Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends
Abstract
:1. Introduction
2. Natural Health Products and Natural Compounds Used in the Treatment of Breast Cancer
2.1. Prevalence Statistics, Prognosis, and Downsides of Conventional Treatments
2.2. Bioactive Compounds
3. Natural Health Products and Natural Compounds Used in the Treatment of Melanoma
3.1. Prevalence Statistics, Prognosis, and Downsides of Conventional Treatments
3.2. Anticancer Effects of Natural Compounds In-Vitro and In-Vivo
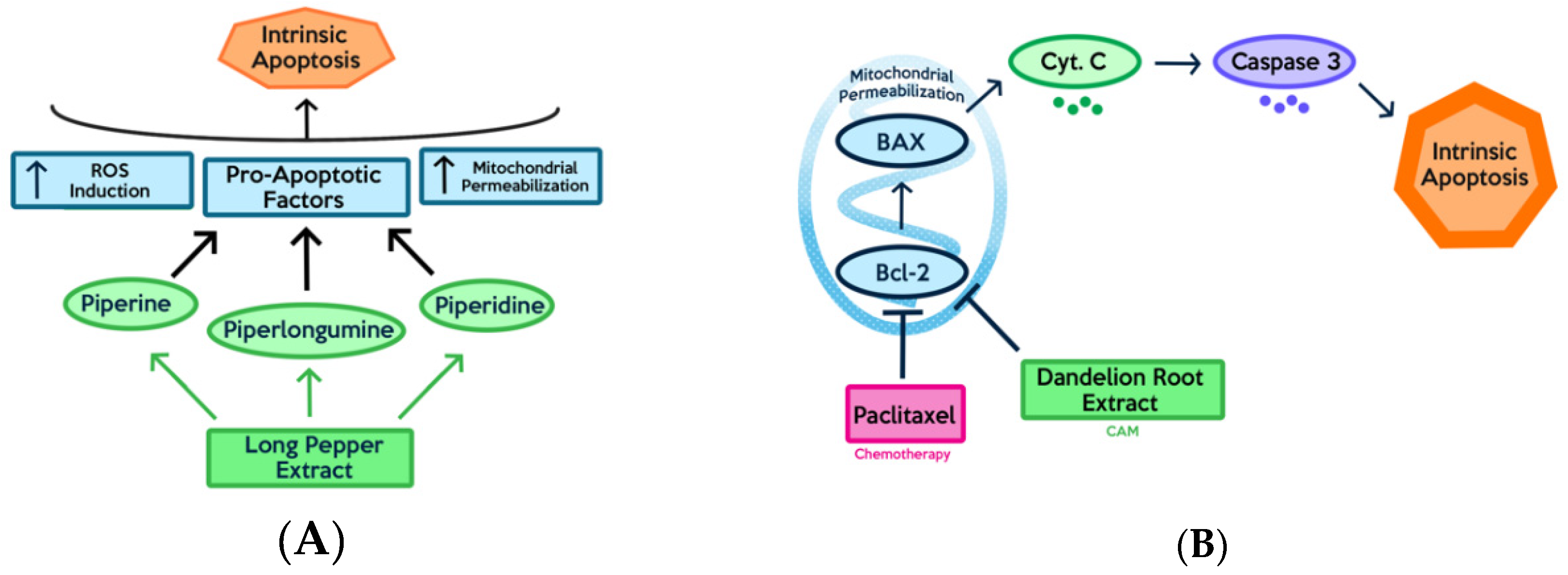
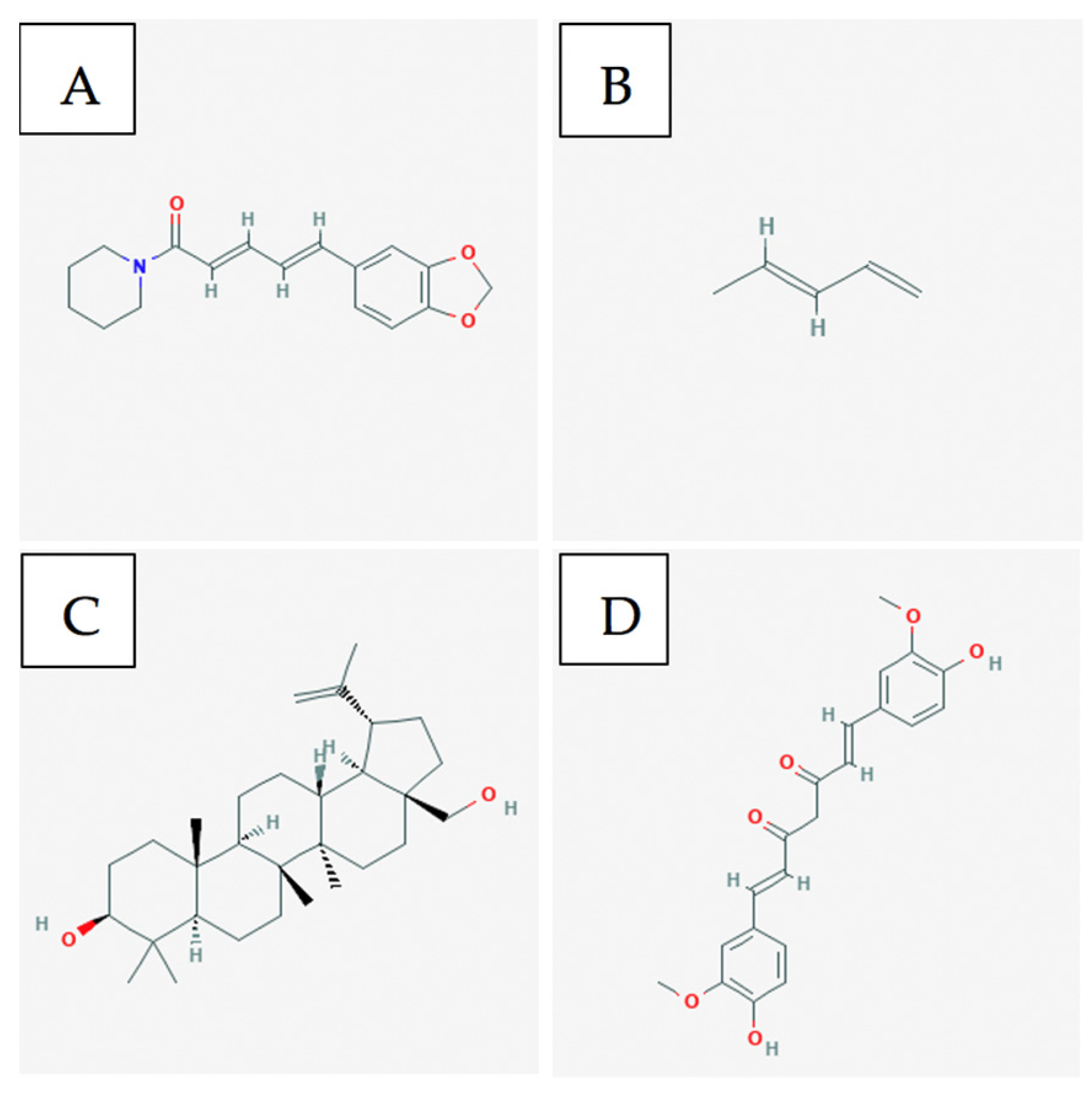
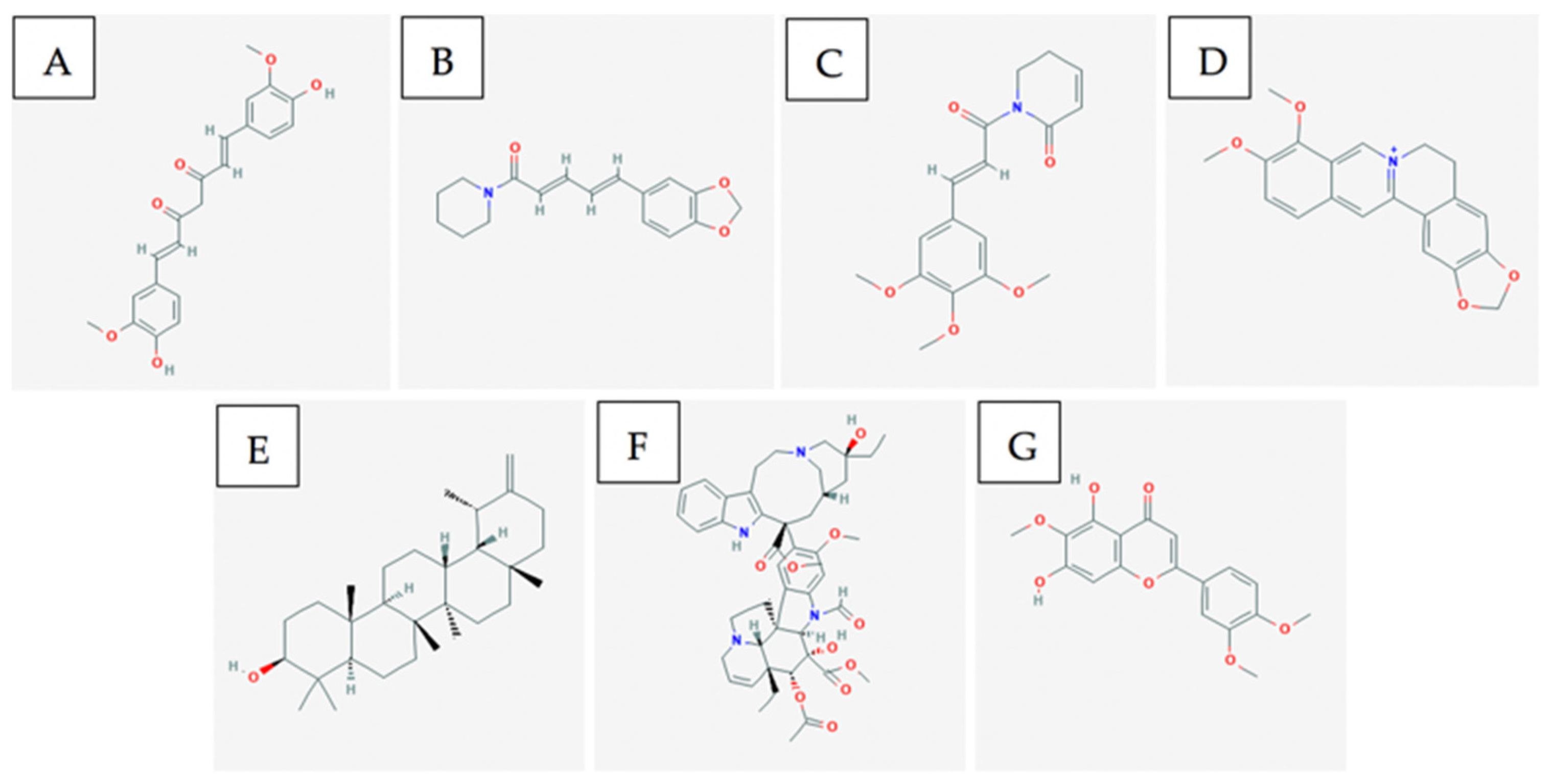
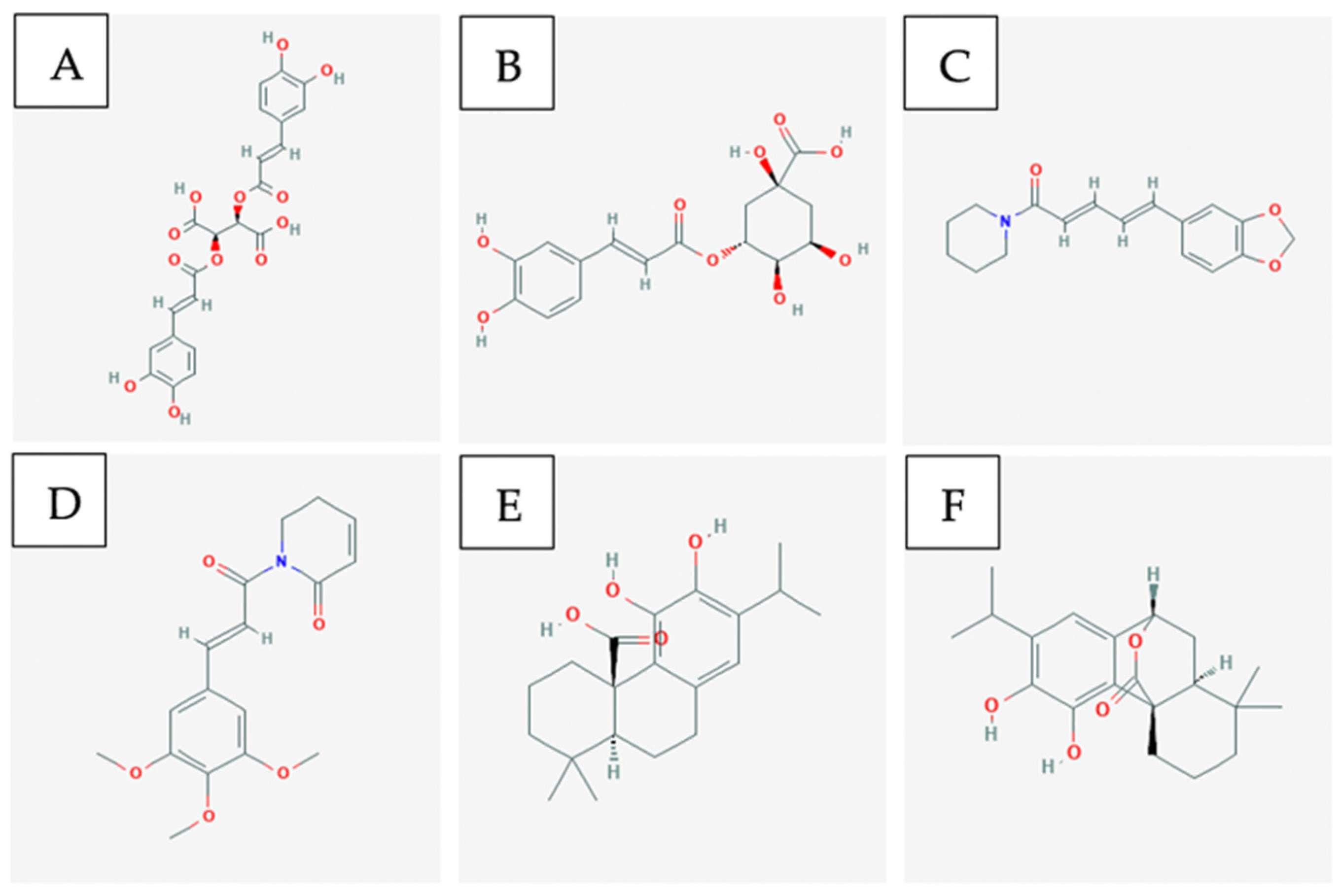
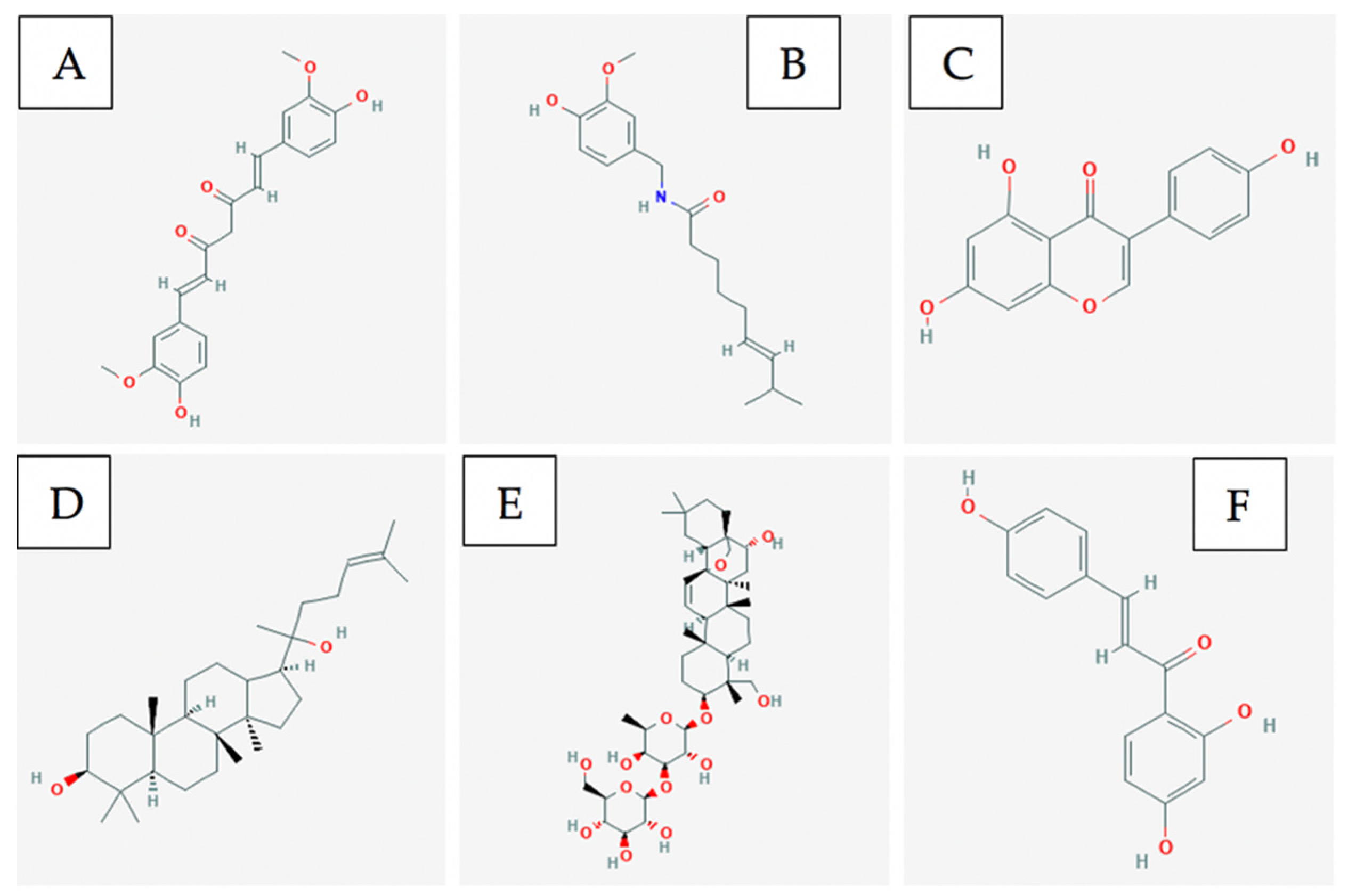
3.3. Bioactive Compounds
3.4. Single vs. Multiple Compounds and Collective Activities Targeting Multiple Pathways
3.5. Combination/Supplemental Therapy Involving Natural Compounds and Chemotherapeutic Drugs
4. Natural Health Products and Natural Compounds Used in the Treatment of Leukemia and Lymphoma
4.1. Prevalence Statistics, Prognosis, and Downsides of Conventional Treatments
4.2. Anticancer Effects of Natural Compounds In-Vitro and In-Vivo
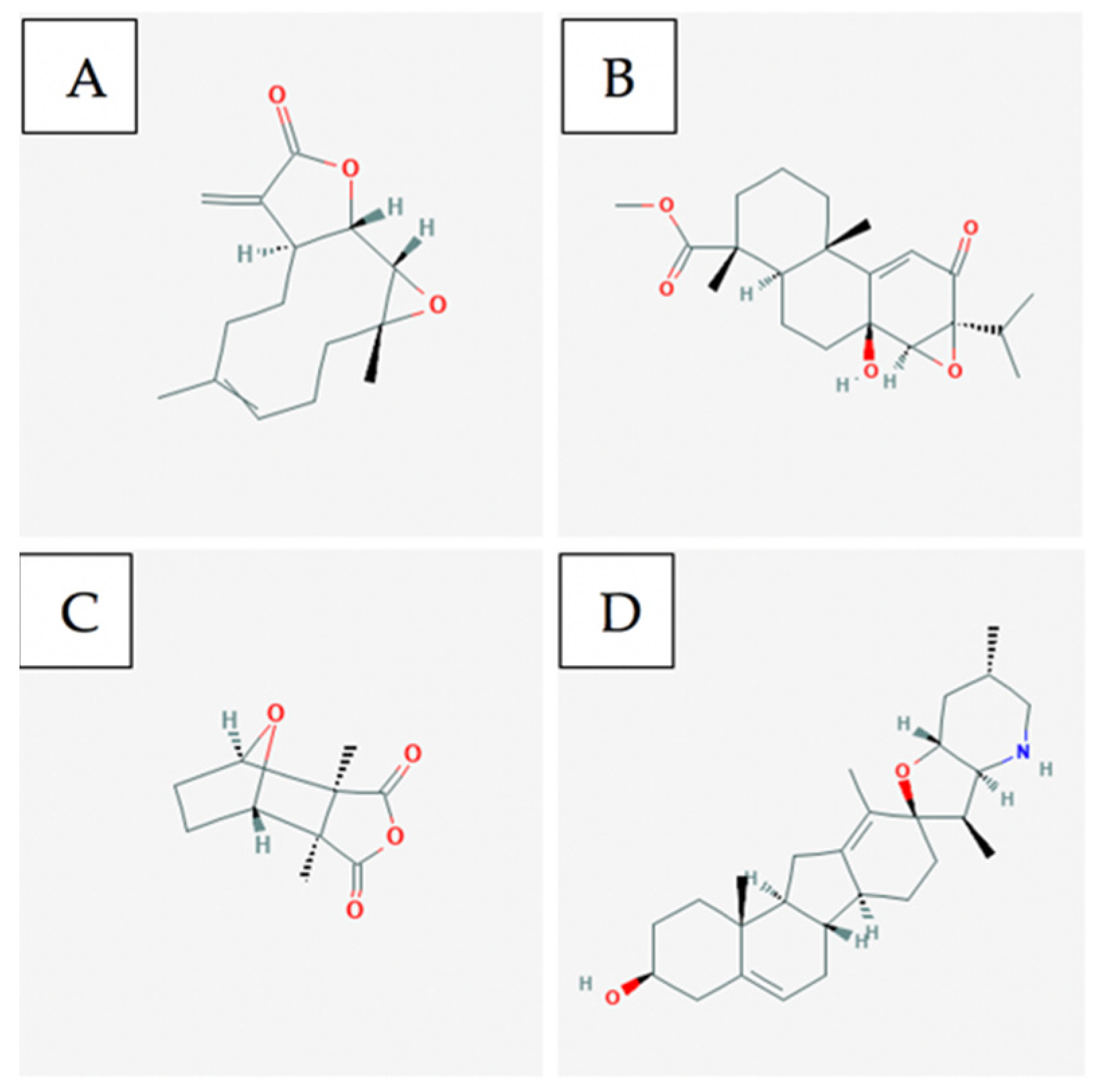
4.3. Bioactive Compounds
