoot, C.A.; Johnson, N.D.; Lucero, J.; Huang, Y.; Dwork, A.J.; Schultz, M.D.; et al. Global Epigenomic Reconfiguration During Mammalian Brain Development. Science 2013, 341, 1237905. [Google Scholar] [CrossRef]
- Yaish, M.W.; Al-Lawati, A.; Al-Harrasi, I.; Patankar, H.V. Genome-wide DNA Methylation analysis in response to salinity in the model plant caliph medic (Medicago truncatula). BMC Genom. 2018, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Thomas, P.D. PANTHER in 2013: Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2012, 41, D377–D386. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, T.; Tang, T.-J.; Song, H.-X.; Guan, C.-Y.; Huang, J.-Y.; Hua, Y.-P. A multiomics approach reveals the pivotal role of subcellular reallocation in determining rapeseed resistance to cadmium toxicity. J. Exp. Bot. 2019, 70, 5437–5455. [Google Scholar] [CrossRef]
- Maillard, A.; Etienne, P.; Diquélou, S.; Trouverie, J.; Billard, V.; Yvin, J.C.; Ourry, A. Nutrient deficiencies in Brassica napus modify the ionomic composition of plant tissues: A focus on cross-talk between molybdenum and other nutrients. J. Exp. Bot. 2016, 67, 5631–5641. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Huang, S.; Guo, T.; Deng, L.; Hua, W. Selection and evaluation of novel reference genes for quantitative reverse transcription PCR (qRT-PCR) based on genome and transcriptome data in Brassica napus L. Gene 2014, 538, 113–122. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Cheng, H.; Hao, M.; Wang, W.; Mei, D.; Wells, R.; Liu, J.; Wang, H.; Sang, S.-F.; Tang, M.; Zhou, R.; et al. Integrative RNA and miRNA-Profile Analysis Reveals a Likely Role of BR and Auxin Signaling in Branch Angle Regulation of B. napus. Int. J. Mol. Sci. 2017, 18, 887. [Google Scholar] [CrossRef]




| Tissue | Chr | Start | End | Length | ML 1_72 h | ML_0 h | DM 2 | C_Context | Gene ID | Region | Annotation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | chrC07 | 18318906 | 18318977 | 72 | 0.12 | 0.43 | hypo | CG | BnaC07g12690D | promoter | NRT1.6/NPF2.12 |
| chrC07 | 18319032 | 18319214 | 183 | 0.03 | 0.10 | hypo | CHH | BnaC07g12690D | promoter | NRT1.6/NPF2.12 | |
| chrC06 | 31598782 | 31598876 | 95 | 0.23 | 0.13 | hyper | CHH | BnaC06g30920D | promoter | NRT1.7/ NPF2.13 | |
| chrCnn 3 | 57013740 | 57013864 | 125 | 0.24 | 0.36 | hyper | CHH | BnaCnng57240D | promoter | NRT1.11/NPF1.2 | |
| chrC04 | 32096889 | 32096944 | 56 | 0.34 | 0.10 | hyper | CG | BnaC04g30250D | intron | Gln1;1 | |
| chrC04 | 32096889 | 32096944 | 56 | 0.34 | 0.10 | hyper | CG | BnaC04g30250D | exon | Gln1;1 | |
| chrA05 | 17101604 | 17101779 | 176 | 0.14 | 0.05 | hyper | CHH | BnaA05g22420D | promoter | Gln1;3 | |
| chrC06 | 2820212 | 2820332 | 121 | 0.05 | 0.01 | hyper | CHH | BnaC06g02010D | promoter | Gln1;5 | |
| Root | chrC08 | 19833588 | 19833747 | 160 | 0.14 | 0.23 | hypo | CHH | BnaC08g15370D | promoter | NRT1.1/NPF6.3 |
| chrA06 | 2701052 | 2701177 | 126 | 0.04 | 0.11 | hypo | CHH | BnaA06g04560D | promoter | NRT2.1 | |
| chrC05 | 19042585 | 19042712 | 128 | 0.04 | 0.01 | hyper | CHH | BnaC05g24580D | promoter | NRT1.5/NPF7.3 | |
| chrC08 | 33965938 | 33966022 | 85 | 0.17 | 0.42 | hypo | CHG | BnaC08g36990D | promoter | NRT1.9/NPF2.9 | |
| chrCnn | 14859936 | 14860033 | 98 | 0.10 | 0.04 | hyper | CHH | BnaCnng15890D | promoter | NAXT1/NPF2.7 | |
| chrA02 | 11371814 | 11371951 | 138 | 0.09 | 0.18 | hypo | CHH | BnaA02g18610D | intron | NIA1/NR | |
| chrC04 | 32095812 | 32095894 | 83 | 0.34 | 0.06 | hyper | CG | BnaC04g30250D | exon | Gln1;1//GS1;1 | |
| chrA07 | 13217084 | 13217222 | 139 | 0.05 | 0.01 | hyper | CHH | BnaA07g15180D | promoter | CLCa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Y.-p.; Zhou, T.; Huang, J.-y.; Yue, C.-p.; Song, H.-x.; Guan, C.-y.; Zhang, Z.-h. Genome-Wide Differential DNA Methylation and miRNA Expression Profiling Reveals Epigenetic Regulatory Mechanisms Underlying Nitrogen-Limitation-Triggered Adaptation and Use Efficiency Enhancement in Allotetraploid Rapeseed. Int. J. Mol. Sci. 2020, 21, 8453. https://doi.org/10.3390/ijms21228453
Hua Y-p, Zhou T, Huang J-y, Yue C-p, Song H-x, Guan C-y, Zhang Z-h. Genome-Wide Differential DNA Methylation and miRNA Expression Profiling Reveals Epigenetic Regulatory Mechanisms Underlying Nitrogen-Limitation-Triggered Adaptation and Use Efficiency Enhancement in Allotetraploid Rapeseed. International Journal of Molecular Sciences. 2020; 21(22):8453. https://doi.org/10.3390/ijms21228453
Chicago/Turabian StyleHua, Ying-peng, Ting Zhou, Jin-yong Huang, Cai-peng Yue, Hai-xing Song, Chun-yun Guan, and Zhen-hua Zhang. 2020. "Genome-Wide Differential DNA Methylation and miRNA Expression Profiling Reveals Epigenetic Regulatory Mechanisms Underlying Nitrogen-Limitation-Triggered Adaptation and Use Efficiency Enhancement in Allotetraploid Rapeseed" International Journal of Molecular Sciences 21, no. 22: 8453. https://doi.org/10.3390/ijms21228453
APA StyleHua, Y.-p., Zhou, T., Huang, J.-y., Yue, C.-p., Song, H.-x., Guan, C.-y., & Zhang, Z.-h. (2020). Genome-Wide Differential DNA Methylation and miRNA Expression Profiling Reveals Epigenetic Regulatory Mechanisms Underlying Nitrogen-Limitation-Triggered Adaptation and Use Efficiency Enhancement in Allotetraploid Rapeseed. International Journal of Molecular Sciences, 21(22), 8453. https://doi.org/10.3390/ijms21228453






