Intermittent Fasting Aggravates Lupus Nephritis through Increasing Survival and Autophagy of Antibody Secreting Cells in MRL/lpr Mice
Abstract
1. Introduction
2. Results
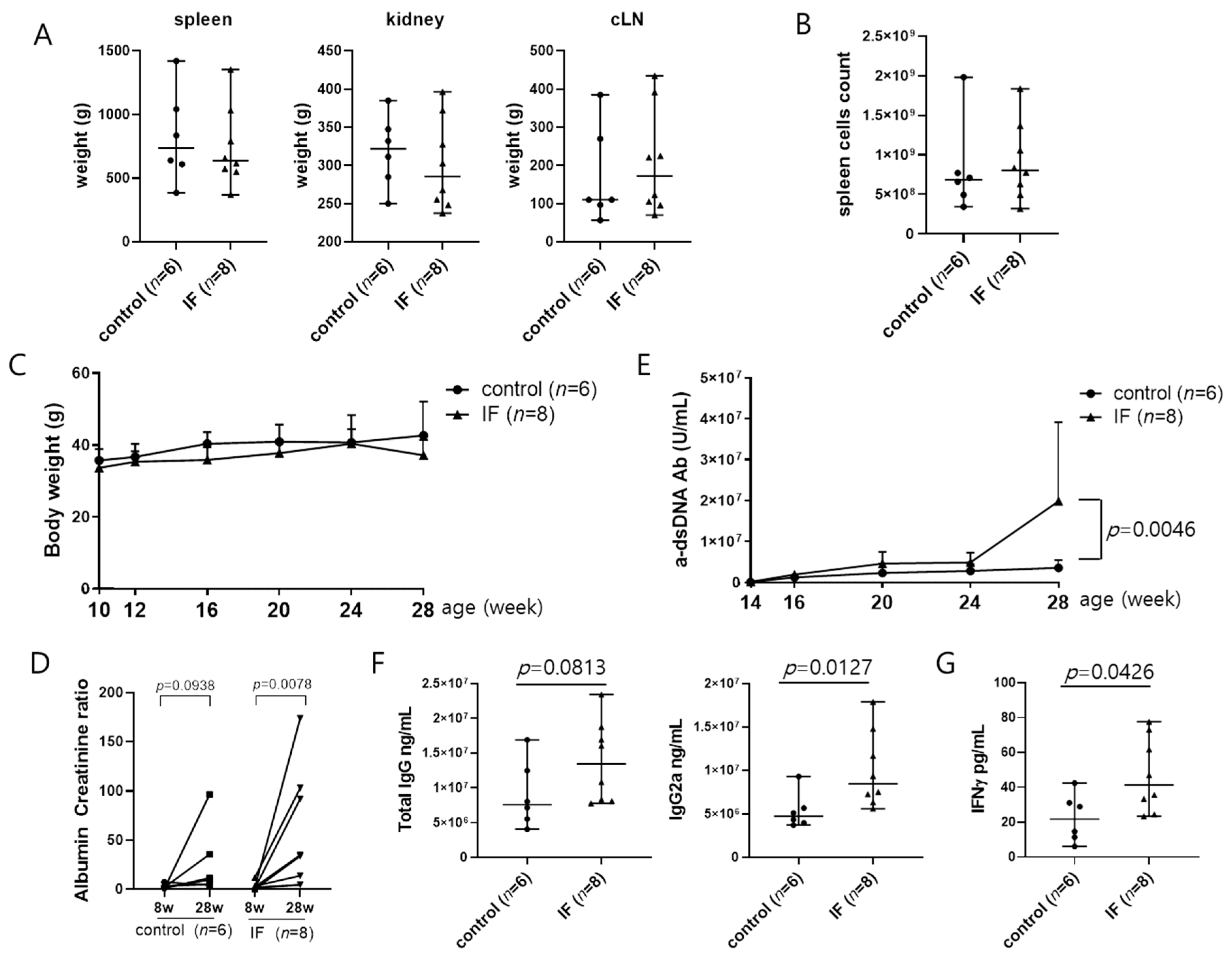
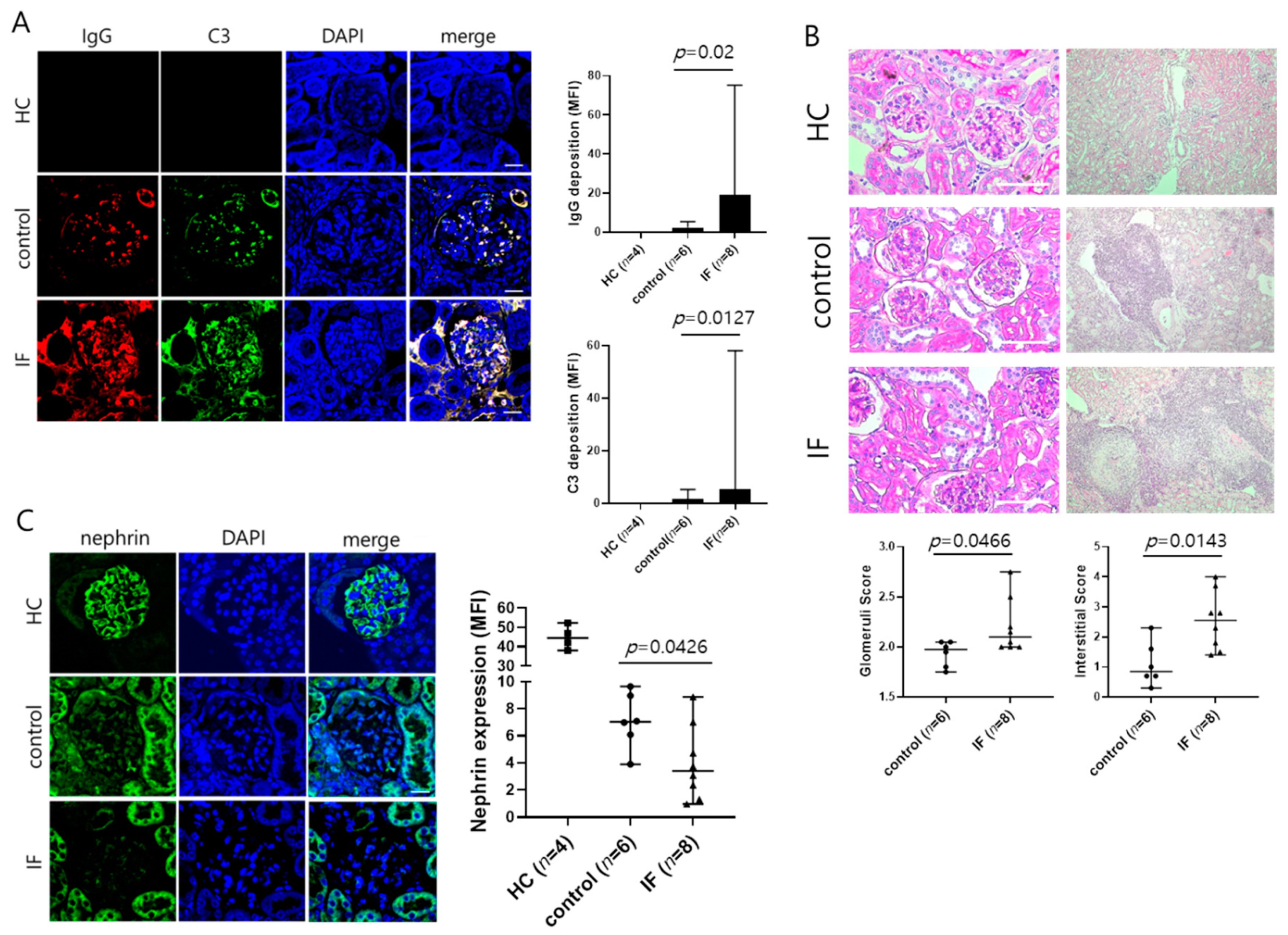
2.1. IF Increases Serum Anti-dsDNA Antibody Concentration and Aggravates Lupus Nephritis in MRL/lpr Mice
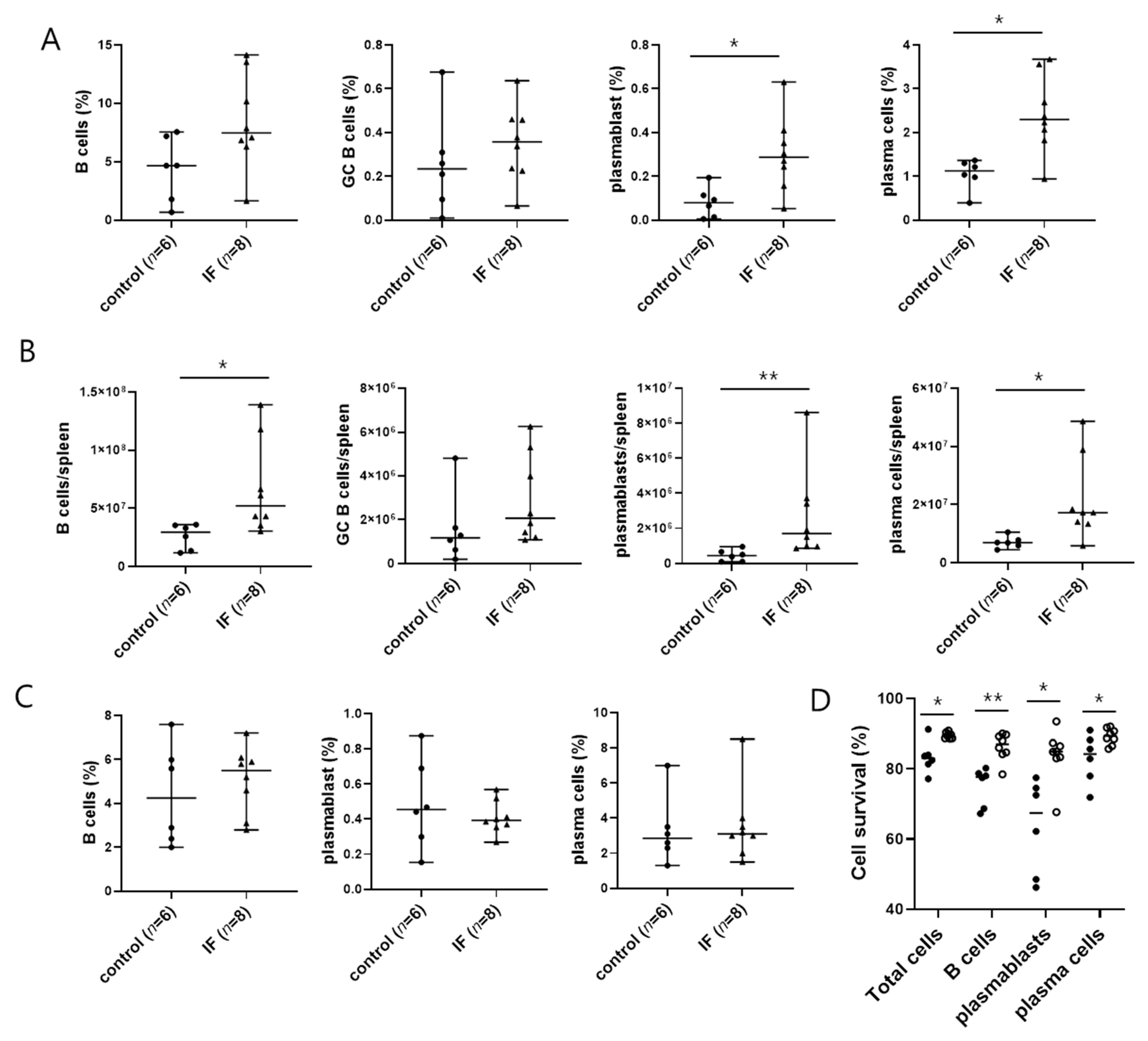
2.2. IF Increases the Abundance of Spleen Plasmablasts and Plasma Cells
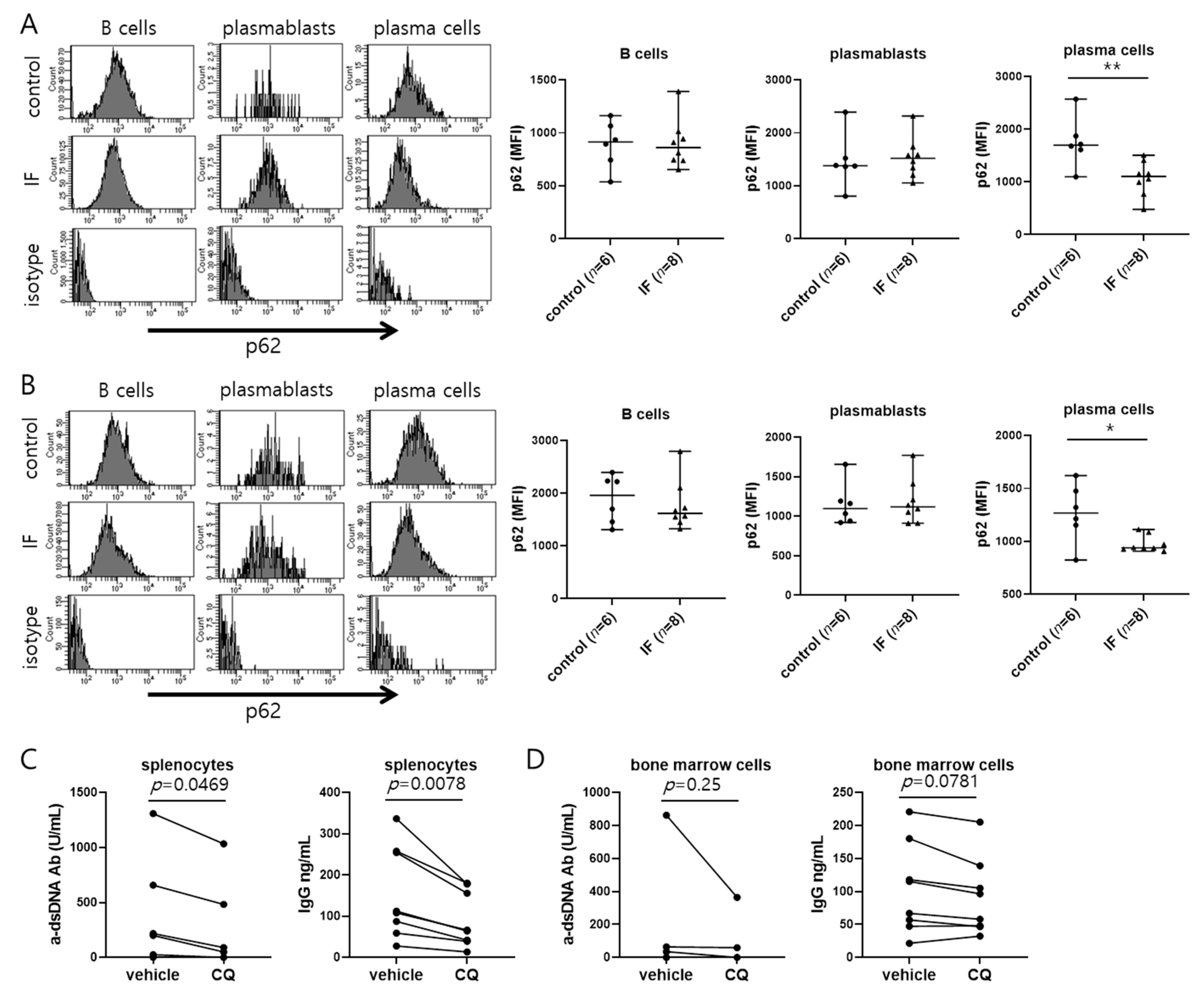
2.3. IF Elevates Autophagy in Plasma Cells
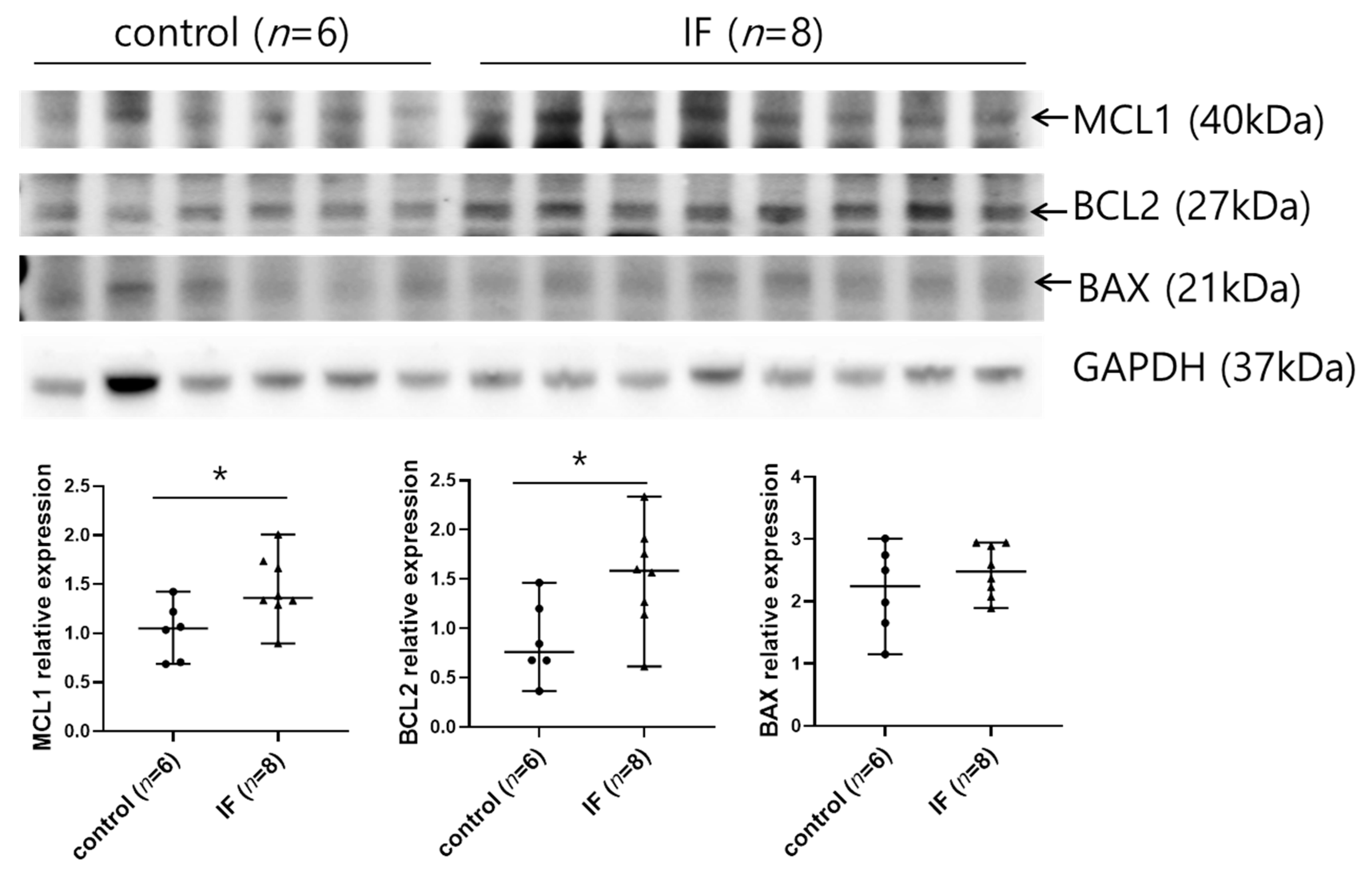
2.4. IF Increases the Expression of Antiapoptotic Factors in Spleen
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Measurement of Serum Antibodies and Cytokine
4.3. Measurement of Urine Albumin to Creatinine Ratio
4.4. Immunofluorescence Microscopy
4.5. Histologic Assessment of Kidney
4.6. Flow Cytometry
4.7. Chloroquine Treatment of Cultured Cells
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bertsias, G.; Cervera, R.; Boumpas, D.T. Systemic lupus erythematosus: Pathogenesis and clinical features. Eular. Textb. Rheum. Dis. 2012, 5, 476–505. [Google Scholar]
- Hahn, B.H. Antibodies to DNA. N. Engl. J. Med. 1998, 338, 1359–1368. [Google Scholar] [CrossRef]
- Durcan, L.; O’Dwyer, T.; Petri, M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019, 393, 2332–2343. [Google Scholar] [CrossRef]
