Abstract
Of all human infertility cases, up to 50% show contributing factors leading to defects in the male reproductive physiology. Seminal plasma (SP) is the biological fluid derived from the male accessory sex gland which carries spermatozoa passing throughout the male and female reproductive tract during ejaculation. It contains a complicated set of heterogeneous molecular structures, including proteins, cell-free nucleic acid (DNA, microRNA and LncRNA), and small-molecule metabolites as well as inorganic chemicals (ions). For a long time, the substantial significance of seminal plasma factors’ functions has been underestimated, which is restricted to spermatozoa transport and protection. Notably, significant advancements have been made in dissecting seminal plasma components, revealing new insights into multiple aspects of sperm function, as well as fertilization and pregnancy outcomes in recent years. In this review, we summarize the state-of-art discoveries regarding SP compositions and their implications in male fertility, particularly describing the novel understanding of seminal plasma components and related modifications using “omics” approaches and mainly focusing on proteome and RNA-seq data in the latest decade. Meanwhile, we highlighted the proposed mechanism of the regulation of SP molecules on immunomodulation in the female reproductive tract. Moreover, we also discussed the proteins investigated as non-invasive diagnosis biomarkers for male infertility in the clinic.
1. Introduction
Over the past few decades, worldwide, gradually decreased human fertility has attracted much more interest and became an important medical and social issue. Approximately 10–15% of couples of the reproductive-aged population are considered infertile, which is defined as the failure to conceive within at least one year of unprotected sexual intercourse [1,2]. For all infertility cases, up to 50% of them show contributing factors leading to defects in male reproductive physiology [3]. However, most male infertility causative factors remain elusive and the underlying mechanisms are also largely unknown. Thus, it is imperative to determine factors affecting male fertility, dissect regulatory mechanisms, search for useful diagnostic biomarkers, and, eventually, develop effective therapeutic tools.
To date, the majority of male infertility research has mainly focused on abnormality related to the testes, where the male gametes (spermatozoa) are produced through spermatogenesis and spermiogenesis [4]. For human male reproduction, apart from the testes, the accessory glands, including the prostate, seminal vesicles, and bulbourethral glands, are also essential, secreting a biological fluid termed as seminal plasma (SP) around sperm from ejaculation to the fertilization process [5]. Seminal plasma makes up more than 95% of human semen, whereas testicular secretions containing spermatozoa compose about 5%. Clinically, male fertility is often evaluated by routine semen analysis, which serves as a baseline marker with data regarding sperm quantity and quality, including sperm count, concentration, viability, motility, and morphology, while decreased fertility frequently associates with aberrant semen parameters [6].
However, limitations exist in investigating male infertility exclusively using routine semen analysis parameters for a certain patient since unexplained male infertility (UMI) cases have an average prevalence of approximately 15% among all male infertility cases [7]. Unexplained male infertility refers to the diagnosis for an individual whose semen fulfills WHO criteria but fails to conceive offspring. Thus, it is necessary and urgent to develop novel evaluation tests assessing male factor infertility besides examining the structure and function of spermatozoa and, furthermore, the possible involvement of seminal plasma factors should be considered [8]. Seminal plasma is the fluid part of semen, which carries spermatozoa passing throughout the male and female reproductive tract during ejaculation, eventually reaching the oocyte for successful fertilization. It is composed of a complicated set of heterogeneous molecular structures, including proteins, lipids, sugars (fructose), cell-free nucleic acid (DNA, microRNA, and LncRNA), and small-molecule metabolites as well as inorganic chemicals (ions). In general, seminal plasma factors provide energy for spermatozoa metabolism and motility and modulate spermatozoa function by regulating a cascade of molecular events, such as sperm maturation in the epididymis and capacitation during transport. Plasma molecules can give an idea about sperm concentration, motility, morphology, and cause of infertility. Notably, emerging evidence has indicated that seminal plasma is not merely the beneficial medium of spermatozoa but also contains essential spermatozoa function modulators (Figure 1). Recently, advancing technologies in the “omics” fields, such as genomics, transcriptomics, proteomics, and metabolomics, have allowed uncovering of novel aspects and improved understanding of seminal plasma involved in male sub- and infertility.