4.4. Single vs. Multiple Compounds and Collective Activities Targeting Multiple Pathways
5. Natural Health Products and Natural Compounds Used in the Treatment of Colorectal Cancer
5.1. Prevalence Statistics, Prognosis, and Downsides of Conventional Treatments
5.2. Anticancer Effects of Natural Compounds In-Vitro and In-Vivo
5.3. Bioactive Compounds
5.4. Single vs. Multiple Compounds and Collective Activities Targeting Multiple Pathways
6. Natural Health Products, Natural Compounds, and Natural Extracts Used in the Treatment of Lung and Pancreatic Cancer
6.1. Prevalence Statistics, Prognosis, and Downsides of Conventional Treatments
6.2. Anticancer Effects of Natural Compounds In Vitro and In Vivo
6.3. Bioactive Compounds
6.4. Single vs. Multiple Compounds and Collective Activities Targeting Multiple Pathways
7. Natural Health Products and Natural Compounds Used in the Treatment of Prostate Cancer
7.1. Prevalence Statistics, Prognosis, and Downsides of Conventional Treatments
7.2. Bioactive Compounds
7.3. Single vs. Multiple Compounds and Collective Activities Targeting Multiple Pathways
8. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NHP | Natural Health Products |
| CT | Chemotherapy |
| RT | Radiation therapy |