- Liossis, S.; Kovacs, B.; Dennis, G.; Kammer, G.M.; Tsokos, G.C. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J. Clin. Investig. 1996, 98, 2549–2557. [Google Scholar] [CrossRef]
- Cappione, A.; Anolik, J.H.; Pugh-Bernard, A.; Barnard, J.; Dutcher, P.; Silverman, G.; Sanz, I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Investig. 2005, 115, 3205–3216. [Google Scholar] [CrossRef] [PubMed]
- Desai-Mehta, A.; Lu, L.; Ramsey-Goldman, R.; Datta, S.K. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J. Clin. Investig. 1996, 97, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Du, W.; Wang, X.; Yuan, S.; Cai, X.; Liu, D.; Li, J.; Lu, L. Multiple functions of B cells in the pathogenesis of systemic lupus erythematosus. Int. J. Mol. Sci. 2019, 20, 6021. [Google Scholar] [CrossRef]
- Katewa, A.; Wang, Y.; Hackney, J.A.; Huang, T.; Suto, E.; Ramamoorthi, N.; Austin, C.D.; Bremer, M.; Chen, J.Z.; Crawford, J.J. Btk-specific inhibition blocks pathogenic plasma cell signatures and myeloid cell–associated damage in IFNα-driven lupus nephritis. JCI Insight 2017, 2, e90111. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, S.; Bai, B.; Zhang, L.; Xue, L.; Lin, Z.; Yang, X.; Zhu, F.; He, P.; Tang, W. Therapeutic effects of the artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition of TLR-triggered B-cell activation and plasma cell formation. Cell. Mol. Immunol. 2016, 13, 379–390. [Google Scholar] [CrossRef]