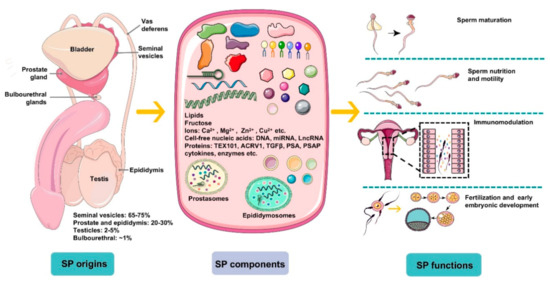
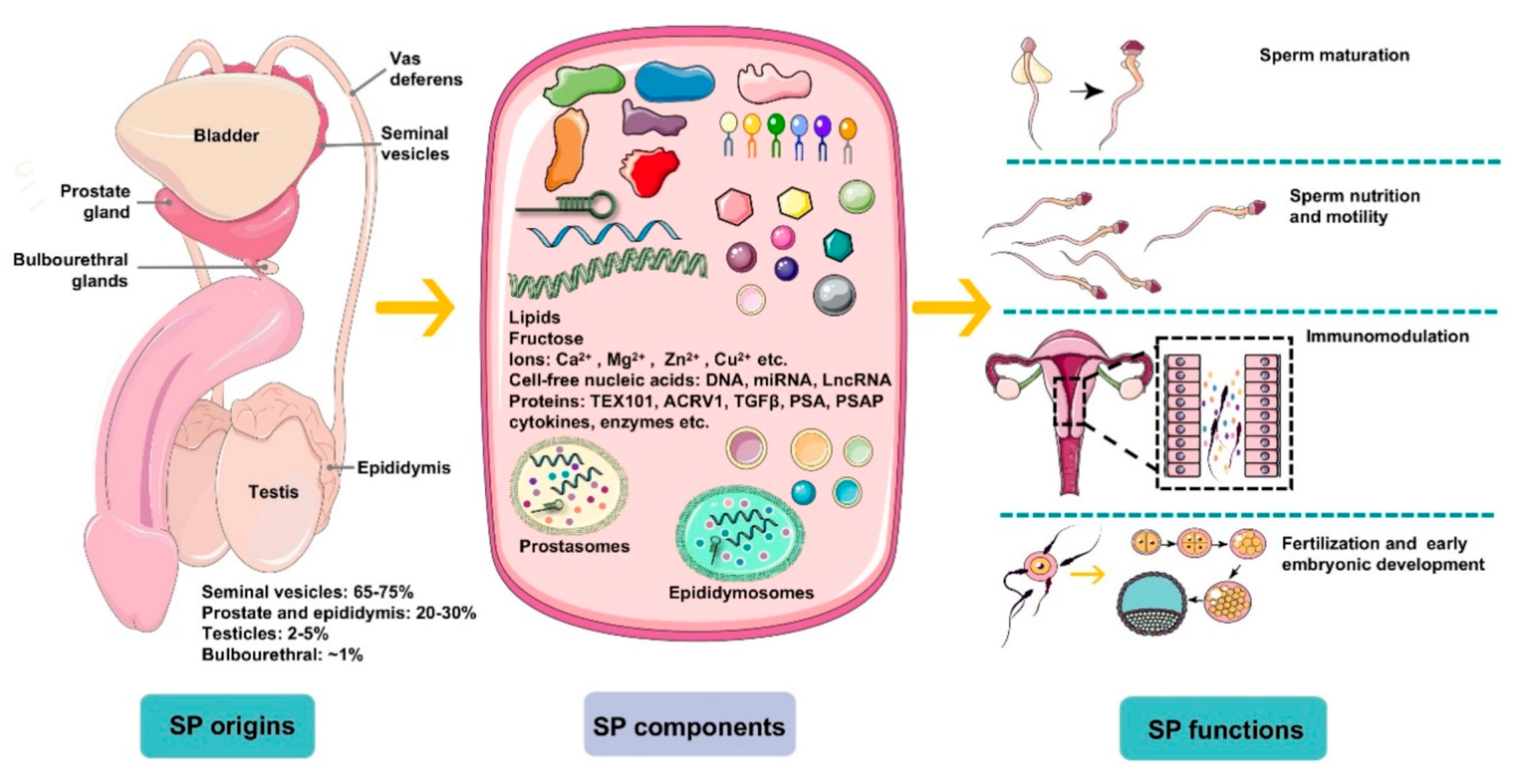
Figure 1.