| CAM | Complementary and alternative medicine |
| CRC | Colorectal cancer |
| DRE | Dandelion root extract |
| SERM | Selective estrogen receptor modulator |
| ER+ | Estrogen-receptor positive cell |
| TNBC | Triple negative breast cancer cell |
| ATP | Adenosine triphosphate |
| CSC | Cancer stem cell |
| AMPK | AMP-activated protein kinase |
| ERK | Extracellular signal-related kinase |
| ERV-EA | Epstein-Barr virus early antigen |
| EMT | Epithelial-mesenchymal transitions |
| DNMT1 | DNA Methyltransferase 1 |
| FAK | Focal adhesion kinase |
| VEGF | Vascular endothelial growth factor |
| PST | Pancratistatin |
| TAM | Tamoxifen |
| ROS | Reactive oxygen species |
| NHL | Non-Hodgkin’s lymphoma |
| CAR | Chimeric antigen receptor |
| AML | Acute myeloid leukemia |
| FOLFOX | Combination Chemotherapy drug: Consists of folinic acid, 5-FU, and oxaliplatin |
| 5-FU | 5-fluorouracil |
| PPLGM | Piperlongumine |
| CA | Carnosic acid |
| CAR | Carnosol |
| JNK | c-Jun N-terminal kinase |
| BA | Betulinic acid |
| UA | Ursolic acid |
| SSa | Saikosaponin-a |
| SSd | Saikosaponin-d |
| Hsp | Heat shock protein |
References
- GBD Results Tool|GHDx. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 12 June 2020).
