Structural and Functional Basis for Understanding the Biological Significance of P2X7 Receptor
Abstract
:1. Introduction
2. The Human P2X7 Receptor: Particular Structure and Genetic Polymorphisms
2.1. The Homo-Trimeric P2X7 Receptor Protein
2.2. Polymorphisms and Transcriptional Regulation of P2X7 Receptor
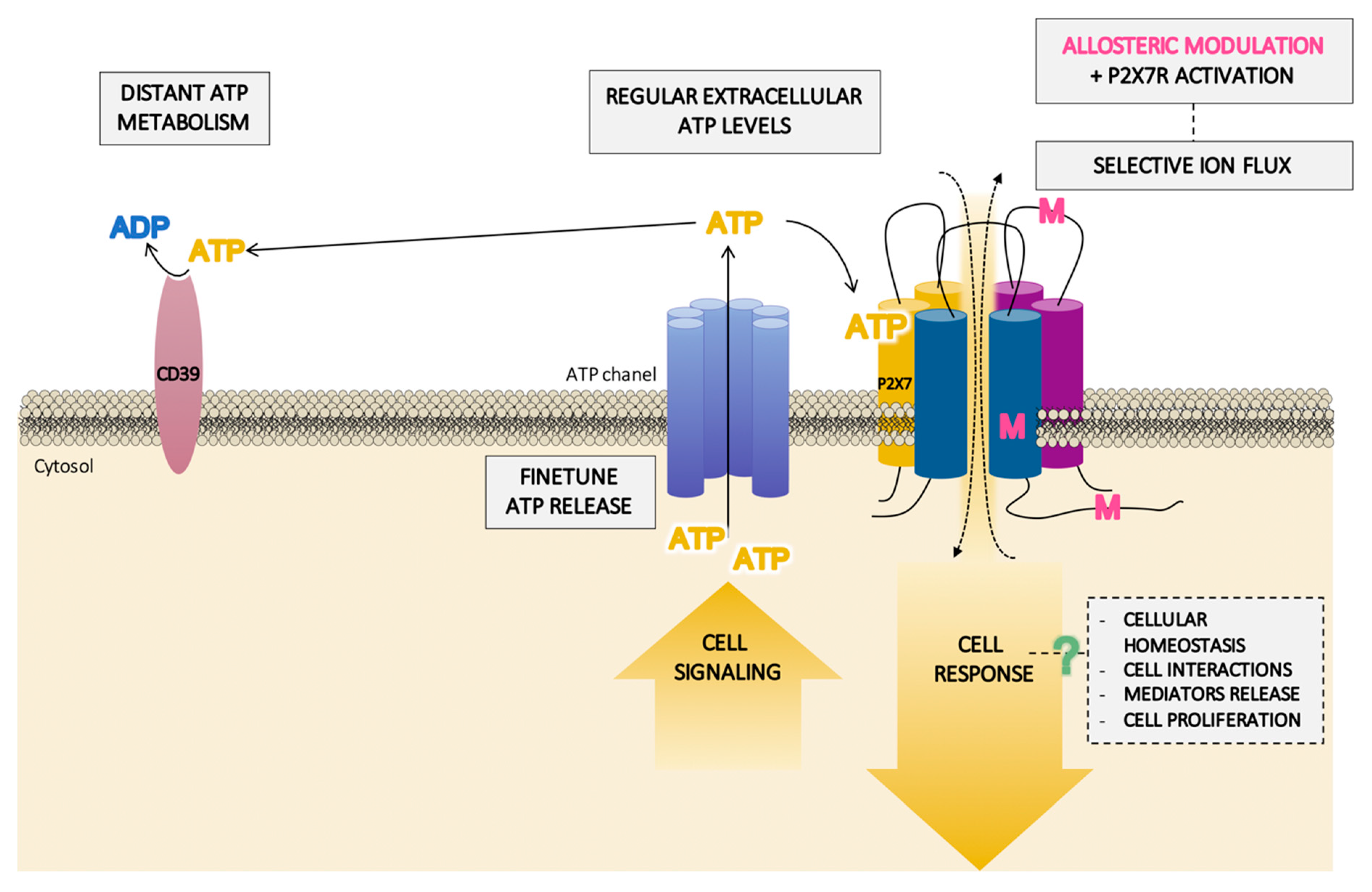
3. Extracellular ATP Levels Are Critical to the Full Activation of the P2X7 Receptor
4. Cell Localization of the P2X7 Receptor Is Crucial for the Nearby Interactions
5. P2X7 Receptor Activation Theories and Transduction Pathways
5.1. Is P2X7 Receptor a Regular Ligand-Gated Cation Channel?
5.2. Does Permeation Occur through P2X7 Channel Receptor Itself or to Membrane Pore Proteins?
5.3. Does P2X7 Receptor Directly Trigger Intracellular Signaling Pathways?
5.4. Can P2X7 Receptor Be Allosterically Modulated by Endogenous Molecules?
6. Pathophysiological Role of P2X7 Receptor and Its Biological Significance
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Adenosine triphosphate-binding cassette |
| ADP | Adenosine diphosphate |
| AKT | Serine/Threonine Kinase |
| AMP | Adenosine monophoshate |
| ATP | Adenosine triphosphate |
| BzATP | 2′(3′)-O-(4-Benzoylbenzoyl)adenosine 5′-triphosphate |
| CALHM | Calcium homeostasis modulator channels |
| Casp | Caspases |
| CHO | Chinese hamster ovary |
| COX | Cyclooxygenase |
| EC50 | Effective concentration 50 |
| EMPs | Epithelial membrane proteins |
| ERK | Extracellular signal-regulated kinase |
| HEK | Human embryonic kidney cell line |
| HIF-1α | Hypoxia–inducible factor 1 alpha |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| LOX | Lipoxygenase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| NAD | Nicotinamide adenine dinucleotide |
| NEK | Never-in-mitosis A- related kinases |
| NF-κB | Nuclear factor κB |
| NLRP | NLR family pyrin domain containing |
| NMDG | N-methyl-D-glucamine |
| P2X7R | P2X7 receptor |
| Pannexin | Panx |
| PBK | Protein Kinase B |
| SNPs | Single-nucleotide polymorphisms |
| SP1 | Specific protein 1 |
| TM | Transmembrane |
| TNF-α | Tumor necrosis factor |
| Tris | Tris(hidroximetil)aminometano |
References
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The Cytolytic P2Z Receptor for Extracellular ATP Identified as a P2X Receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G. Immune Cell Regulation by Autocrine Purinergic Signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.; Ganesan, J.; Müller, T.; Dürr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Jüttner, E.; et al. Graft-Versus-Host Disease is Enhanced by Extracellular ATP Activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; North, R.A. P2X Receptors as Cell-Surface ATP Sensors in Health and Disease. Nature 2006, 442, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Dubyak, G.R. Go it Alone no More--P2X7 Joins the Society of Heteromeric ATP-Gated Receptor Channels. Mol. Pharmacol. 2007, 72, 1402–1405. [Google Scholar] [CrossRef] [Green Version]
- Eltzschig, H.K.; Sitkovsky, M.V.; Robson, S.C. Purinergic Signaling during Inflammation. N. Engl. J. Med. 2012, 367, 2322–2333. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G.; Ralevic, V. Purinergic Signaling and Blood Vessels in Health and Disease. Pharmacol. Rev. 2014, 66, 102–192. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 Receptor: A Main Player in Inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The Potential of P2X7 Receptors as a Therapeutic Target, Including Inflammation and Tumour Progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novitskaya, T.; Chepurko, E.; Covarrubias, R.; Novitskiy, S.; Ryzhov, S.V.; Feoktistov, I.; Gumina, R.J. Extracellular Nucleotide Regulation and Signaling in Cardiac Fibrosis. J. Mol. Cell. Cardiol. 2016, 93, 47–56. [Google Scholar] [CrossRef]
- Tewari, M.; Seth, P. Emerging Role of P2X7 Receptors in CNS Health and Disease. Ageing Res. Rev. 2015, 24, 328–342. [Google Scholar] [CrossRef]
- Gordon, J.L. Extracellular ATP: Effects, Sources and Fate. Biochem. J. 1986, 233, 309–319. [Google Scholar] [CrossRef]
- Di Virgilio, F. The P2Z Purinoceptor: An Intriguing Role in Immunity, Inflammation and Cell Death. Immunol. Today 1995, 16, 524–528. [Google Scholar] [CrossRef]
- Buell, G.N.; Talabot, F.; Gos, A.; Lorenz, J.; Lai, E.; Morris, M.A.; Antonarakis, S.E. Gene Structure and Chromosomal Localization of the Human P2X7 Receptor. Recept. Channels 1998, 5, 347–354. [Google Scholar] [PubMed]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Baldwin, J.M.; Roger, S.; Baldwin, S.A. Insights into the Molecular Mechanisms Underlying Mammalian P2X7 Receptor Functions and Contributions in Diseases, Revealed by Structural Modeling and Single Nucleotide Polymorphisms. Front Pharmacol. 2013, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, A.; Kawate, T. Structural Basis for Subtype-Specific Inhibition of the P2X7 Receptor. Elife 2016, 5. [Google Scholar] [CrossRef]