An overview of the origins, components, and main functions of seminal plasma (SP). Seminal plasma makes up more than 95% of the human ejaculate volume, whereas testicular secretions containing spermatozoa compose about 2–5% of semen. Seminal plasma is mainly derived from seminal vesicles, the prostate and epididymis, which produce ~65–75% and ~20–30% of the volume of semen, respectively, with a small proportion generated by bulbourethral glands (~1%). SP is composed of a complicated set of heterogeneous molecular structures, including proteins (enzymes, cytokines, TEX101, ACRV1, TGFΒβ, prostate-specific antigen (PSA), prostatic-specific acid phosphatase (PSAP), etc.), lipids, sugars (fructose), cell-free nucleic acid (DNA, microRNA, and LncRNA), ions (Ca2+, Mg2+, Zn2+, Cu2+, etc.), and small-molecule metabolites. In general, SP is a beneficial medium for spermatozoa maturation, nutrition, and motility and modulates spermatozoa function by regulating a cascade of molecular events, such as sperm maturation in the epididymis and capacitation during transport. More importantly, plasma molecules, such as cytokines, directly recognize receptors on epithelial cells lining the cervix and uterus to induce synthesis of pro-inflammatory cytokines and chemokines that recruit and activate inflammatory leukocytes. Besides, SP modulates the release of cytokines and growth factors that regulate embryo development in the oviduct and uterus before implantation, which is critical for early embryo development and implantation.
This review provides an overview of the state-of-art discoveries regarding SP compositions and their implications in male fertility. We describe the novel understandings of seminal plasma components and related modifications using “omics” approaches, mainly focusing on proteome and RNA-seq data in the latest decade. We will also particularly highlight the functions of SP molecules for immunomodulation in the female reproductive tract and the proposed mechanisms. Subsequently, we discuss seminal plasma’s clinical relevance with particular reference to the potential application as a biomarker to better assess male fertility. Finally, the perspective of seminal plasma on male fertility is briefly summarized.
2. Protein Compositions of SP
Human normal seminal plasma is predominantly enriched with a large diversity of tissue-specific proteins and peptides, directly binding to spermatozoa, thus contributing to maturation, function, and interaction with the female genital tract [9,10]. Seminal plasma proteins are mainly derived from seminal vesicles and the prostate, which produce ~65–75% and ~20–30% of the volume of semen, respectively, with a small proportion generated by the testis, epididymis, and bulbourethral and periurethral glands [11]. The total protein concentration varies depending mainly on the detected technique at an average value of 40–60 mg/mL [12,13,14]. Prostasomes and epididymosomes are distinctive vesicles in SP secreted by the prostate and the epididymal epithelium, filled with a mixture of complex proteins. Prostasome proteome contains 139 proteins, revealed by microLC-MS/MS, including enzymes, transport/structural proteins, chaperone proteins, and signal transduction proteins [15]. The protein composition of epididymosomes differs from prostasomes, which contain 146 different proteins identified by MS [16]. High-abundance proteins in human seminal plasma, such as matrix and adhesion proteins semenogelins, laminin, and enzymes, including prostate-specific antigen (PSA), prostatic-specific acid phosphatase (PSAP), and creatine kinase, can be identified by 2D gel electrophoresis or mass spectrometry (MS) [17,18]. The application of advanced proteomics technologies has gained substantial progress to elucidate relative or absolute quantitation of seminal plasma protein composition and content and provided the underlying mechanistic insights of male reproductive physiology and pathology [7] (Table 1). The proteomics approach largely supports the discriminate differences in protein composition of semen, based on which proteins can potentially be used for noninvasive clinical diagnosis for male infertility disorder.

Table 1.
Distinct proteomes of human seminal plasma in respective conditions reported from 2012 to 2019.