- Adjuvant Therapy: Treatment to Keep Cancer from Returning-Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/cancer/in-depth/adjuvant-therapy/art-20046687 (accessed on 13 June 2020).
- Mayer, E.L. Early and Late Long-Term Effects of Adjuvant Chemotherapy. Am. Soc. Clin. Oncol. Educ. Book 2013, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Burstein, H.J.; Winer, E.P. Side Effects of Chemotherapy and Combined Chemohormonal Therapy in Women with Early-Stage Breast Cancer. JNCI Monogr. 2001, 2001, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity-focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Complementary, Alternative, or Integrative Health: What’s in a Name?|NCCIH. Available online: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name (accessed on 14 June 2020).
- Garland, S.N.; Valentine, D.; Desai, K.; Li, S.; Langer, C.; Evans, T.; Mao, J.J. Complementary and alternative medicine use and benefit finding among cancer patients. J. Altern. Complement. Med. 2013, 19, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Nutritional Assessment and Use of Complementary and Alternative Medicine in...: EBSCOhost. Available online: http://web.b.ebscohost.com.ledproxy2.uwindsor.ca/ehost/pdfviewer/pdfviewer?vid=1&sid=f8bac6c0-449c-4aec-800d-83db0c25dd19%40pdc-v-sessmgr03 (accessed on 15 June 2020).
- Paul, M.; Davey, B.; Senf, B.; Stoll, C.; Münstedt, K.; Mücke, R.; Micke, O.; Prott, F.J.; Buentzel, J.; Huebner, J. Patients with advanced cancer and their usage of complementary and alternative medicine. J. Cancer Res. Clin. Oncol. 2013, 139, 1515–1522. [Google Scholar] [CrossRef]
- Complementary and Alternative Medicine (CAM)-National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/cam (accessed on 15 June 2020).
- Dasgupta, A.; Bernard, D.W. Herbal Remedies Effects on Clinical Laboratory Tests; Allen Press: St. Lawrence, KS, USA, 2006; Volume 130. [Google Scholar]
- Comelli, M.C.; Mengs, U.; Schneider, C.; Prosdocimi, M. Toward the definition of the mechanism of action of silymarin: Activities related to cellular protection from toxic damage induced by chemotherapy. Integr. Cancer Ther. 2007, 6, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Asher, G.N. Complementary and Alternative Medicine Use at a Comprehensive Cancer Center. Integr. Cancer Ther. 2017, 16, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Golden, E.B.; Lam, P.Y.; Kardosh, A.; Gaffney, K.J.; Cadenas, E.; Louie, S.G.; Petasis, N.A.; Chen, T.C.; Schönthal, A.H. Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors. Blood 2009, 113, 5927–5937. [Google Scholar] [CrossRef] [Green Version]
- Sparreboom, A.; Cox, M.C.; Acharya, M.R.; Figg, W.D. Herbal remedies in the United States: Potential adverse interactions with anticancer agents. J. Clin. Oncol. 2004, 22, 2489–2503. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; de Paula, B.; Shafaee, M.N.; Souza, P.H.; Ellis, M.J.; Bines, J. Endocrine therapy for ER-positive/HER2-negative metastatic breast cancer. Chin. Clin. Oncol. 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Brunello, A.; Maruzzo, M.; Basso, U.; Basso, S.M.M. Treatment of estrogen receptor-positive breast cancer. Curr. Med. Chem. 2013, 20, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients with Triple-Negative Breast Cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P. Treatment of Breast Cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar] [PubMed]
- Padma, V.V. An overview of targeted cancer therapy. BioMedicine 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Baskaran, K.; Pupulin, A.; Ruvinov, I.; Zaitoon, O.; Grewal, S.; Scaria, B.; Mehaidli, A.; Vegh, C.; Pandey, S. Hibiscus flower extract selectively induces apoptosis in breast cancer cells and positively interacts with common chemotherapeutics. BMC Complement. Altern. Med. 2019, 19, 98. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- De Souza Grinevicius, V.M.A.; Kviecinski, M.R.; Santos Mota, N.S.R.; Ourique, F.; Porfirio Will Castro, L.S.E.; Andreguetti, R.R.; Gomes Correia, J.F.; Filho, D.W.; Pich, C.T.; Pedrosa, R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016, 189, 139–147. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Burande, A.S.; Viswanadh, M.K.; Jha, A.; Mehata, A.K.; Shaik, A.; Agrawal, N.; Poddar, S.; Mahto, S.K.; Muthu, M.S. EGFR Targeted Paclitaxel and Piperine Co-loaded Liposomes for the Treatment of Triple Negative Breast Cancer. AAPS PharmSciTech 2020, 21, 151. [Google Scholar] [CrossRef]
- Piperine|C17H19NO3-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Piperine (accessed on 21 September 2020).
- 1,3-Pentadiene|C5H8-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1_3-Pentadiene#section=2D-Structure (accessed on 21 September 2020).
- Betulin|C30H50O2-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Betulin (accessed on 21 September 2020).
- Curcumin|IC21H20O6-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Curcumin#section=2D-Structure (accessed on 21 September 2020).