- Lin, W.; Seshasayee, D.; Lee, W.P.; Caplazi, P.; McVay, S.; Suto, E.; Nguyen, A.; Lin, Z.; Sun, Y.; DeForge, L. Dual B cell immunotherapy is superior to individual anti-CD20 depletion or BAFF blockade in murine models of spontaneous or accelerated lupus. Arthritis Rheumatol. 2015, 67, 215–224. [Google Scholar] [CrossRef]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Luan, X.; Zhao, W.; Chandrasekar, B.; Fernandes, G. Calorie restriction modulates lymphocyte subset phenotype and increases apoptosis in MRLlpr mice. Immunol. Lett. 1995, 47, 181–186. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Longo, V. Fasting vs dietary restriction in cellular protection and cancer treatment: From model organisms to patients. Oncogene 2011, 30, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Kristan, D.M. Calorie restriction and susceptibility to intact pathogens. Age 2008, 30, 147. [Google Scholar] [CrossRef]
- Harvie, M.; Howell, A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—A narrative review of human and animal evidence. Behav. Sci. 2017, 7, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Matarese, G.; La Cava, A. Cutting edge: Fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J. Immunol. 2012, 188, 2070–2073. [Google Scholar] [CrossRef]
- Fann, D.Y.-W.; Santro, T.; Manzanero, S.; Widiapradja, A.; Cheng, Y.-L.; Lee, S.-Y.; Chunduri, P.; Jo, D.-G.; Stranahan, A.M.; Mattson, M.P. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp. Neurol. 2014, 257, 114–119. [Google Scholar] [CrossRef]
- Razeghi, J.S.; Ghaemi, A.; Alizadeh, A.; Sabetghadam, F.; Moradi, T.H.; Togha, M. Effects of intermittent fasting on experimental autoimune encephalomyelitis in C57BL/6 mice. Iran J. Allergy Asthma Immunol. 2016, 15, 212–219. [Google Scholar]
- Zenz, G.; Jačan, A.; Reichmann, F.; Farzi, A.; Holzer, P. Intermittent fasting exacerbates the acute immune and behavioral sickness response to the viral mimic poly (I: C) in mice. Front. Neurosci. 2019, 13, 359. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K. Inflammatory cytokines in systemic lupus erythematosus. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Butler, A.E.; Barreto, G.E.; Sahebkar, A. The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res. Rev. 2018, 47, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Codogno, P.; Meijer, A. Autophagy and signaling: Their role in cell survival and cell death. Cell Death Differ. 2005, 12, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.J.; Ellinghaus, U.; Cortini, A.; Stranks, A.; Simon, A.K.; Botto, M.; Vyse, T.J. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann. Rheum. Dis. 2015, 74, 912–920. [Google Scholar] [CrossRef]