- Mansoor, S.E.; Lü, W.; Oosterheert, W.; Shekhar, M.; Tajkhorshid, E.; Gouaux, E. X-Ray Structures Define Human P2X(3) Receptor Gating Cycle and Antagonist Action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Hattori, M.; Gouaux, E. Molecular Mechanism of ATP Binding and Ion Channel Activation in P2X Receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal Structure of the ATP-Gated P2X(4) Ion Channel in the Closed State. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Flores, G.; Lévesque, S.A.; Pacheco, J.; Vaca, L.; Lacroix, S.; Pérez-Cornejo, P.; Arreola, J. The P2X7/P2X4 Interaction Shapes the Purinergic Response in Murine Macrophages. Biochem. Biophys. Res. Commun. 2015, 467, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Masin, M.; Qureshi, O.S.; Murrell-Lagnado, R.D. Evidence for Functional P2X4/P2X7 Heteromeric Receptors. Mol. Pharmacol. 2007, 72, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Boumechache, M.; Masin, M.; Edwardson, J.M.; Górecki, D.C.; Murrell-Lagnado, R. Analysis of Assembly and Trafficking of Native P2X4 and P2X7 Receptor Complexes in Rodent Immune Cells. J. Biol. Chem. 2009, 284, 13446–13454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonio, L.S.; Stewart, A.P.; Xu, X.J.; Varanda, W.A.; Murrell-Lagnado, R.D.; Edwardson, J.M. P2X4 Receptors Interact with both P2X2 and P2X7 Receptors in the Form of Homotrimers. Br. J. Pharmacol. 2011, 163, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Compan, V.; Ulmann, L.; Stelmashenko, O.; Chemin, J.; Chaumont, S.; Rassendren, F. P2X2 and P2X5 Subunits Define a New Heteromeric Receptor with P2X7-Like Properties. J. Neurosci. 2012, 32, 4284–4296. [Google Scholar] [CrossRef] [Green Version]
- Grimes, L.; Young, M.T. Purinergic P2X Receptors: Structural and Functional Features Depicted by X-Ray and Molecular Modelling Studies. Curr. Med. Chem. 2015, 22, 783–798. [Google Scholar] [CrossRef] [Green Version]
- Stelmashenko, O.; Lalo, U.; Yang, Y.; Bragg, L.; North, R.A.; Compan, V. Activation of Trimeric P2X2 Receptors by Fewer than Three ATP Molecules. Mol. Pharmacol. 2012, 82, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, W.J.; Jiang, L.H.; Surprenant, A.; North, R.A. Role of Ectodomain Lysines in the Subunits of the Heteromeric P2X2/3 Receptor. Mol. Pharmacol. 2006, 70, 1159–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elssner, A.; Duncan, M.; Gavrilin, M.; Wewers, M.D. A Novel P2X7 Receptor Activator, the Human Cathelicidin-Derived Peptide LL37, Induces IL-1 Beta Processing and Release. J. Immunol. 2004, 172, 4987–4994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasinsig, L.; Pizzirani, C.; Skerlavaj, B.; Pellegatti, P.; Gulinelli, S.; Tossi, A.; Di Virgilio, F.; Zanetti, M. The Human Cathelicidin LL-37 Modulates the Activities of the P2X7 Receptor in a Structure-Dependent Manner. J. Biol. Chem. 2008, 283, 30471–30481. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.M.; Chiozzi, P.; Ferrari, D.; Colaianna, M.; Idzko, M.; Falzoni, S.; Fellin, R.; Trabace, L.; Di Virgilio, F. Activation of Microglia by Amyloid {Beta} Requires P2X7 Receptor Expression. J. Immunol. 2009, 182, 4378–4385. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.M.; Chiozzi, P.; Di Virgilio, F. Tenidap Enhances P2Z/P2X7 Receptor Signalling in Macrophages. Eur. J. Pharmacol. 1998, 355, 235–244. [Google Scholar] [CrossRef]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Forchap, S.; Sitta, B.; Turchet, L.; Falzoni, S.; Minelli, M.; Baricordi, R.; Di Virgilio, F. The Antibiotic Polymyxin B Modulates P2X7 Receptor Function. J. Immunol. 2004, 173, 4652–4660. [Google Scholar] [CrossRef] [Green Version]
- Helliwell, R.M.; ShioukHuey, C.O.; Dhuna, K.; Molero, J.C.; Ye, J.; Xue, C.C.; Stokes, L. Selected Ginsenosides of the Protopanaxdiol Series are Novel Positive Allosteric Modulators of P2X7 Receptors. Br. J. Pharmacol. 2015, 172, 3326–3340. [Google Scholar] [CrossRef] [PubMed]
- Costa-Junior, H.M.; Sarmento Vieira, F.; Coutinho-Silva, R. C Terminus of the P2X7 Receptor: Treasure Hunting. Purinergic Signal. 2011, 7, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amstrup, J.; Novak, I. P2X7 Receptor Activates Extracellular Signal-Regulated Kinases ERK1 and ERK2 Independently of Ca2+ Influx. Biochem. J. 2003, 374, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Samways, D.S.K.; Wolf, K.; Bowles, E.A.; Richards, J.P.; Bruno, J.; Dutertre, S.; DiPaolo, R.J.; Egan, T.M. Quantifying Ca2+ Current and Permeability in ATP-Gated P2X7 Receptors. J. Biol. Chem. 2015, 290, 7930–7942. [Google Scholar] [CrossRef] [Green Version]
- Allsopp, R.C.; Evans, R.J. Contribution of the Juxtatransmembrane Intracellular Regions to the Time Course and Permeation of ATP-Gated P2X7 Receptor Ion Channels. J. Biol. Chem. 2015, 290, 14556–14566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Li, S.; Liang, Z.; Tomić, M.; Stojilkovic, S.S. The P2X7 Receptor Channel Pore Dilates Under Physiological Ion Conditions. J. Gen. Physiol. 2008, 132, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, L.E.; Shridar, M.; Smith, P.; Murrell-Lagnado, R.D. Plasma Membrane Cholesterol as a Regulator of Human and Rodent P2X7 Receptor Activation and Sensitization. J. Biol. Chem. 2014, 289, 31983–31994. [Google Scholar] [CrossRef] [Green Version]
- Roger, S.; Mei, Z.; Baldwin, J.M.; Dong, L.; Bradley, H.; Baldwin, S.A.; Surprenant, A.; Jiang, L. Single Nucleotide Polymorphisms that were Identified in Affective Mood Disorders Affect ATP-Activated P2X7 Receptor Functions. J. Psychiatr. Res. 2010, 44, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.J.; Stokes, L.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Genetics of the P2X7 Receptor and Human Disease. Purinergic Signal. 2009, 5, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Metzger, M.W.; Walser, S.M.; Dedic, N.; Aprile-Garcia, F.; Jakubcakova, V.; Adamczyk, M.; Webb, K.J.; Uhr, M.; Refojo, D.; Schmidt, M.V.; et al. Heterozygosity for the Mood Disorder-Associated Variant Gln460Arg Alters P2X7 Receptor Function and Sleep Quality. J. Neurosci. 2017, 37, 11688–11700. [Google Scholar] [CrossRef] [PubMed]