SP protein profiling is dynamic and affected by various factors, such as reactive oxygen species (ROS). Aberrant ROS level is a common consequence of diverse pathways that cause sperm dysfunction in the semen of two-thirds of infertile patients [40,41]. One-dimensional gel electrophoresis-coupled LC/MS–MS identified 105 unique, differentially expressed proteins from high-ROS infertile patients compared with fertile controls, which is much more than a comparison between medium-ROS or low-ROS patients with a control [42]. Rakesh Sharmae et al. categorized seminal plasma into ROS-positive (ROS+) or ROS-negative (ROS−) groups from both fertile and infertile men and then profiled proteins to uncover molecular mechanisms underlying oxidative stress and sperm dysfunction. They identified four ROS+−specific proteins: cystatin S precursor, albumin preprotein, lactotransferrin precursor-1 peptide, and prostate-specific antigen isoform 4 preprotein [35].
Besides, distinct protein content in SP is associated with semen parameters. Many studies have explored the association of seminal plasma protein profile and routine semen analysis. Analysis of the proteins profiled in the following groups: (1) NN (normal sperm count and normal morphology), (2) NA (normal sperm count and abnormal morphology), (3) ON (oligozoospermia and normal morphology), and (4) OA (oligozoospermia and abnormal morphology) by LC-MS/MS revealed 20 proteins differentially expressed, which indicates a correlation between seminal plasma protein and sperm concentration and morphology [43]. It is reasonable that SP protein is associated with sperm motility because sperm are surrounded by SP and directly bound by specific proteins on the surface. From asthenozoospermic patients, a total of 29 differentially expressed proteins were identified by quantitative proteomes, which could be used as candidate targets for studying the molecular bases of sperm motility [20].
Proteins in seminal plasma are closed related to male fertility status and, thus, may provide predicted information of male reproductive health. Investigating the differential expression of proteins of distinct fertility states can provide potential mechanism implications underlying the decreased male fertility. In oligoasthenozoospermic patients, a proteome comprising 734 proteins in seminal plasma was established with 22 upregulated, and 20 downregulated through comparative proteomics using the label-free high-throughput iTRAQ approach [27]. Another study on seminal extracellular vesicles proteome from asthenozoospermia patients identified a list of 91 differentially expressed proteins, of which 11 proteins were significantly upregulated and 80 were downregulated [23]. For a male with primary infertility and one with secondary infertility, 48 and 53 proteins in seminal plasma were differentially expressed to the control group, respectively. ANXA2, CDC42, and SEMG2 are dysregulated in primary infertility and ANXA2 and APP were overexpressed in secondary infertility, which may serve as diagnostic biomarkers [44].
An abnormal male reproductive system anatomy structure induces the alteration of SP proteins. Proteomic analysis demonstrates that 91 proteins are differentially expressed in varicocele compared to 12 months after varicocelectomy samples from the same individuals [45]. In prostatitis patient seminal plasma, a total of 59 proteins were identified, 33 of which were significantly increased, whereas 26 were decreased in prostatitis compared to normal controls [46]. In varicocele-associated infertility, such as bilateral varicocele patients, global proteomic analysis of SP proteins revealed that the SP homeostasis is compromised due to the dysregulation of proteins involved in ROS response and tissue homeostasis compared with healthy men [19]. Further, it seems that smoking has a synergistic effect with varicocele on SP proteome, and a cross-sectional study revealed that varicocele smokers presented lower mitochondrial activity and acrosome integrity and higher DNA fragmentation compared with non-smokers. Ninety-one proteins, including neprilysin, beta-defensin 106A, and histone H4A, were dysregulated, which indicated that smoking triggers the establishment of inflammatory protein pathways in the testis/epididymis with varicocele [47]. Additionally, for men with normal fertility, smoking has an adverse impact on functional aspects of sperms such as sperm DNA damage and the destruction of acrosomes’ integrity. Proteomic analysis by LC/MS detected 422 proteins, of which 34 are differentially expressed and associated with an inflammatory state [31]. Obesity is another factor resulting from an unhealthy lifestyle which is believed to be potentially related to male fertility decline. Proteomics analysis of seminal plasma from obese men revealed 69 proteins differentially expressed, enriched in pathways such as apoptosis, activation of immune and inflammatory responses, and antioxidant activity compared with males with a normal BMI. Simultaneously, sperm in the obesity group presented decreased non-progressive motility and acrosome integrity and increased sperm DNA fragmentation [25].