- Pengo, N.; Scolari, M.; Oliva, L.; Milan, E.; Mainoldi, F.; Raimondi, A.; Fagioli, C.; Merlini, A.; Mariani, E.; Pasqualetto, E. Plasma cells require autophagy for sustainable immunoglobulin production. Nat. Immunol. 2013, 14, 298–305. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.-s.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.-I.; Ezaki, J.; Murata, S. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Vikström, I.B.; Slomp, A.; Carrington, E.M.; Moesbergen, L.M.; Chang, C.; Kelly, G.L.; Glaser, S.P.; Jansen, J.M.; Leusen, J.H.; Strasser, A. MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL. Cell Death Dis. 2016, 7, e2345. [Google Scholar] [CrossRef]
- Niiya, T.; Akbar, S.M.F.; Yoshida, O.; Miyake, T.; Matsuura, B.; Murakami, H.; Abe, M.; Hiasa, Y.; Onji, M. Impaired dendritic cell function resulting from chronic undernutrition disrupts the antigen-specific immune response in mice. J. Nutr. 2007, 137, 671–675. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of childhood malnutrition on host defense and infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef] [PubMed]
- Kuballa, P.; Nolte, W.M.; Castoreno, A.B.; Xavier, R.J. Autophagy and the immune system. Annu. Rev. Immunol. 2012, 30, 611–646. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wu, H.; Chen, Y.; Zhang, J.; Zheng, M.; Chen, G.; Li, L.; Lu, Q. The therapeutic and pathogenic role of autophagy in autoimmune diseases. Front. Immunol. 