- Stokes, L.; Fuller, S.J.; Sluyter, R.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Two Haplotypes of the P2X(7) Receptor Containing the Ala-348 to Thr Polymorphism Exhibit a Gain-of-Function Effect and Enhanced Interleukin-1beta Secretion. FASEB J. 2010, 24, 2916–2927. [Google Scholar] [CrossRef]
- Bernier, L.; Ase, A.R.; Séguéla, P. P2X Receptor Channels in Chronic Pain Pathways. Br. J. Pharmacol. 2018, 175, 2219–2230. [Google Scholar] [CrossRef]
- Peverini, L.; Beudez, J.; Dunning, K.; Chataigneau, T.; Grutter, T. New Insights into Permeation of Large Cations through ATP-Gated P2X Receptors. Front Mol. Neurosci. 2018, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandonà, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic Activity of a Naturally Occurring Truncated Isoform of the P2X7 Receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef]
- Cheewatrakoolpong, B.; Gilchrest, H.; Anthes, J.C.; Greenfeder, S. Identification and Characterization of Splice Variants of the Human P2X7 ATP Channel. Biochem. Biophys. Res. Commun. 2005, 332, 17–27. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Wang, L.; Zhou, L.; Gorodeski, G.I. A Truncated P2X7 Receptor Variant (P2X7-J) Endogenously Expressed in Cervical Cancer Cells Antagonizes the Full-Length P2X7 Receptor through Hetero-Oligomerization. J. Biol. Chem. 2006, 281, 17228–17237. [Google Scholar] [CrossRef] [Green Version]
- García-Huerta, P.; Díaz-Hernandez, M.; Delicado, E.G.; Pimentel-Santillana, M.; Miras-Portugal, M.T.; Gómez-Villafuertes, R. The Specificity Protein Factor Sp1 Mediates Transcriptional Regulation of P2X7 Receptors in the Nervous System. J. Biol. Chem. 2012, 287, 44628–44644. [Google Scholar] [CrossRef] [Green Version]
- Engel, T.; Brennan, G.P.; Sanz-Rodriguez, A.; Alves, M.; Beamer, E.; Watters, O.; Henshall, D.C.; Jimenez-Mateos, E.M. A Calcium-Sensitive Feed-Forward Loop Regulating the Expression of the ATP-Gated Purinergic P2X7 Receptor Via Specificity Protein 1 and microRNA-22. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 255–266. [Google Scholar] [CrossRef]
- Tafani, M.; Schito, L.; Pellegrini, L.; Villanova, L.; Marfe, G.; Anwar, T.; Rosa, R.; Indelicato, M.; Fini, M.; Pucci, B.; et al. Hypoxia-Increased RAGE and P2X7R Expression Regulates Tumor Cell Invasion through Phosphorylation of Erk1/2 and Akt and Nuclear Translocation of NF-{Kappa}B. Carcinogenesis 2011, 32, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, Y.; Koizumi, S. Hypoxia-Independent Mechanisms of HIF-1α Expression in Astrocytes after Ischemic Preconditioning. Glia 2017, 65, 523–530. [Google Scholar] [CrossRef]
- Cauwels, A.; Rogge, E.; Vandendriessche, B.; Shiva, S.; Brouckaert, P. Extracellular ATP Drives Systemic Inflammation, Tissue Damage and Mortality. Cell Death Dis. 2014, 5, e1102. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, K.S. Beginnings of a Good Apoptotic Meal: The Find-Me and Eat-Me Signaling Pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides Released by Apoptotic Cells Act as a Find-Me Signal to Promote Phagocytic Clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Bradley, H.J.; Browne, L.E.; Yang, W.; Jiang, L. Pharmacological Properties of the Rhesus Macaque Monkey P2X7 Receptor. Br. J. Pharmacol. 2011, 164, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Roman, S.; Cusdin, F.S.; Fonfria, E.; Goodwin, J.A.; Reeves, J.; Lappin, S.C.; Chambers, L.; Walter, D.S.; Clay, W.C.; Michel, A.D. Cloning and Pharmacological Characterization of the Dog P2X7 Receptor. Br. J. Pharmacol. 2009, 158, 1513–1526. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, X.; Coddou, C.; Geng, X.; Wei, J.; Sun, J. Molecular Characterization and Expression Analysis of ATP-Gated P2X7 Receptor Involved in Japanese Flounder (Paralichthys Olivaceus) Innate Immune Response. PLoS ONE 2014, 9, e96625. [Google Scholar] [CrossRef]
- López-Castejón, G.; Young, M.T.; Meseguer, J.; Surprenant, A.; Mulero, V. Characterization of ATP-Gated P2X7 Receptors in Fish Provides New Insights into the Mechanism of Release of the Leaderless Cytokine Interleukin-1 Beta. Mol. Immunol. 2007, 44, 1286–1299. [Google Scholar] [CrossRef]
- Chessell, I.P.; Simon, J.; Hibell, A.D.; Michel, A.D.; Barnard, E.A.; Humphrey, P.P. Cloning and Functional Characterisation of the Mouse P2X7 Receptor. FEBS Lett. 1998, 439, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Fonfria, E.; Clay, W.C.; Levy, D.S.; Goodwin, J.A.; Roman, S.; Smith, G.D.; Condreay, J.P.; Michel, A.D. Cloning and Pharmacological Characterization of the Guinea Pig P2X7 Receptor Orthologue. Br. J. Pharmacol. 2008, 153, 544–556. [Google Scholar] [CrossRef] [Green Version]
- Paukert, M.; Hidayat, S.; Gründer, S. The P2X(7) Receptor from Xenopus Laevis: Formation of a Large Pore in Xenopus Oocytes. FEBS Lett. 2002, 513, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular Nucleotides and Nucleosides as Signalling Molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef]
- Diezmos, E.F.; Bertrand, P.P.; Liu, L. Purinergic Signaling in Gut Inflammation: The Role of Connexins and Pannexins. Front Neurosci. 2016, 10, 311. [Google Scholar] [CrossRef] [Green Version]