In addition to protein expression, post-translational modification is a remarkable aspect of protein function regulation. Glycoproteins on the cell surface predominantly mediate cell–cell and cell–protein interactions. A typical example is Gd-S, the male-specific form of glycodelin S (Gd-S), highly expressed in seminal vesicles [48]. Glycosylated glycodelin binds to the sperm to inhibit capacitated until ejaculation pass through the cervix. Conversely, reversible binding enables the immediate disassociation of Gd-S from the sperm surface immediately when capacitation and fertilization are demanded [49]. Employing enrichment of N-linked glycosylated peptide-coupled LC-MS/MS analysis, Yang et al. found the first N-linked glycoproteome in human SP, in which 372 proteins were identified at 720 N-glycosylated sites [32].
3. Cell-Free Nucleic Acids in SP
Seminal plasma cell-free nucleic acids mainly include cell-free DNAs (cfDNAs) and cell-free RNAs (cfRNAs), comprising messenger RNAs (mRNAs) and long non-coding RNA (LncRNAs), microRNAs (miRNAs), and piwi-interacting RNAs (piRNAs). cfDNA can be detected in semen by modified capillary gel electrophoresis (CE) followed by SYBR-Gold staining. Interestingly, low-molecular-weight seminal cfDNA content is significantly positively associated with sperm rapid progression, curvilinear velocity, morphology, and capacitation index [50]. The average concentration of cfDNA from normozoospermic semen is 1.34 ± 0.65 μg/mL, whereas a higher concentration can be detected in azoospermia with a value of 2.56 ± 1.43 μg/mL [51]. Moreover, testis-specific methylated gene promoters (CCNA1, ACRBP, CIB1, DMRT1, and HSF1) can be detected from seminal cfDNA, which correlated with methylation percentage in testicular DNA and displayed dynamic changes among nonobstructive azoospermia (NOA) patients and normal controls [52]. This study demonstrated that cfDNA carries epigenetic information originating from the testes and could be a candidate as a non-invasive biomarker for spermatogenesis abnormalities. More recently, a mitochondrial DNA copy number in seminal plasma was detected in semen, significantly decreased in asthenozoospermia and oligoasthenozoospermia patients compared with normal controls, and positively correlated with semen parameters, such as sperm concentration and motility [53].
MicroRNAs (miRNAs) are a type of small-molecule RNA that are widely involved in a variety of physiological pathways of the body, including stress response, cell differentiation, cell metabolism, cell apoptosis, and the generation and metastasis of tumors [54] as well as the much-implicated spermatogenesis process [55,56]. Recent growing studies have uncovered abundant and stable miRNAs in seminal plasma, but only about 20% of free miRNAs are stored in microvesicles (MV) and exist in the form of seminal corpuscles, and most of the remaining seminal plasma is free miRNA that exists as a protein complex [57]. Simultaneously, the seminal plasma miRNA expression profile of male infertile patients is significantly different from normal controls. Some specific seminal plasma miRNA changes are closely related to male infertility and spermatogenic disorders, which can be used as new diagnostic markers of male infertility. The study of seminal plasma miRNA of specific changes can provide a new direction for studying the molecular mechanism of male infertility (Table 2).

Table 2.
Functional microRNAs presented in seminal plasma.
In 2011, 19 miRNAs with a significant difference in seminal plasma between infertile and fertile men were screened by Wang et al., of which seven kinds of miRNA (miR-34c-5p, miR-122, miR-146b-5p, miR-181a, miR-374b, miR-509-5p, and miR-513a-5p) were significantly decreased in azoospermia patients [68]. Subsequently, in another study, Wu et al. found that the expression of miR-19b and miR-7a was significantly upregulated in the seminal plasma of NOA patients, but there was no significant difference between asthenospermia patients and normal controls [69]. Further, in vitro experiments showed that miR-141 down-regulated the expression of CBL and TGFβ-2 protein, and miR-7-1-3p down-regulated the expression of RB1 and PIK3R3 [70]. It has been reported that there are significant changes in miRNA in the epididymis and seminal plasma of vasectomized men [71]. The above results show that the seminal plasma miRNA expression profile of male infertile patients is significantly different from that of the normal control group and the seminal plasma miRNA with specific changes can be used as a potential marker for the diagnosis of male infertility.