- Lin, H.-H.; Chen, J.-H.; Kuo, W.-H.; Wang, C.-J. Chemopreventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem. Biol. Interact. 2007, 165, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Amran, N.; Rani, A.A.; Mahmud, R.; Yin, K. Antioxidant and cytotoxic effect of Barringtonia racemosa and Hibiscus sabdariffa fruit extracts in MCF-7 human breast cancer cell line. Pharmacogn. Res. 2016, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afiune, L.A.F.; Leal-Silva, T.; Sinzato, Y.K.; Moraes-Souza, R.Q.; Soares, T.S.; Campos, K.E.; Fujiwara, R.T.; Herrera, E.; Damasceno, D.C.; Volpato, G.T. Beneficial effects of Hibiscus rosa-sinensis L. flower aqueous extract in pregnant rats with diabetes. PLoS ONE 2017, 12, e0179785. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Nithya, V. Phytochemical Screening and In Vitro Antioxidant Activities of the Ethanolic Extract of Hibiscus Rosa Sinensis L. Sch. Res. Libr. Ann. Biol. Res. 2011, 2, 653–661. [Google Scholar]
- Hsu, R.-J.; Hsu, Y.-C.; Chen, S.-P.; Fu, C.-L.; Yu, J.-C.; Chang, F.-W.; Chen, Y.-H.; Liu, J.-M.; Ho, J.-Y.; Yu, C.-P. The triterpenoids of Hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complement. Altern. Med. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Nik Abd Rahman, N.M.A. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafra-Polo, M.C.; González, M.C.; Estornell, E.; Sahpaz, S.; Cortes, D. Acetogenins from annonaceae, inhibitors of mitochondrial complex I. Phytochemistry 1996, 42, 253–271. [Google Scholar] [CrossRef]
- Parashar, K.; Sood, S.; Mehaidli, A.; Curran, C.; Vegh, C.; Nguyen, C.; Pignanelli, C.; Wu, J.; Liang, G.; Wang, Y.; et al. Evaluating the Anti-cancer Efficacy of a Synthetic Curcumin Analog on Human Melanoma Cells and Its Interaction with Standard Chemotherapeutics. Molecules 2019, 24, 2483. [Google Scholar] [CrossRef] [Green Version]
- Pignanelli, C.; Ma, D.; Noel, M.; Ropat, J.; Mansour, F.; Curran, C.; Pupulin, S.; Larocque, K.; Wu, J.; Liang, G.; et al. Selective Targeting of Cancer Cells by Oxidative Vulnerabilities with Novel Curcumin Analogs. Sci. Rep. 2017, 7, 1105. [Google Scholar] [CrossRef] [Green Version]
- Nakhjavani, M.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Yool, A.J.; Pei, J.V.; Townsend, A.R.; Hardingham, J.E. Stereoselective anti-cancer activities of ginsenoside rg3 on triple negative breast cancer cell models. Pharmaceuticals 2019, 12, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Townsend, A.R. Ginsenoside Rg3: Potential Molecular Targets and Therapeutic Indication in Metastatic Breast Cancer. Medicines 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, T.T.; Moon, J.Y.; Song, Y.W.; Viet, P.Q.; Van Phuc, P.; Lee, J.M.; Yi, T.H.; Cho, M.; Cho, S.K. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012, 321, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Song, K.H.; Woo, J.K.; Park, M.H.; Rhee, M.H.; Choi, C.; Oh, S.H. Ginsenoside Rp1 from Panax ginseng Exhibits Anti-cancer Activity by Down-regulation of the IGF-1R/Akt Pathway in Breast Cancer Cells. Plant Foods Hum. Nutr. 2011, 66, 298–305. [Google Scholar] [CrossRef]
- Palethorpe, H.M.; Smith, E.; Tomita, Y.; Nakhjavani, M.; Yool, A.J.; Price, T.J.; Young, J.P.; Townsend, A.R.; Hardingham, J.E. Bacopasides I and II act in synergy to inhibit the growth, migration and invasion of breast cancer cell lines. Molecules 2019, 24, 3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. CMAJ 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandru, A.; Voinea, S.; Panaitescu, E.; Blidaru, A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life 2014, 7, 572–576. [Google Scholar] [PubMed]
- Rosenberg, S.A. Cancer Treatment Reports-Google Books, 2nd ed.; U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health: Bethesda, MD, USA, 1976; Volume 60.
- Jiang, G.; Li, R.H.; Sun, C.; Liu, Y.Q.; Zheng, J.N. Dacarbazine combined targeted therapy versus dacarbazine alone in patients with malignant melanoma: A meta-analysis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selby, M.; Engelhardt, J.; Lu, L.-S.; Quigley, M.; Wang, C.; Chen, B.; Korman, A.J. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J. Clin. Oncol. 2013, 31, 3061. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.J.; McNulty, J.; Pandey, S. Sensitization of human melanoma cells by tamoxifen to apoptosis induction by pancratistatin, a nongenotoxic natural compound. Melanoma Res. 2011, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.A.; Cheung, K.J.J.; Li, G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 2001, 271, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Himes, R.H.; Wilson, L. Comparison of the Effects of Vinblastine, Vincristine, Vindesine, and Vinepidine on Microtubule Dynamics and Cell Proliferation in Vitro. Cancer Res. 1985, 45, 2741–2747. [Google Scholar] [PubMed]
- Leung, G.P.; Feng, T.; Sigoillot, F.D.; Geyer, F.C.; Shirley, M.D.; Ruddy, D.A.; Rakiec, D.P.; Freeman, A.K.; Engelman, J.A.; Jaskelioff, M.; et al. Hyperactivation of MAPK Signaling Is Deleterious to RAS/RAF-mutant Melanoma. Mol. Cancer Res. 2019, 17, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soengas, M.S.; Lowe, S.W. Apoptosis and melanoma chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigler, M.; Villares, G.J.; Lev, D.C.; Melnikova, V.O.; Bar-Eli, M. Tumor Immunotherapy in Melanoma. Am. J. Clin. Dermatol. 2008, 9, 307–311. [Google Scholar] [CrossRef]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy resistance mechanisms in advanced skin cancer. Oncol. Rev. 2017, 11, 19–25. [Google Scholar] [CrossRef]
- Lesueur, P.; Lequesne, J.; Barraux, V.; Kao, W.; Geffrelot, J.; Grellard, J.M.; Habrand, J.L.; Emery, E.; Marie, B.; Thariat, J.; et al. Radiosurgery or hypofractionated stereotactic radiotherapy for brain metastases from radioresistant primaries (melanoma and renal cancer). Radiat. Oncol. 2018, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Marconcini, R.; Spagnolo, F.; Stucci, L.S.; Ribero, S.; Marra, E.; De Rosa, F.; Picasso, V.; Di Guardo, L.; Cimminiello, C.; Cavalieri, S.; et al. Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget 2017, 9, 12452–12470. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.; Holden, R. Characteristics and management of immune-related adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma. Cancer Manag. Res. 2012, 4, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Mehaidli, A.; Baskaran, K.; Grewal, S.; Pupulin, A.; Ruvinov, I.; Scaria, B.; Parashar, K.; Vegh, C.; Pandey, S. Dandelion Root and Lemongrass Extracts Induce Apoptosis, Enhance Chemotherapeutic Efficacy, and Reduce Tumour Xenograft Growth In Vivo in Prostate Cancer. Available online: https://www.hindawi.com/journals/ecam/2019/2951428/?fbclid=IwAR0EXxzbbdw5M2y_GjMAHxwo9X0MyVBItBRDpO04_0uTLebIbonAfk60w6M (accessed on 24 June 2020).
- Alqathama, A.; Prieto, J.M. Natural products with therapeutic potential in melanoma metastasis. Nat. Prod. Rep. 2015, 32, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Tabasum, S.; Singh, R.P. Berberine in combination with doxorubicin suppresses growth of murine melanoma B16F10 cells in culture and xenograft. Phytomedicine 2014, 21, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.L. The discovery of the vinca alkaloids-Chemotherapeutic agents against cancer. Biochem. Cell Biol. 1990, 68, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Legha, S.S.; Ring, S.; Papadopoulos, N.; Plager, C.; Chawla, S.; Benjamin, R. A Prospective Evaluation of a Triple-Drug Regimen Containing Cisplatin, Vinblastine, and Dacarbazine (CVD) for Metastatic Melanoma. Cancer 1989, 64. [Google Scholar] [CrossRef]
- Sawada, N.; Kataoka, K.; Kondo, K.; Arimochi, H.; Fujino, H.; Takahashi, Y.; Miyoshi, T.; Kuwahara, T.; Monden, Y.; Ohnishi, Y. Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br. J. Cancer 2004, 90, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Piperlongumine|C17H19NO5-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Piperlongumine#section=2D-Structure (accessed on 21 September 2020).
- Berberine|C20H18NO4+-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2353 (accessed on 21 September 2020).
- Taraxasterol|C30H50O-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Taraxasterol (accessed on 21 September 2020).
- Vincristine|C46H56N4O10-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vincristine (accessed on 21 September 2020).