- Fortes, F.S.A.; Pecora, I.L.; Persechini, P.M.; Hurtado, S.; Costa, V.; Coutinho-Silva, R.; Braga, M.B.M.; Silva-Filho, F.C.; Bisaggio, R.C.; De Farias, F.P.; et al. Modulation of Intercellular Communication in Macrophages: Possible Interactions between GAP Junctions and P2 Receptors. J. Cell. Sci. 2004, 117, 4717–4726. [Google Scholar] [CrossRef] [Green Version]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic Control of T Cell Activation by ATP Released through Pannexin-1 Hemichannels. Sci. Signal. 2008, 1, ra6. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, R.; Locovei, S.; Roque, A.; Alberto, A.P.; Dahl, G.; Spray, D.C.; Scemes, E. P2X7 Receptor-Pannexin1 Complex: Pharmacology and Signaling. Am. J. Physiol. Cell Physiol. 2008, 295, 752. [Google Scholar] [CrossRef] [Green Version]
- Pelegrin, P.; Surprenant, A. Pannexin-1 Mediates Large Pore Formation and Interleukin-1beta Release by the ATP-Gated P2X7 Receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parzych, K.; Zetterqvist, A.V.; Wright, W.R.; Kirkby, N.S.; Mitchell, J.A.; Paul-Clark, M.J. Differential Role of Pannexin-1/ATP/P2X7 Axis in IL-1β Release by Human Monocytes. FASEB J. 2017, 31, 2439–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Leguillier, T.; Dargère, D.; Auzeil, N.; Laprévote, O.; Rat, P. P2X7-Pannexin-1 and Amyloid Β-Induced Oxysterol Input in Human Retinal Cell: Role in Age-Related Macular Degeneration? Biochimie 2016, 127, 70–78. [Google Scholar] [CrossRef]
- Aquilino, M.S.; Whyte-Fagundes, P.; Zoidl, G.; Carlen, P.L. Pannexin-1 Channels in Epilepsy. Neurosci. Lett. 2019, 695, 71–75. [Google Scholar] [CrossRef]
- Draganov, D.; Gopalakrishna-Pillai, S.; Chen, Y.; Zuckerman, N.; Moeller, S.; Wang, C.; Ann, D.; Lee, P.P. Modulation of P2X4/P2X7/Pannexin-1 Sensitivity to Extracellular ATP Via Ivermectin Induces a Non-Apoptotic and Inflammatory Form of Cancer Cell Death. Sci. Rep. 2015, 5, 16222. [Google Scholar] [CrossRef] [Green Version]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin Channels are Not Gap Junction Hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef]
- Locovei, S.; Bao, L.; Dahl, G. Pannexin 1 in Erythrocytes: Function without a Gap. Proc. Natl. Acad. Sci. USA 2006, 103, 7655–7659. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Grinspan, J.B.; Abrams, C.K.; Scherer, S.S. Pannexin1 is Expressed by Neurons and Glia but does Not Form Functional Gap Junctions. Glia 2007, 55, 46–56. [Google Scholar] [CrossRef]
- Scemes, E.; Suadicani, S.O.; Dahl, G.; Spray, D.C. Connexin and Pannexin Mediated Cell-Cell Communication. Neuron Glia Biol. 2007, 3, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Hui, H.; Pelegrin, P.; Surprenant, A. Pharmacological Characterization of Pannexin-1 Currents Expressed in Mammalian Cells. J. Pharmacol. Exp. Ther. 2009, 328, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Locovei, S.; Scemes, E.; Qiu, F.; Spray, D.C.; Dahl, G. Pannexin1 is Part of the Pore Forming Unit of the P2X(7) Receptor Death Complex. FEBS Lett. 2007, 581, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in Immunity and Inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Robson, S.C.; Sévigny, J.; Zimmermann, H. The E-NTPDase Family of Ectonucleotidases: Structure Function Relationships and Pathophysiological Significance. Purinergic Signal. 2006, 2, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Regateiro, F.S.; Cobbold, S.P.; Waldmann, H. CD73 and Adenosine Generation in the Creation of Regulatory Microenvironments. Clin. Exp. Immunol. 2013, 171, 1–7. [Google Scholar] [CrossRef]
- Sluyter, R.; Barden, J.A.; Wiley, J.S. Detection of P2X Purinergic Receptors on Human B Lymphocytes. Cell Tissue Res. 2001, 304, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Cesaro, A.; Brest, P.; Hofman, V.; Hébuterne, X.; Wildman, S.; Ferrua, B.; Marchetti, S.; Doglio, A.; Vouret-Craviari, V.; Galland, F.; et al. Amplification Loop of the Inflammatory Process is Induced by P2X7R Activation in Intestinal Epithelial Cells in Response to Neutrophil Transepithelial Migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 32. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Marcos, M.; Dehaye, J.; Marino, A. Membrane Compartments and Purinergic Signalling: The Role of Plasma Membrane Microdomains in the Modulation of P2XR-Mediated Signalling. FEBS J. 2009, 276, 330–340. [Google Scholar] [CrossRef]
- Garcia-Marcos, M.; Pérez-Andrés, E.; Tandel, S.; Fontanils, U.; Kumps, A.; Kabré, E.; Gómez-Muñoz, A.; Marino, A.; Dehaye, J.; Pochet, S. Coupling of Two Pools of P2X7 Receptors to Distinct Intracellular Signaling Pathways in Rat Submandibular Gland. J. Lipid Res. 2006, 47, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Bradley, H.J.; Liu, X.; Collins, V.; Owide, J.; Goli, G.R.; Smith, M.; Surprenant, A.; White, S.J.; Jiang, L. Identification of an Intracellular Microdomain of the P2X7 Receptor that is Crucial in Basolateral Membrane Targeting in Epithelial Cells. FEBS Lett. 2010, 584, 4740–4744. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Intracellular Expression of Purinoceptors. Purinergic Signal. 2015, 11, 275–276. [Google Scholar] [CrossRef] [Green Version]
- Gudipaty, L.; Humphreys, B.D.; Buell, G.; Dubyak, G.R. Regulation of P2X(7) Nucleotide Receptor Function in Human Monocytes by Extracellular Ions and Receptor Density. Am. J. Physiol. Cell Physiol. 2001, 280, 943. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.J.; Zhang, W.Y.; Bendall, L.J.; Chessell, I.P.; Buell, G.N.; Wiley, J.S. Expression of P2X(7) Purinoceptors on Human Lymphocytes and Monocytes: Evidence for Nonfunctional P2X(7) Receptors. Am. J. Physiol. Cell Physiol. 2000, 279, 1189. [Google Scholar] [CrossRef]