2018, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yue, Y.; Dong, C.; Shi, Y.; Xiong, S. Blockade of macrophage autophagy ameliorates activated lymphocytes-derived DNA induced murine lupus possibly via inhibition of proinflammatory cytokine production. Clin. Exp. Rheumatol. 2014, 32, 705–714. [Google Scholar] [PubMed]
- Weindel, C.G.; Richey, L.J.; Bolland, S.; Mehta, A.J.; Kearney, J.F.; Huber, B.T. B cell autophagy mediates TLR7-dependent autoimmunity and inflammation. Autophagy 2015, 11, 1010–1024. [Google Scholar] [CrossRef]
- Balomenos, D.; Rumold, R.; Theofilopoulos, A.N. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J. Clin. Investig. 1998, 101, 364–371. [Google Scholar] [CrossRef]
- Bossie, A.; Vitetta, E.S. IFN-γ enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: Implications for the role of IFN-γ in class switching. Cell. Immunol. 1991, 135, 95–104. [Google Scholar] [CrossRef]
- Snapper, C.M.; Peschel, C.; Paul, W.E. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 1988, 140, 2121–2127. [Google Scholar]
- Su, L.; David, M. Inhibition of B cell receptor-mediated apoptosis by IFN. J. Immunol. 1999, 162, 6317–6321. [Google Scholar]
- Hasbold, J.; Hong, J.S.-Y.; Kehry, M.R.; Hodgkin, P.D. Integrating signals from IFN-γ and IL-4 by B cells: Positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J. Immunol. 1999, 163, 4175–4181. [Google Scholar]