- Eupatilin|C18H16O7-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Eupatilin (accessed on 21 September 2020).
- Khandhar, A.; Patel, S.G.; Zaveri, M.; Patel, S.; Patel, A. Chemistry and Pharmacology of Piper Longum L. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 67–76. [Google Scholar]
- Yoo, E.S.; Choo, G.S.; Kim, S.H.; Woo, J.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.O.O.; Kim, S.K.; Park, B.K.; Cho, S.D.; et al. Antitumor and Apoptosis-inducing Effects of Piperine on Human Melanoma Cells. Anticancer Res. 2019, 39, 1883–1892. [Google Scholar] [CrossRef]
- Sunila, E.S.; Kuttan, G. Piper longum inhibits VEGF and proinflammatory cytokines and tumor-induced angiogenesis in C57BL/6 mice. Int. Immunopharmacol. 2006, 6, 733–741. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; De Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef]
- Meng, F.C.; Wu, Z.F.; Yin, Z.Q.; Lin, L.G.; Wang, R.; Zhang, Q.W. Coptidis rhizoma and its main bioactive components: Recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin. Med. (UK) 2018, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, M.J.; Kim, E.J.; Yang, Y.; Lee, M.S.; Lim, J.S. Berberine-induced AMPK activation inhibits the metastatic potential of melanoma cells via reduction of ERK activity and COX-2 protein expression. Biochem. Pharmacol. 2012, 83, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The physiological effects of dandelion (Taraxacum officinale) in type 2 diabetes. Rev. Diabet. Stud. 2016, 13, 113–131. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.; Tezuka, Y.; Banskota, A.H.; Le Tran, Q.; Tran, Q.K.; Harimaya, Y.; Saiki, I.; Kadota, S. Antiproliferative Activity of Vietnamese Medicinal Plants. Biol. Pharm. Bull. 2002, 25, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Groth-Pedersen, L.; Ostenfeld, M.S.; Høyer-Hansen, M.; Nylandsted, J.; Jäättelä, M. Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine. Cancer Res. 2007, 67, 2217–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, K.R.S.; Pacheco, N.M.; Casao, T.D.R.L.; De Melo, F.C.S.A.; Novaes, R.D.; Gonçalves, R.V. Applicability of Plant Extracts in Preclinical Studies of Melanoma: A Systematic Review. Mediat. Inflamm. 2018, 2018, 1–28. [Google Scholar] [CrossRef]
- Al Shawi, A.; Rasul, A.; Khan, M.; Iqbal, F.; Tonghui, M. Eupatilin: A flavonoid compound isolated from the artemisia plant, induces apoptosis and G2/M phase cell cycle arrest in human melanoma A375 cells. Afr. J. Pharm. Pharmacol. 2011, 5, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Hata, K.; Ishikawa, K.; Hori, K.; Konishi, T. Differentiation-inducing activity of lupeol, a lupane-type triterpene from Chinese dandelion root (Hokouei-kon), on a mouse melanoma cell line. Biol. Pharm. Bull. 2000, 23, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Takasaki, M.; Konoshima, T.; Tokuda, H.; Masuda, K.; Arai, Y.; Shiojima, K.; Ageta, H. Anti-carcinogenic activity of Taraxacum plant. II. Biol. Pharm. Bull. 1999, 22, 606–610. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Cao, Z.; Pan, Y.; Zhang, G.; Yang, P.; Guo, P.; Zhou, Q. Jatrorrhizine hydrochloride inhibits the proliferation and neovascularization of C8161 metastatic melanoma cells. Anticancer. Drugs 2013, 24, 667–676. [Google Scholar] [CrossRef]
- Yan, L.; Yee, J.A.; Li, D.; Mcguire, M.H.; Graef, G.L. Dietary flaxseed supplementation and experimental metastasis of melanoma cells in mice. Cancer Lett. 1998, 124, 181–186. [Google Scholar] [CrossRef]
- Gofita, E.; Calina, D.; Blendea, A.; Brandusa, C.; Mitrut, R. Curcumin in the Treatment of Melanoma. Trends Toxicol. Relat. Sci. 2017, 1, 21–40. [Google Scholar]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef]
- Selimovic, D.; Badura, H.E.; El-Khattouti, A.; Soell, M.; Porzig, B.B.O.W.; Spernger, A.; Ghanjati, F.; Santourlidis, S.; Haikel, Y.; Hassan, M. Vinblastine-induced apoptosis of melanoma cells is mediated by Ras homologous A protein (Rho A) via mitochondrial and non-mitochondrial-dependent mechanisms. Apoptosis 2013, 18, 980–997. [Google Scholar] [CrossRef]
- Faião-Flores, F.; Suarez, J.A.Q.; Fruet, A.C.; Maria-Engler, S.S.; Pardi, P.C.; Maria, D.A. Curcumin analog DM-1 in monotherapy or combinatory treatment with dacarbazine as a strategy to inhibit in vivo melanoma progression. PLoS ONE 2015, 10, e0118702. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Rybarczyk, A.; Tuckey, R.C.; Slominski, A.T.; Zmijewski, M.A. Vitamin D and its low calcemic analogs modulate the anticancer properties of cisplatin and dacarbazine in the human melanoma A375 cell line. Int. J. Oncol. 2019, 54, 1481–1495. [Google Scholar] [CrossRef] [Green Version]
- Baharara, J.; Amini, E.; Nikdel, N.; Salek-Abdollahi, F. The cytotoxicity of dacarbazine potentiated by sea cucumber saponin in resistant B16F10 melanoma cells through apoptosis induction. Avicenna J. Med. Biotechnol. 2016, 8, 112–119. [Google Scholar]
- Ellison, L.F. Increasing survival from leukemia among adolescents and adults in Canada: A closer look. Health Rep. 2016, 27, 19–26. [Google Scholar]
- IIsCanada.org. Leukemia & Lymphoma Society of Canada. Available online: https://www.llscanada.org/disease-information/facts-and-statistics (accessed on 16 November 2015).
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019; Canadian Cancer Society: Toronto, ON, Canada, 2019. [Google Scholar]
- Stöppler, M. Leukemia Treatment, Diagnosis, Causes, Symptoms & Prognosis. 11 September 2019. Available online: https://www.medicinenet.com/leukemia/article.htm (accessed on 3 June 2020).
- Titov, D.; He, Q. Solving A Traditional Chinese Medicine Mystery-03/02/2011. 2 March 2011. Available online: https://www.hopkinsmedicine.org/news/media/releases/solving_a_traditional_chinese_medicine_mystery (accessed on 5 June 2020).
- Amato, I. An Eye on Cancer, A Smiling Death. Available online: https://cen.acs.org/articles/87/i35/Eye-Cancer-Smiling-Death.html. (accessed on 31 August 2009).
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, D. Epigenetic Dietary Interventions for Cancer Prevention. In Epigenetics of Cancer Prevention; Bishayee, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 419–427. [Google Scholar]
- Parthenolide|C15H20O3-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/108068 (accessed on 21 September 2020).
- Triptolide Analog|C21H30O5-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Triptolide-analog (accessed on 21 September 2020).
- Cantharidin|C10H12O4-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cantharidin (accessed on 21 September 2020).
- Cyclopamine|C27H41NO2-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyclopamine (accessed on 21 September 2020).