- Sánchez-Nogueiro, J.; Marín-García, P.; Bustillo, D.; Olivos-Oré, L.A.; Miras-Portugal, M.T.; Artalejo, A.R. Subcellular Distribution and Early Signalling Events of P2X7 Receptors from Mouse Cerebellar Granule Neurons. Eur. J. Pharmacol. 2014, 744, 190–202. [Google Scholar] [CrossRef]
- Lee, H.Y.; Bardini, M.; Burnstock, G. Distribution of P2X Receptors in the Urinary Bladder and the Ureter of the Rat. J. Urol. 2000, 163, 2002–2007. [Google Scholar] [CrossRef]
- Gröschel-Stewart, U.; Bardini, M.; Robson, T.; Burnstock, G. Localisation of P2X5 and P2X7 Receptors by Immunohistochemistry in Rat Stratified Squamous Epithelia. Cell Tissue Res. 1999, 296, 599–605. [Google Scholar] [CrossRef]
- Menzies, J.; Paul, A.; Kennedy, C. P2X7 Subunit-Like Immunoreactivity in the Nucleus of Visceral Smooth Muscle Cells of the Guinea Pig. Auton. Neurosci. 2003, 106, 103–109. [Google Scholar] [CrossRef]
- Kuehnel, M.P.; Rybin, V.; Anand, P.K.; Anes, E.; Griffiths, G. Lipids Regulate P2X7-Receptor-Dependent Actin Assembly by Phagosomes via ADP Translocation and ATP Synthesis in the Phagosome Lumen. J. Cell. Sci. 2009, 122, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Biswas, D.; Qureshi, O.S.; Lee, W.Y.; Croudace, J.E.; Mura, M.; Lammas, D.A. ATP-Induced Autophagy is Associated with Rapid Killing of Intracellular Mycobacteria within Human Monocytes/Macrophages. BMC Immunol. 2008, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Ugur, M.; Ugur, Ö. A Mechanism-Based Approach to P2X7 Receptor Action. Mol. Pharmacol. 2019, 95, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Rassendren, F.; Mackenzie, A.; Zhang, Y.; Surprenant, A.; North, R.A. N-Methyl-D-Glucamine and Propidium Dyes Utilize Different Permeation Pathways at Rat P2X(7) Receptors. Am. J. Physiol. Cell Physiol. 2005, 289, 1295. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, T.H.; Newman, A.S.; Swanson, J.A.; Silverstein, S.C. ATP4-Permeabilizes the Plasma Membrane of Mouse Macrophages to Fluorescent Dyes. J. Biol. Chem. 1987, 262, 8884–8888. [Google Scholar] [PubMed]
- Schachter, J.; Motta, A.P.; de Souza Zamorano, A.; da Silva-Souza, H.A.; Guimarães, M.Z.P.; Persechini, P.M. ATP-Induced P2X7-Associated Uptake of Large Molecules Involves Distinct Mechanisms for Cations and Anions in Macrophages. J. Cell. Sci. 2008, 121, 3261–3270. [Google Scholar] [CrossRef] [Green Version]
- Virginio, C.; MacKenzie, A.; North, R.A.; Surprenant, A. Kinetics of Cell Lysis, Dye Uptake and Permeability Changes in Cells Expressing the Rat P2X7 Receptor. J. Physiol. 1999, 519, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Riedel, T.; Lozinsky, I.; Schmalzing, G.; Markwardt, F. Kinetics of P2X7 Receptor-Operated Single Channels Currents. Biophys. J. 2007, 92, 2377–2391. [Google Scholar] [CrossRef] [Green Version]
- Riedel, T.; Schmalzing, G.; Markwardt, F. Influence of Extracellular Monovalent Cations on Pore and Gating Properties of P2X7 Receptor-Operated Single-Channel Currents. Biophys. J. 2007, 93, 846–858. [Google Scholar] [CrossRef] [Green Version]
- Harkat, M.; Peverini, L.; Cerdan, A.H.; Dunning, K.; Beudez, J.; Martz, A.; Calimet, N.; Specht, A.; Cecchini, M.; Chataigneau, T.; et al. On the Permeation of Large Organic Cations through the Pore of ATP-Gated P2X Receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E3786–E3795. [Google Scholar] [CrossRef] [Green Version]
- Pippel, A.; Stolz, M.; Woltersdorf, R.; Kless, A.; Schmalzing, G.; Markwardt, F. Localization of the Gate and Selectivity Filter of the Full-Length P2X7 Receptor. Proc. Natl. Acad. Sci. USA 2017, 114, E2156–E2165. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Misaghi, S.; Newton, K.; Gilmour, L.L.; Louie, S.; Cupp, J.E.; Dubyak, G.R.; Hackos, D.; Dixit, V.M. Pannexin-1 is Required for ATP Release during Apoptosis but Not for Inflammasome Activation. J. Immunol. 2011, 186, 6553–6561. [Google Scholar] [CrossRef] [Green Version]
- Alberto, A.V.P.; Faria, R.X.; Couto, C.G.C.; Ferreira, L.G.B.; Souza, C.a.M.; Teixeira, P.C.N.; Fróes, M.M.; Alves, L.A. Is Pannexin the Pore Associated with the P2X7 Receptor? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, P.J.; Kronlage, M.; Kirschning, C.; del Rey, A.; Di Virgilio, F.; Leipziger, J.; Chessell, I.P.; Sargin, S.; Filippov, M.A.; Lindemann, O.; et al. Transient P2X7 Receptor Activation Triggers Macrophage Death Independent of Toll-Like Receptors 2 and 4, Caspase-1, and Pannexin-1 Proteins. J. Biol. Chem. 2012, 287, 10650–10663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janks, L.; Sprague, R.S.; Egan, T.M. ATP-Gated P2X7 Receptors Require Chloride Channels to Promote Inflammation in Human Macrophages. J. Immunol. 2019, 202, 883–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Hide, I.; Ido, K.; Kohsaka, S.; Inoue, K.; Nakata, Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J. Neurosci. 2004, 24, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, Z.A.; Aga, M.; Prabhu, U.; Watters, J.J.; Hall, D.J.; Bertics, P.J. The Nucleotide Receptor P2X7 Mediates Actin Reorganization and Membrane Blebbing in RAW 264.7 Macrophages Via p38 MAP Kinase and Rho. J. Leukoc. Biol. 2004, 75, 1173–1182. [Google Scholar] [CrossRef]
- Alzola, E.; Pérez-Etxebarria, A.; Kabré, E.; Fogarty, D.J.; Métioui, M.; Chaïb, N.; Macarulla, J.M.; Matute, C.; Dehaye, J.P.; Marino, A. Activation by P2X7 Agonists of Two Phospholipases A2 (PLA2) in Ductal Cells of Rat Submandibular Gland. Coupling of the Calcium-Independent PLA2 with Kallikrein Secretion. J. Biol. Chem. 1998, 273, 30208–30217. [Google Scholar] [CrossRef] [Green Version]