- Jackson, S.W.; Jacobs, H.M.; Arkatkar, T.; Dam, E.M.; Scharping, N.E.; Kolhatkar, N.S.; Hou, B.; Buckner, J.H.; Rawlings, D.J. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J. Exp. Med. 2016, 213, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, L.; Tomasi, C.; Greco, M. Fasting-induced apoptosis in rat liver is blocked by cycloheximide. Eur. J. Cell Biol. 1999, 78, 573–579. [Google Scholar] [CrossRef]
- Park, J.M.; Kakimoto, T.; Kuroki, T.; Shiraishi, R.; Fujise, T.; Iwakiri, R.; Fujimoto, K. Suppression of intestinal mucosal apoptosis by ghrelin in fasting rats. Exp. Biol. Med. 2008, 233, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Uchida, H.; Yokote, T.; Ohtake, K.; Kobayashi, J. Fasting-induced intestinal apoptosis is mediated by inducible nitric oxide synthase and interferon-γ in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G916–G926. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Yu, K.S.; Bak, D.H.; Lee, J.H.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; Kim, J.J.; Han, S.Y. Intermittent fasting is neuroprotective in focal cerebral ischemia by minimizing autophagic flux disturbance and inhibiting apoptosis. Exp. Ther. Med. 2016, 12, 3021–3028. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-Y.; Park, K.T.; Jang, S.Y.; Lee, K.H.; Byun, J.-Y.; Suh, K.H.; Lee, Y.-M.; Kim, Y.H.; Hwang, K.W. HM71224, a selective Bruton’s tyrosine kinase inhibitor, attenuates the development of murine lupus. Arthrit. Res. Ther. 2017, 19, 1–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-M.; Lee, J.; Jang, S.G.; Song, Y.; Kim, M.; Lee, J.; Cho, M.