- Rauh, R.; Kahl, S.; Boechzelt, H.; Bauer, R.; Kaina, B.; Efferth, T. Molecular biology of cantharidin in cancer cells. Chin. Med. 2007, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.T.; Welchrl, K.D.; Panter, K.; Gardner, D.R.; Garrossian, M.; Chang, C.-W.T. Cyclopamine: From cyclops lambs to cancer treatment. J. Agric. Food Chem. 2014, 62, 7355–7362. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Talcott, S.T.; Percival, S.S. Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells. J. Nutr. 2003, 133, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Percival, S.S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett. 2005, 218, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Bray, F.; Pisani, P.; Parkin, D. Cancer mortality and mortality worldwide: Sources, methods, and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- MacDonald, V. Chemotherapy: Managing side effects and safe handling. Can. Vet. J. 2009, 50, 665–668. [Google Scholar]
- Ovadje, P.; Ammar, S.; Guerrero, J.A.; Arnason, J.T.; Pandey, S. Dandelion root extract affects colorectal cancer proliferaion and survival through the activation of multiple death signalling pathways. Oncotarget 2016, 7, 73080–73100. [Google Scholar] [CrossRef] [Green Version]
- Ruvinov, I.; Nguyen, C.; Scaria, B.; Vegh, C.; Zaitoon, O.; Baskaran, K.; Mehaidli, A.; Nunes, M.; Pandey, S. Lemongrass extract possesses potent anticancer activity against human colon cancers, inhibits tumorigenesis, enhances efficacy of FOLFOX, and reduces its adverse effects. Integr. Cancer Ther. 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wen, C.; Bai, H.; Wang, X.; Zhang, X.; Huang, L.; Yang, X.; Iwamoto, A.; Liu, H. JNK signaling pathway is involved in piperlongumine-mediated apoptosis in human colorectal cancer HCT116 cells. Oncol. Lett. 2015, 10, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Ovadje, P.; Ma, D.; Tremblay, P.; Roma, A.; Steckle, M.; Guerrero, J.A.; Arnason, J.T.; Pandey, S. Evaluation of the efficacy & biochemical mechanism of cell death induction by Piper longum extract selectively in in-vitro and in-vivo models of human cancer cells. PLoS ONE 2014, 9, e113250. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mosieniak, G.; Adamowicz, M.; Alster, O.; Jaskowiak, H.; Szczepankiewicz, A.A.; Wilczynski, G.M.; Ciechomska, I.A.; Sikora, E. Curcumin induces permanent growth arrest of human colon cancer cells: Link between senescence and autophagy. Mech. Ageing Dev. 2012, 133, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Lee, C. Curcumin induces downregulation of E2F4 expression and apoptotic cell death in HCT116 human colon cancer cells; involvement of reactive oxygen species. Korean J. Physiol. Pharmacol. 2010, 14, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, J.; Drummond, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.J.; Chen, B.; Kumar, N. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol. 2013, 86, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [Green Version]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, R.A.; Asmis, T.R. Overview of systemic therapy for colorectal cancer. Clin. Colon Rectal Surg. 2009, 22, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef]
- Randhawa, H.; Kibble, K.; Zeng, H.; Moyer, M.P.; Reindl, K.M. Activation of ERK signaling and induction of colon cancer cell death by piperlongumine. Toxicol. Vitr. 2013, 27, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.M.; Pan, F.; Li, L.; Liu, Q.R.; Chen, Y.; Xiong, X.X.; Cheng, K.; Bin, Y.S.; Shi, Z.; Yu, A.C.-H.; et al. Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem. Biophys. Res. Commun. 2013, 437, 87–93. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, W.; Barszcz, B. Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia 2000, 71, 269–273. [Google Scholar] [CrossRef]
- Watson, J.L.; Hill, R.; Yaffe, P.B.; Greenshields, A.; Walsh, M.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010, 297, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J.L.; Klauber, N.; Rohan, R.; van Leeuwen, R.; Huang, M.T.; Fisher, C.; Flynn, E.; Byers, H.R. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998, 4, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ieso, M.L.; Pei, J.V.; Nourmohammadi, S.; Smith, E.; Chow, P.H.; Kourghi, M.; Hardingham, J.E.; Yool, A.J. Combined pharmacological administration of AQP1 ion channel blocker AqB011 and water channel blocker Bacopaside II amplifies inhibition of colon cancer cell migration. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Castejon, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef]
- Chicoric Acid|C22H18O12-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chicoric-acid (accessed on 21 September 2020).
- Chlorogenic Acid|C16H18O9-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorogenic-acid (accessed on 21 September 2020).
- Carnosic Acid|C20H28O4-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carnosic-acid (accessed on 21 September 2020).
- Carnosol|C20H26O4-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carnosol (accessed on 21 September 2020).
- Halabi, M.F.; Sheikh, B.Y. Anti-proliferative effect and phytochemical analysis of Cymbopogon citratus extract. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Olorunnisola, K.S.; Hammed, A.M.; Asiyanbi-Hammed, T.; Simsek, S. Biological properties of lemongrass: An overview. Int. Food Res. J. 2014, 21, 455–462. [Google Scholar]
- Hu, R.; Kim, B.R.; Chen, C.; Hebbar, V.; Kong, A.N. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1361–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Shen, G.; Yuan, X.; Kim, J.H.; Gopalkrishnan, A.; Keum, Y.S.; Nair, S.; Kong, A.T. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis 2006, 27, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visanji, J.M.; Thompson, D.G.; Padfield, P.J. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006, 237, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J. Carnosol: A promising anti-cancer and anti- inflammatory agent. Cancer Lett. 2011, 305, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdés, A.; García-Cañas, V.; Simó, C.; Ibáñez, C.; Micol, V.; Ferragut, J.A.; Cifuentes, A. Comprehensive foodomics study on the mechanisms operating at various molecular levels in cancer cells in response to individual rosemary polyphenols. Anal. Chem. 2014, 86, 9807–9815. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Su, C.C.; Lin, J.G.; Li, T.M.; Chung, J.G.; Yang, J.S.; Ip, S.W.; Lin, W.C.; Chen, G.W. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006, 26, 4379–4389. [Google Scholar]
- Collett, G.P.; Campbell, F.C. Overexpression of p65/RelA potentiates curcumin-induced apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2006, 27, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Gandhy, S.U.; Kim, K.; Larsen, L.; Rosengren, R.J.; Safe, S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp.) transcription factors by targeting microRNAs. BMC Cancer 2012, 12, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.M.; Jin, K.S.; Lee, Y.W.; Song, Y.S. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur. J. Pharmacol. 2011, 660, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Philion, C.; Ma, D.; Ruvinov, I.; Mansour, F.; Pignanelli, C.; Noel, M.; Saleem, A.; Arnason, J.; Rodrigues, M.; Singh, I.; et al. Cymbopogon citratus and Camellia sinensis extracts selectively induce apoptosis in cancer cells and reduce growth of lymphoma xenografts in vivo. Oncotarget 2017, 8, 110756–110773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 2006, 52, 23–28. [Google Scholar] [CrossRef]
- Nautiyal, J.; Kanwar, S.S.; Yu, Y.; Majumdar, A.P. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J. Mol. Signal. 2011, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Xie, B.; Li, Y.; Shi, L.; Wan, J.; Chen, X.; Wang, H. Orally deliverable nanotherapeutics for the synergistic treatment of colitis-associated colorectal cancer. Theranostics 2019, 9, 7458–7473. [Google Scholar] [CrossRef]
- Pancreatic Cancer Statistics-Canadian Cancer Society. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/pancreatic/statistics/?region=on (accessed on 20 August 2020).
- Survival Rates for Pancreatic Cancer. American Cancer Society. Published 2020. Available online: https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html#references (accessed on 30 August 2020).
- Lung Cancer Statistics-Canadian Cancer Society. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/lung/statistics/?region=pe (accessed on 30 August 2020).
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Kunk, P.R.; Bauer, T.W.; Slingluff, C.L.; Rahma, O.E. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J. Immunother. Cancer 2016, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Cooper, S.; Spiro, S.G. Small cell lung cancer: Treatment review. Respirology 2006, 11, 241–248. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kummalue, T. Molecular Mechanism of Herbs in Human Lung Cancer Cells. 2005. Available online: https://www.researchgate.net/publication/51373862 (accessed on 30 August 2020).