- Gargett, C.E.; Cornish, E.J.; Wiley, J.S. Phospholipase D Activation by P2Z-Purinoceptor Agonists in Human Lymphocytes is Dependent on Bivalent Cation Influx. Biochem. J. 1996, 313, 529–535. [Google Scholar] [CrossRef] [Green Version]
- El-Moatassim, C.; Dubyak, G.R. A Novel Pathway for the Activation of Phospholipase D by P2z Purinergic Receptors in BAC1.2F5 Macrophages. J. Biol. Chem. 1992, 267, 23664–23673. [Google Scholar]
- Kim, M.; Jiang, L.H.; Wilson, H.L.; North, R.A.; Surprenant, A. Proteomic and Functional Evidence for a P2X7 Receptor Signalling Complex. EMBO J. 2001, 20, 6347–6358. [Google Scholar] [CrossRef] [Green Version]
- Wilson, H.L.; Wilson, S.A.; Surprenant, A.; North, R.A. Epithelial Membrane Proteins Induce Membrane Blebbing and Interact with the P2X7 Receptor C Terminus. J. Biol. Chem. 2002, 277, 34017–34023. [Google Scholar] [CrossRef] [Green Version]
- Franceschini, A.; Capece, M.; Chiozzi, P.; Falzoni, S.; Sanz, J.M.; Sarti, A.C.; Bonora, M.; Pinton, P.; Di Virgilio, F. The P2X7 Receptor Directly Interacts with the NLRP3 Inflammasome Scaffold Protein. FASEB J. 2015, 29, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 Receptors Mediate NLRP3 Inflammasome-Dependent IL-1β Secretion in Response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotjumlong, P.; Bolscher, J.G.; Nazmi, K.; Reutrakul, V.; Supanchart, C.; Buranaphatthana, W.; Krisanaprakornkit, S. Involvement of the P2X7 Purinergic Receptor and C-Jun N-Terminal and Extracellular Signal-Regulated Kinases in Cyclooxygenase-2 and Prostaglandin E2 Induction by LL-37. J. Innate Immun. 2013, 5, 72–83. [Google Scholar] [CrossRef]
- Wan, M.; Soehnlein, O.; Tang, X.; van der Does, A.M.; Smedler, E.; Uhlén, P.; Lindbom, L.; Agerberth, B.; Haeggström, J.Z. Cathelicidin LL-37 Induces Time-Resolved Release of LTB4 and TXA2 by Human Macrophages and Triggers Eicosanoid Generation in Vivo. FASEB J. 2014, 28, 3456–3467. [Google Scholar] [CrossRef] [Green Version]
- Dalgarno, R.; Leduc-Pessah, H.; Pilapil, A.; Kwok, C.H.; Trang, T. Intrathecal Delivery of a Palmitoylated Peptide Targeting Y382-384 within the P2X7 Receptor Alleviates Neuropathic Pain. Mol. Pain 2018, 14. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Liu, L.; Wei, D.; Lv, Y.; Wang, G.; Xiong, W.; Wang, X.; Altaf, A.; Wang, L.; He, D.; et al. P2X7R is Involved in the Progression of Atherosclerosis by Promoting NLRP3 Inflammasome Activation. Int. J. Mol. Med. 2015, 35, 1179–1188. [Google Scholar] [CrossRef] [Green Version]
- Stachon, P.; Heidenreich, A.; Merz, J.; Hilgendorf, I.; Wolf, D.; Willecke, F.; von Garlen, S.; Albrecht, P.; Härdtner, C.; Ehrat, N.; et al. P2X7 Deficiency Blocks Lesional Inflammasome Activity and Ameliorates Atherosclerosis in Mice. Circulation 2017, 135, 2524–2533. [Google Scholar] [CrossRef]
- Gicquel, T.; Robert, S.; Loyer, P.; Victoni, T.; Bodin, A.; Ribault, C.; Gleonnec, F.; Couillin, I.; Boichot, E.; Lagente, V. IL-1β Production is Dependent on the Activation of Purinergic Receptors and NLRP3 Pathway in Human Macrophages. FASEB J. 2015, 29, 4162–4173. [Google Scholar] [CrossRef] [Green Version]
- Englezou, P.C.; Rothwell, S.W.; Ainscough, J.S.; Brough, D.; Landsiedel, R.; Verkhratsky, A.; Kimber, I.; Dearman, R.J. P2X7R Activation Drives Distinct IL-1 Responses in Dendritic Cells Compared to Macrophages. Cytokine 2015, 74, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xiao, Y.; Li, Z. P2X7 Receptor Positively Regulates MyD88-Dependent NF-κB Activation. Cytokine 2011, 55, 229–236. [Google Scholar] [CrossRef]
- Minkiewicz, J.; de Rivero Vaccari, J.P.; Keane, R.W. Human Astrocytes Express a Novel NLRP2 Inflammasome. Glia 2013, 61, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Salaro, E.; Rambaldi, A.; Falzoni, S.; Amoroso, F.S.; Franceschini, A.; Sarti, A.C.; Bonora, M.; Cavazzini, F.; Rigolin, G.M.; Ciccone, M.; et al. Involvement of the P2X7-NLRP3 Axis in Leukemic Cell Proliferation and Death. Sci. Rep. 2016, 6, 26280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Gassart, A.; Martinon, F. Pyroptosis: Caspase-11 Unlocks the Gates of Death. Immunity 2015, 43, 835–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adinolfi, E.; Callegari, M.G.; Ferrari, D.; Bolognesi, C.; Minelli, M.; Wieckowski, M.R.; Pinton, P.; Rizzuto, R.; Di Virgilio, F. Basal Activation of the P2X7 ATP Receptor Elevates Mitochondrial Calcium and Potential, Increases Cellular ATP Levels, and Promotes Serum-Independent Growth. Mol. Biol. Cell 2005, 16, 3260–3272. [Google Scholar] [CrossRef] [Green Version]
- Coddou, C.; Stojilkovic, S.S.; Huidobro-Toro, J.P. Allosteric Modulation of ATP-Gated P2X Receptor Channels. Rev. Neurosci. 2011, 22, 335–354. [Google Scholar] [CrossRef] [Green Version]
- Acuña-Castillo, C.; Coddou, C.; Bull, P.; Brito, J.; Huidobro-Toro, J.P. Differential Role of Extracellular Histidines in Copper, Zinc, Magnesium and Proton Modulation of the P2X7 Purinergic Receptor. J. Neurochem. 2007, 101, 17–26. [Google Scholar] [CrossRef]
- Virginio, C.; Church, D.; North, R.A.; Surprenant, A. Effects of Divalent Cations, Protons and Calmidazolium at the Rat P2X7 Receptor. Neuropharmacology 1997, 36, 1285–1294. [Google Scholar] [CrossRef]