-L.; Kwok, S.-K.; Park, S.-H. Intermittent Fasting Aggravates Lupus Nephritis through Increasing Survival and Autophagy of Antibody Secreting Cells in MRL/lpr Mice. Int. J. Mol. Sci. 2020, 21, 8477. https://doi.org/10.3390/ijms21228477
Hong S-M, Lee J, Jang SG, Song Y, Kim M, Lee J, Cho M-L, Kwok S-K, Park S-H. Intermittent Fasting Aggravates Lupus Nephritis through Increasing Survival and Autophagy of Antibody Secreting Cells in MRL/lpr Mice. International Journal of Molecular Sciences. 2020; 21(22):8477. https://doi.org/10.3390/ijms21228477
Chicago/Turabian StyleHong, Seung-Min, Jaeseon Lee, Se Gwang Jang, Youngseok Song, Minjun Kim, Jennifer Lee, Mi-La Cho, Seung-Ki Kwok, and Sung-Hwan Park. 2020. "Intermittent Fasting Aggravates Lupus Nephritis through Increasing Survival and Autophagy of Antibody Secreting Cells in MRL/lpr Mice" International Journal of Molecular Sciences 21, no. 22: 8477. https://doi.org/10.3390/ijms21228477
APA StyleHong, S.-M., Lee, J., Jang, S. G., Song, Y., Kim, M., Lee, J., Cho, M.-L., Kwok, S.-K., & Park, S.-H. (2020). Intermittent Fasting Aggravates Lupus Nephritis through Increasing Survival and Autophagy of Antibody Secreting Cells in MRL/lpr Mice. International Journal of Molecular Sciences, 21(22), 8477. https://doi.org/10.3390/ijms21228477