- Li, L.; Leung, P.S. Use of herbal medicines and natural products: An alternative approach to overcoming the apoptotic resistance of pancreatic cancer. Int. J. Biochem. Cell Biol. 2014, 53, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Lai, F.-J.; Chen, H.; Luo, J.; Zhang, R.-Y.; Bu, H.-Q.; Wang, Z.-H.; Lin, H.-H.; Lin, S.-Z. Involvement of the phosphoinositide 3-kinase/Akt pathway in apoptosis induced by capsaicin in the human pancreatic cancer cell line PANC-1. Oncol. Lett. 2013, 5, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zheng, X.-L.; Yang, L.; Shi, F.; Gao, L.; Zhong, Y.-J.; Sun, H.; He, F.; Lin, Y.; Wang, X. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.-L.; Kuo, P.-L.; Chiang, L.-C.; Lin, C.-C. Isoliquiritigenin Inhibits the Proliferation and Induces the Apoptosis of Human Non-Small Cell Lung Cancer a549 Cells. Clin. Exp. Pharmacol. Physiol. 2004, 31, 414–418. [Google Scholar] [CrossRef]
- Capsaicin|C18H27NO3-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Capsaicin (accessed on 21 September 2020).
- Genistein|C15H10O5-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Genistein (accessed on 21 September 2020).
- Ginsenosides|C30H52O2-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ginsenosides (accessed on 21 September 2020).
- Saikosaponin D|C42H68O13-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Saikosaponin-D (accessed on 21 September 2020).
- Isoliquiritigenin|C15H12O4-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isoliquiritigenin (accessed on 21 September 2020).
- Meng, F.-C.; Zhou, Y.-Q.; Ren, D.; Wang, R.; Wang, C.; Lin, L.-G.; Zhang, X.-Q.; Ye, W.-C.; Zhang, Q.-W. Turmeric: A Review of Its Chemical Composition, Quality Control, Bioactivity, and Pharmaceutical Application. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 299–350. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B. Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.). Fac Publ. Published Online 1 January 2011. Available online: https://scholarworks.sfasu.edu/agriculture_facultypubs/1 (accessed on 30 August 2020).
- Hwang, S.-H.; Lee, B.-H.; Kim, H.-J.; Cho, H.-J.; Shin, H.-C.; Im, K.-S.; Choi, S.-H.; Shin, T.-J.; Lee, S.-M.; Nam, S.W.; et al. Suppression of metastasis of intravenously-inoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: Involvement of autotaxin inhibition. Int. J. Oncol. 2012, 42, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012: Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Thompson, I.; Thrasher, J.B.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; Cookson, M.S.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; et al. Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. J. Urol. 2007, 177, 2106–2131. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, X. Prostate Cancer: Current Treatment and Prevention Strategies. Iran. Red Crescent Med. J. 2013, 15, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and Prooxidant Properties of Flavonoids. In Fitoterapia; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Lafay, S.; Gueux, E.; Rayssiguier, Y.; Mazur, A.; Rémésy, C.; Scalbert, A. Caffeic Acid Inhibits Oxidative Stress and Reduces Hypercholesterolemia Induced by Iron Overload in Rats. Int. J. Vitam. Nutr. Res. 2005, 75, 119–125. [Google Scholar] [CrossRef]
- Caffeic Acid|C9H8O4-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Caffeic-acid#section=2D-Structure (accessed on 21 September 2020).
- Elemicin|C12H16O3-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Elemicin#section=2D-Structure (accessed on 21 September 2020).
- Gallic Acid|C7H6O5-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gallic-acid#section=2D-Structure (accessed on 21 September 2020).
- You, Q.; Ni, H.; Sharp, J.L.; Wang, X.; You, Y.; Zhang, C. High-Performance Liquid Chromatography-Mass Spectrometry and Evaporative Light-Scattering Detector to Compare Phenolic Profiles of Muscadine Grapes. J. Chromatogr. A 2012, 1240, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hudson, T.S.; Hartle, D.K.; Hursting, S.D.; Nunez, N.P.; Wang, T.T.; Young, H.A.; Arany, P.; Green, J.E. Inhibition of Prostate Cancer Growth by Muscadine Grape Skin Extract and Resveratrol through Distinct Mechanisms. Cancer Res. 2007, 67, 8396–8405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stępkowski, T.M.; Brzóska, K. The Role of Natural Polyphenols in Cell Signaling and Cytoprotection against Cancer Development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, O.; Doğanlar, Z.B.; Serttas, R.; Turkekul, K.; Dibirdik, I.; Bilir, A. The Flavonoid Apigenin Reduces Prostate Cancer CD44+ Stem Cell Survival and Migration through PI3K/Akt/NF-ΚB Signaling. Life Sci. 2016, 162, 77–86. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Kenney, P.M.; Lam, L.K.T. Potential Anticarcinogenic Natural Products Isolated from Lemongrass Oil and Galanga Root Oil. J. Agric. Food Chem. 1993, 41, 153–156. [Google Scholar] [CrossRef]
- Sharmila, G.; Bhat, F.; Arunkumar, R.; Elumalai, P.; Singh, P.R.; Senthilkumar, K.; Arunakaran, J. Chemopreventive Effect of Quercetin, a Natural Dietary Flavonoid on Prostate Cancer in Invivo Model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Ignacio, D.N.; Mason, K.D.; Hackett-Morton, E.C.; Albanese, C.; Ringer, L.; Wagner, W.D.; Wang, P.C.; Carducci, M.A.; Kachhap, S.K.; Paller, C.J.; et al. Muscadine Grape Skin Extract Inhibits Prostate Cancer Cells by Inducing Cell-Cycle Arrest, and Decreasing Migration through Heat Shock Protein 40. Heliyon 2019, 5, e01128. [Google Scholar] [CrossRef] [Green Version]
- Madonna, G.; Ullman, C.D.; Gentilcore, G.; Palmieri, G.; Ascierto, P.A. NF-κB as potential target in the treatment of melanoma. J. Transl. Med. 2012, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Afshari, K.; Haddadi, N.S.; Haj-Mirzaian, A.; Farzaei, M.H.; Rohani, M.M.; Akramian, F.; Naseri, R.; Sureda, A.; Ghanaatian, N.; Abdolghaffari, A.H. Natural flavonoids for the prevention of colon cancer: A comprehensive review of preclinical and clinical studies. J. Cell. Physiol. 2019, 234, 21519–21546. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Lansigan, F.; Ruta, S.; Lamb, L.; Mezes, M.; Elligers, K.; Grant, N.; Jiang, Z.L.; Liu, S.H.; Cheng, Y.C. Phase I study of the botanical formulation PHY906 with capecitabine in advanced pancreatic and other gastrointestinal malignancies. Phytomedicine 2010, 17, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Li, J.; Lamb, L.; Kaley, K.; Elligers, K.; Jiang, Z.; Bussom, S.; Liu, S.H.; Cheng, Y.C. First-in-human phase II trial of the botanical formulation PHY906 with capecitabine as second-line therapy in patients with advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2014, 73, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Gao, G.; Zou, G.; Yu, H.; Zheng, X. Natural Products as Adjunctive Treatment for Pancreatic Cancer: Recent Trends and Advancements. BioMed Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaria, B.; Sood, S.; Raad, C.; Khanafer, J.; Jayachandiran, R.; Pupulin, A.; Grewal, S.; Okoko, M.; Arora, M.; Miles, L.; et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. Int. J. Mol. Sci. 2020, 21, 8480. https://doi.org/10.3390/ijms21228480
Scaria B, Sood S, Raad C, Khanafer J, Jayachandiran R, Pupulin A, Grewal S, Okoko M, Arora M, Miles L, et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. International Journal of Molecular Sciences. 2020; 21(22):8480. https://doi.org/10.3390/ijms21228480
Chicago/Turabian StyleScaria, Benjamin, Siddhartha Sood, Christopher Raad, Jana Khanafer, Rahul Jayachandiran, Alaina Pupulin, Sahibjot Grewal, Michael Okoko, Mansi Arora, Lauren Miles, and et al. 2020. "Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends" International Journal of Molecular Sciences 21, no. 22: 8480. https://doi.org/10.3390/ijms21228480
APA StyleScaria, B., Sood, S., Raad, C., Khanafer, J., Jayachandiran, R., Pupulin, A., Grewal, S., Okoko, M., Arora, M., Miles, L., & Pandey, S. (2020). Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. International Journal of Molecular Sciences, 21(22), 8480. https://doi.org/10.3390/ijms21228480