- Zhao, Q.; Logothetis, D.E.; Séguéla, P. Regulation of ATP-Gated P2X Receptors by Phosphoinositides. Pflugers Arch. 2007, 455, 181–185. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, M.; Ting, A.T.; Logothetis, D.E. PIP(2) Regulates the Ionic Current of P2X Receptors and P2X(7) Receptor-Mediated Cell Death. Channels 2007, 1, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Michel, A.D.; Fonfria, E. Agonist Potency at P2X7 Receptors is Modulated by Structurally Diverse Lipids. Br. J. Pharmacol. 2007, 152, 523–537. [Google Scholar] [CrossRef] [Green Version]
- Moura, G.; Lucena, S.V.; Lima, M.A.; Nascimento, F.D.; Gesteira, T.F.; Nader, H.B.; Paredes-Gamero, E.J.; Tersariol, I. Post-Translational Allosteric Activation of the P2X7 Receptor through Glycosaminoglycan Chains of CD44 Proteoglycans. Cell Death Dis. 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denlinger, L.C.; Fisette, P.L.; Sommer, J.A.; Watters, J.J.; Prabhu, U.; Dubyak, G.R.; Proctor, R.A.; Bertics, P.J. Cutting Edge: The Nucleotide Receptor P2X7 Contains Multiple Protein- and Lipid-Interaction Motifs Including a Potential Binding Site for Bacterial Lipopolysaccharide. J. Immunol. 2001, 167, 1871–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Schwarz, N.; Brass, A.; Seman, M.; Haag, F.; Koch-Nolte, F.; Schilling, W.P.; Dubyak, G.R. Differential Regulation of P2X7 Receptor Activation by Extracellular Nicotinamide Adenine Dinucleotide and Ecto-ADP-Ribosyltransferases in Murine Macrophages and T Cells. J. Immunol. 2009, 183, 578–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.J.; Boumechache, M.; Robinson, L.E.; Marschall, V.; Gorecki, D.C.; Masin, M.; Murrell-Lagnado, R.D. Splice Variants of the P2X7 Receptor Reveal Differential Agonist Dependence and Functional Coupling with Pannexin-1. J. Cell. Sci. 2012, 125, 3776–3789. [Google Scholar] [CrossRef] [Green Version]
- Nörenberg, W.; Hempel, C.; Urban, N.; Sobottka, H.; Illes, P.; Schaefer, M. Clemastine Potentiates the Human P2X7 Receptor by Sensitizing it to Lower ATP Concentrations. J. Biol. Chem. 2011, 286, 11067–11081. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, M.; Mori, T.; Nishikawa, K.; Sawada, M.; Kuno, M.; Asada, A. The Effects of General Anesthetics on P2X7 and P2Y Receptors in a Rat Microglial Cell Line. Anesth. Analg. 2007, 104, 1136–1144. [Google Scholar] [CrossRef]
- Ferrari, D.; Pizzirani, C.; Gulinelli, S.; Callegari, G.; Chiozzi, P.; Idzko, M.; Panther, E.; Di Virgilio, F. Modulation of P2X7 Receptor Functions by Polymyxin B: Crucial Role of the Hydrophobic Tail of the Antibiotic Molecule. Br. J. Pharmacol. 2007, 150, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Bidula, S.M.; Cromer, B.A.; Walpole, S.; Angulo, J.; Stokes, L. Mapping a Novel Positive Allosteric Modulator Binding Site in the Central Vestibule Region of Human P2X7. Sci. Rep. 2019, 9, 3231. [Google Scholar] [CrossRef]
- Dhuna, K.; Felgate, M.; Bidula, S.M.; Walpole, S.; Bibic, L.; Cromer, B.A.; Angulo, J.; Sanderson, J.; Stebbing, M.J.; Stokes, L. Ginsenosides Act as Positive Modulators of P2X4 Receptors. Mol. Pharmacol. 2019, 95, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Fischer, W.; Urban, N.; Immig, K.; Franke, H.; Schaefer, M. Natural Compounds with P2X7 Receptor-Modulating Properties. Purinergic Signal. 2014, 10, 313–326. [Google Scholar] [CrossRef]
- Nörenberg, W.; Sobottka, H.; Hempel, C.; Plötz, T.; Fischer, W.; Schmalzing, G.; Schaefer, M. Positive Allosteric Modulation by Ivermectin of Human but Not Murine P2X7 Receptors. Br. J. Pharmacol. 2012, 167, 48–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, A.D.; Chambers, L.J.; Walter, D.S. Negative and Positive Allosteric Modulators of the P2X(7) Receptor. Br. J. Pharmacol. 2008, 153, 737–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virginio, C.; MacKenzie, A.; Rassendren, F.A.; North, R.A.; Surprenant, A. Pore Dilation of Neuronal P2X Receptor Channels. Nat. Neurosci. 1999, 2, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bidula, S.; Dhuna, K.; Helliwell, R.; Stokes, L. Positive Allosteric Modulation of P2X7 Promotes Apoptotic Cell Death over Lytic Cell Death Responses in Macrophages. Cell Death Dis. 2019, 10, 882. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Giuliani, A.L. Purinergic Signalling in Autoimmunity: A Role for the P2X7R in Systemic Lupus Erythematosus? Biomed. J. 2016, 39, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, S.; Akhtar, U.; Naseem, S.; Khalid, M.; Mazhar, M.; Parvaiz, F.; Khaliq, S. Ionotropic Purinergic Receptors P2X4 and P2X7: Proviral or Antiviral? An Insight into P2X Receptor Signaling and Hepatitis C Virus Infection. Viral Immunol. 2016, 29, 401–408. [Google Scholar] [CrossRef]
- Alvarez, A.; Rios-Navarro, C.; Blanch-Ruiz, M.A.; Collado-Diaz, V.; Andujar, I.; Martinez-Cuesta, M.A.; Orden, S.; Esplugues, J.V. Abacavir Induces Platelet-Endothelium Interactions by Interfering with Purinergic Signalling: A Step from Inflammation to Thrombosis. Antivir. Res. 2017, 141, 179–185. [Google Scholar] [CrossRef]
- Esplugues, J.V.; De Pablo, C.; Collado-Diaz, V.; Hernandez, C.; Orden, S.; Alvarez, A. Interference with Purinergic Signalling: An Explanation for the Cardiovascular Effect of Abacavir? AIDS 2016, 30, 1341–1351. [Google Scholar] [CrossRef]
- Alvarez, A.; Orden, S.; Andujar, I.; Collado-Diaz, V.; Nunez-Delgado, S.; Galindo, M.J.; Estrada, V.; Apostolova, N.; Esplugues, J.V. Cardiovascular Toxicity of Abacavir: A Clinical Controversy in Need of a Pharmacological Explanation. AIDS 2017, 31, 1781–1795. [Google Scholar] [CrossRef]
- Collado-Diaz, V.; Andujar, I.; Sanchez-Lopez, A.; Orden, S.; Blanch-Ruiz, M.A.; Martinez-Cuesta, M.A.; Blas-Garcia, A.; Esplugues, J.V.; Alvarez, A. Abacavir Induces Arterial Thrombosis in a Murine Model. J. Infect. Dis. 2018, 218, 228–233. [Google Scholar] [CrossRef]
- Hechler, B.; Gachet, C. Purinergic Receptors in Thrombosis and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2307–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperlagh, B.; Illes, P. P2X7 Receptor: An Emerging Target in Central Nervous System Diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, L.; Li, L.; Chen, L. The P2X7 Purinergic Receptor: An Emerging Therapeutic Target in Cardiovascular Diseases. Clin. Chim. Acta 2018, 479, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Minns, M.S.; Trinkaus-Randall, V. Purinergic signaling in corneal wound healing. J. Ocular Pharmacol. Therap. 2016, 32, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Williams, D.R.; Lee, J.H.; Lee, S.D.; Lee, J.H.; Ko, H.; Lee, G.E.; Kim, S.; Lee, J.M.; Abdelrahman, A.; et al. Potent Suppressive Effects of 1-Piperidinylimidazole Based Novel P2X7 Receptor Antagonists on Cancer Cell Migration and Invasion. J. Med. Chem. 2016, 59, 7410–7430. [Google Scholar] [CrossRef]
- Ferrari, D.; Malavasi, F.; Antonioli, L. A Purinergic Trail for Metastases. Trends Pharmacol. Sci. 2017, 38, 277–290. [Google Scholar] [CrossRef]
- Lara, R.; Adinolfi, E.; Harwood, C.A.; Philpott, M.; Barden, J.A.; Di Virgilio, F.; McNulty, S. P2X7 in Cancer: From Molecular Mechanisms to Therapeutics. Front. Pharmacol. 2020, 11, 793. [Google Scholar] [CrossRef]
- Roger, S.; Jelassi, B.; Coullin, I.; Pelegrin, P.; Besson, P.; Jiang, L.H. Understanding the roles of P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim. Biophys. Acta 2015, 1848, 2584–2602. [Google Scholar] [CrossRef] [Green Version]
- Jelassi, B.; Anchelin, M.; Chamouton, J.; Cayuela, M.L.; Clarysse, L.; Li, J.; Gore, J.; Jiang, L.H.; Roger, S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 2013, 34, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Jelassi, B.; Chantome, A.; Alcaraz-Pérez, F.; Baroja-Mazo, A.; Cayuela, M.L.; Pelegrin, P.; Suprenant, A.; Roger, S. P2X(7) receptor activation enhances SK3 channels-and cysteine cathepsin-dependent cancer cells invasiveness. Oncogene 2011, 30, 2108–2122. [Google Scholar] [CrossRef] [Green Version]
- Adinofi, E.; Melchiorri, L.; Falzoni, S.; Chiozzi, P.; Morelli, A.; Tieghi, A.; Cuneo, A.; Castoldi, G.; Di Virgilio, F.; Baricordi, O.R. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood 2002, 99, 706–708. [Google Scholar] [CrossRef]
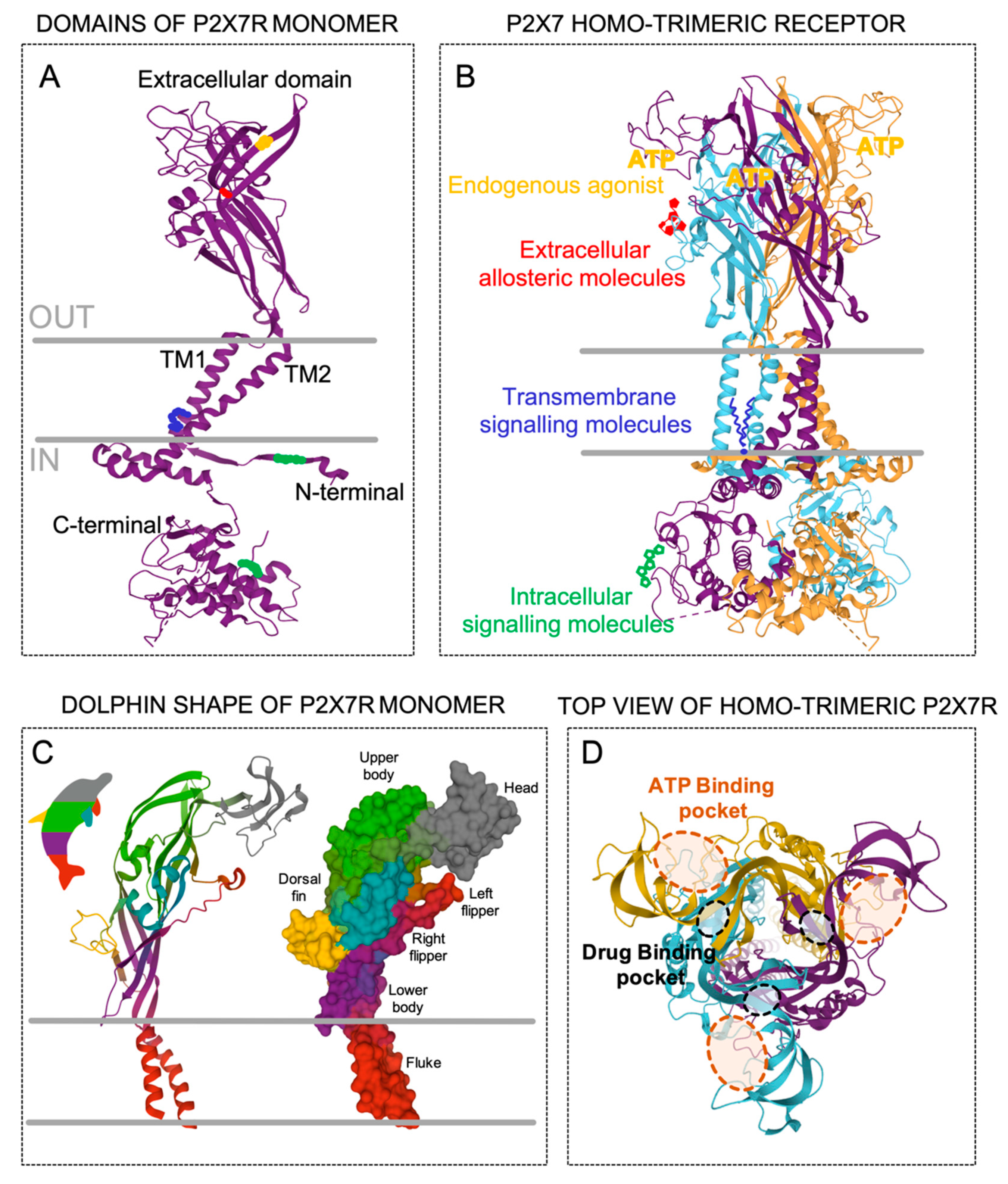
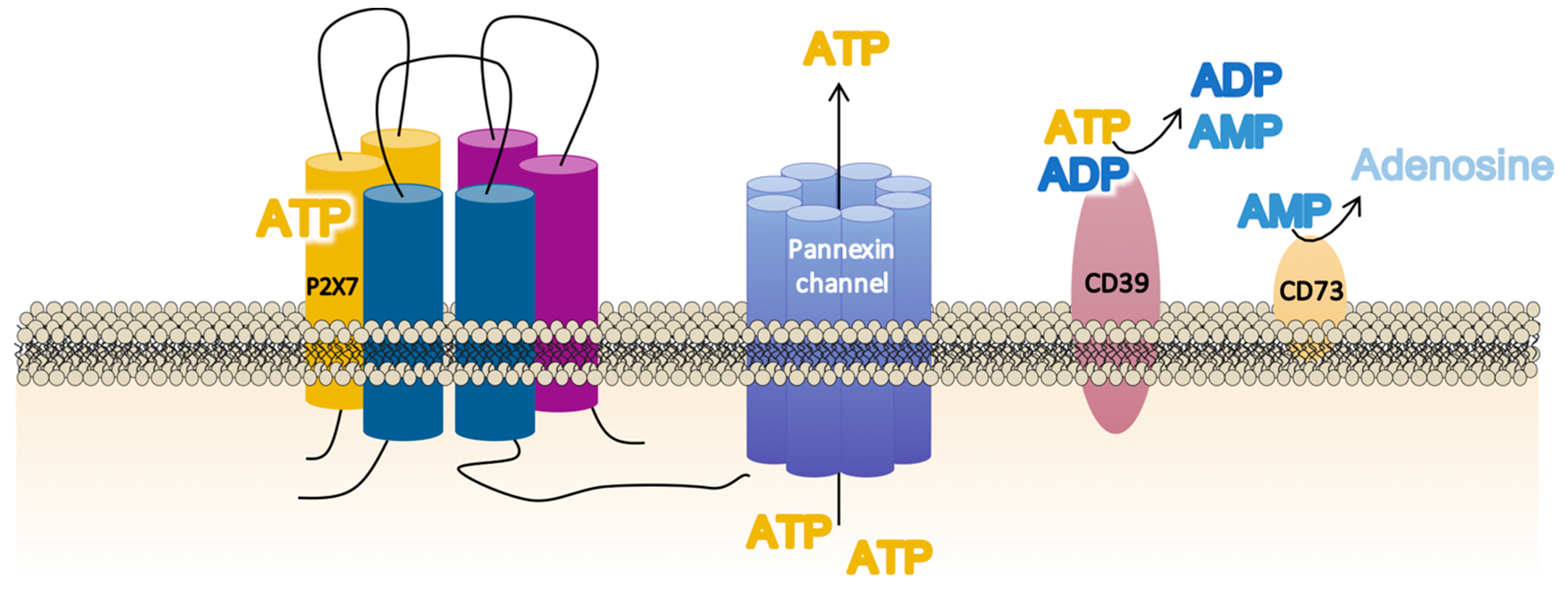
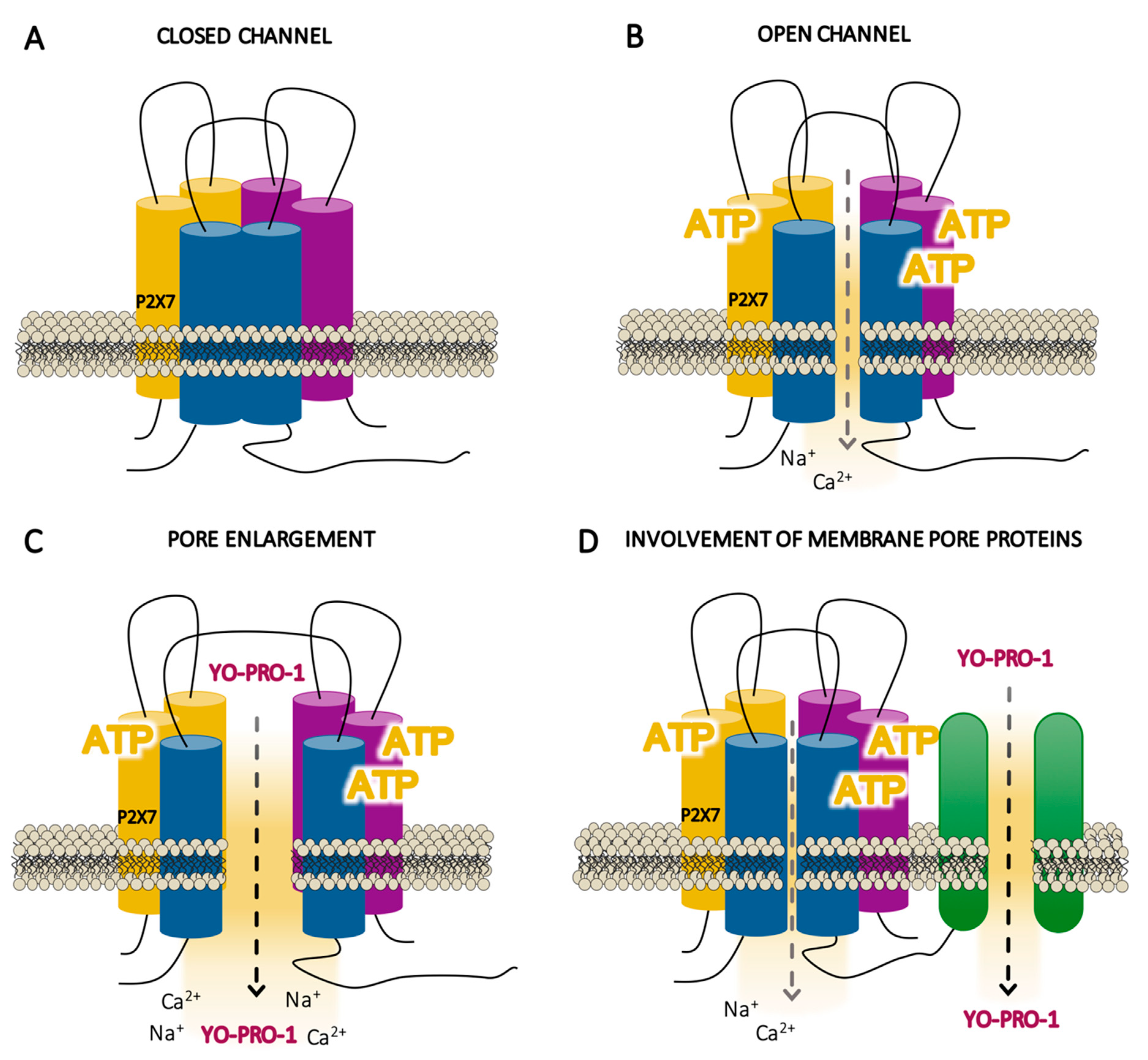
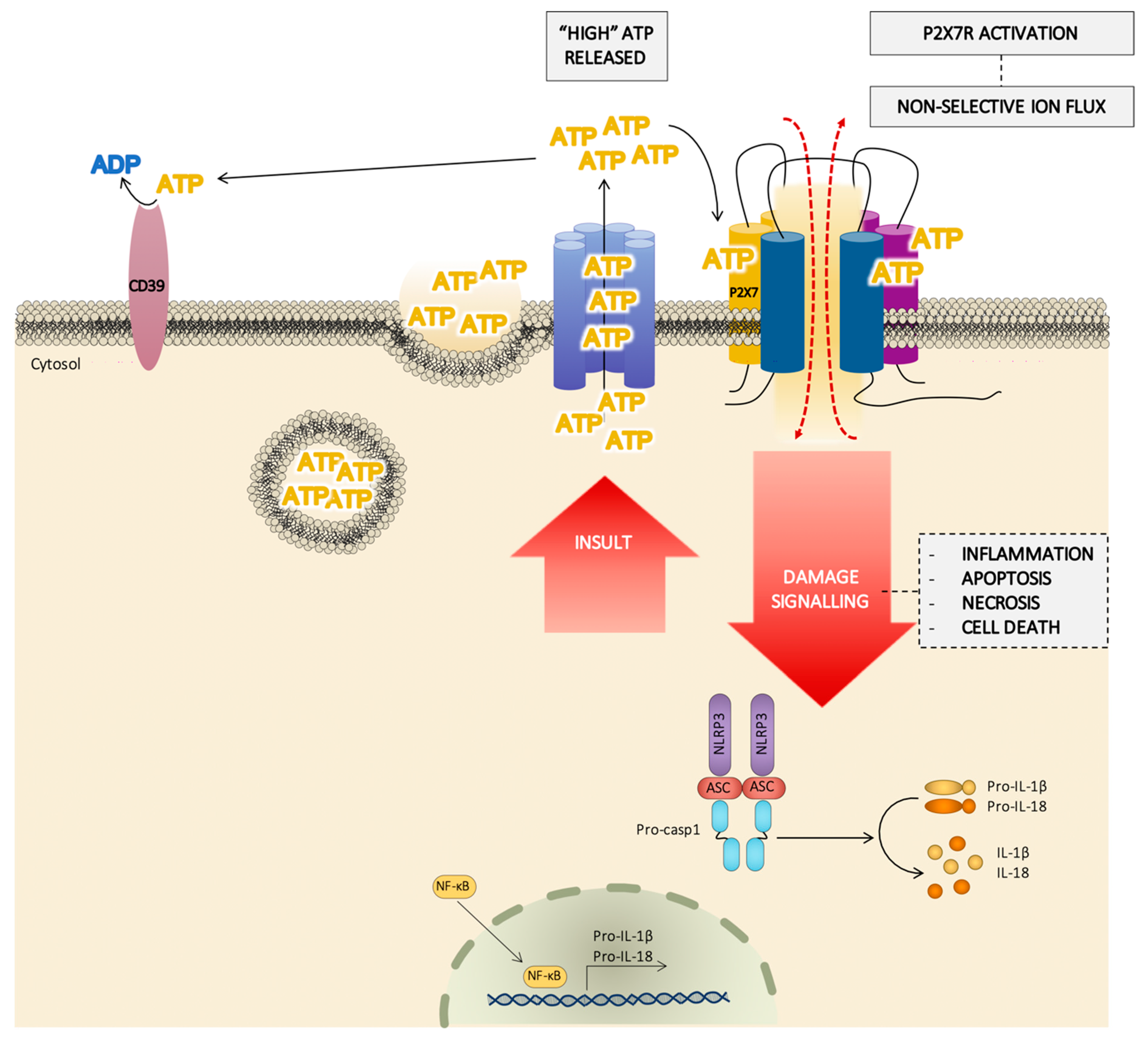
| Endogenous Molecules | P2X7R Modulation | Specie/Cell Type | References |
| Divalent cations Protons | (−) | HEK-293 transfected with rat P2X7R | [136,153] |
| Phosphoinositides | (+) | HEK transfected Macrophage cell line Primary T cells | [139] |
| Lysophosphatidyl-choline PLA2 lysolipids | (+) | HEK-293 expressing rat, mouse or human recombinant P2X7R | [140] |
| Glycosaminoglycans | (+) | CHO-K1 cell line | [141] |
| Lipoglycans * | (+) | Murine RAW 264.7 macrophages | [142] |
| Nicotinamide adenine dinucleotide (NAD) | (+) | Murine BW5147 T lymphoma Murine bone marrow-derived macrophages HEK 293 transfected with murine P2X7R | [143] |
| Drug Name | P2X7R Modulation | Specie/Cell Type | References |
| Clemastine | (+) S | HEK-293 transfected with human P2X7R Human-monocyte-derived macrophages Murine bone marrow-derived macrophages | [145] |
| Tenidap | (+) E/S | J774 mouse macrophage cell line | [35] |
| Propofol Ketamine | (+) | GMI-R1 rat microglia cell line | [146] |
| Polymyxin B | (+) E/S | HEK-293 transfected with P2X7R K-562 erythroid transfected with P2X7R J-774 mouse macrophages Human-monocyte-derived macrophages Lymphocytes from lymphocytic leukemia | [36] |
| Ginsenoside | (+) E/S | J774 mouse macrophage cell line HEK-293 Macrophages from C57BL/6 mice | [37,148,149,154] |
| Agelasine Garcinolic acid | (+) | HEK-293 transfected with human P2X7R A-375 human melanoma cell line | [150] |
| Ivermectin | (+) E/S | Human-monocyte-derived macrophages | [151] |
| GW791343 | (+) E/S (−) | HEK-293 transfected with rat P2X7R HEK-293 transfected with human P2X7R | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cuesta, M.Á.; Blanch-Ruiz, M.A.; Ortega-Luna, R.; Sánchez-López, A.; Álvarez, Á. Structural and Functional Basis for Understanding the Biological Significance of P2X7 Receptor. Int. J. Mol. Sci. 2020, 21, 8454. https://doi.org/10.3390/ijms21228454
Martínez-Cuesta MÁ, Blanch-Ruiz MA, Ortega-Luna R, Sánchez-López A, Álvarez Á. Structural and Functional Basis for Understanding the Biological Significance of P2X7 Receptor. International Journal of Molecular Sciences. 2020; 21(22):8454. https://doi.org/10.3390/ijms21228454
Chicago/Turabian StyleMartínez-Cuesta, María Ángeles, María Amparo Blanch-Ruiz, Raquel Ortega-Luna, Ainhoa Sánchez-López, and Ángeles Álvarez. 2020. "Structural and Functional Basis for Understanding the Biological Significance of P2X7 Receptor" International Journal of Molecular Sciences 21, no. 22: 8454. https://doi.org/10.3390/ijms21228454
APA StyleMartínez-Cuesta, M. Á., Blanch-Ruiz, M. A., Ortega-Luna, R., Sánchez-López, A., & Álvarez, Á. (2020). Structural and Functional Basis for Understanding the Biological Significance of P2X7 Receptor. International Journal of Molecular Sciences, 21(22), 8454. https://doi.org/10.3390/ijms21228454