For patients with sperm disorders caused by varicocele, miR-210-3p can be used as a biomarker for screening, which is the key to determine effective early treatment and protect patients’ fertility [62]. miR-192a induces apoptosis of GC-2 cells by activating caspase-3 protein, which is also a potential marker to successfully indicate the sperm present in patients’ ejaculatory fluid with varicocele after microsurgery [64]. Indeed, the levels of miR-122, miR-181a, and miR-34c-5p in seminal plasma were significantly decreased in infertile men with varicocele and oligozoospermia and were related to apoptosis markers (BAX and BCL2) and oxidative stress (OS) [72].
Since the content of RNA molecules, especially miRNA, in the seminal plasma of exosomes changes with the origin cell, it can accurately reflect the pathological and physiological changes of reproductive organs and can be used as a potential reliable biomarker. Using ultracentrifugation technology, Abu-Halima et al. isolated extracellular microbubbles, including exosomes from seminal plasma, analyzed the expression of 1205 miRNAs by chip technology, and identified 36 miRNAs with altered expression levels, including 7 miRNAs with high expression and 29 with low expression in oligoasthenospermia [73]. Recently, Barcelo et al. compared the miRNA expression profiles of seminal plasma exosomes between patients with different pathological types of azoospermia (obstructive azoospermia and endocrine azoospermia). Five miRNAs (miR-182-3p, miR-205-5p, miR-31-5p, miR-539-5p, and miR-941) were found to be potential biomarkers in patients with endocrine azoospermia [74]. The above results suggest that the change in miRNA expression in seminal plasma exosomes will contribute to the development of male infertility molecular markers and molecular mechanisms.
The sperm DNA fragmentation index (SDF), as an important supplement to semen routine parameters, has been proposed to distinguish fertile and infertile men and to predict the outcome of natural pregnancy and in vitro fertilization. Che et al. found that the level of miR-424 (mouse homologous miR-322) in the seminal plasma of infertile patients with high DNA fragmentation index (DFI) was much lower than that of the fertility group and established a GC-2 cell model of down-regulation of miR-322, which leads to spermatogenesis disturbance. It was observed that after inhibition of miR-322, the survival rate of GC-2 cells decreased significantly and apoptosis increased significantly. MiR-322 plays a crucial role in promoting the apoptosis of GC-2 cells by directly regulating the expression of DDX3X. The downregulation of miR-424 expression in infertile men may directly affect spermatogenic cell apoptosis and sperm DNA damage [60]. Other studies have shown that the decreased expression of miR-374b and miR-26b can be used as the first indicator of the increase in SDF and they can be used as auxiliary biomarkers to diagnose male idiopathic infertility [75].
Long non-coding RNAs (lncRNAs) are a class of RNAs with a length longer than 200 nucleotides, acting as gene expression regulators from both transcription and post-transcription aspects [76,77]. RNA-sequencing of seminal plasma exosomes revealed the existence of lncRNAs, some of which differentially expressed in asthenozoospermia compared with a normal group, indicating a pivotal role of lncRNAs as molecular mechanisms of asthenozoospermia [78]. In addition, the expression profile of extracellular vesicle lncRNAs in nonobstructive azoospermia (NOA) patients is significantly altered, of which a set of differentially expressed testis-specific lncRNAs displayed valuable prediction potential to assess the presence of testicular spermatozoa in NOA patients [79].
4. Regulation of Hormones on SP Components
Sex steroid hormones, including androgen and estrogen, are present in seminal plasma, in cooperation with other constituents necessary for normal spermatogenesis, protection, and spermatozoa maturation. As early as the 1970s, levels of steroid hormones in seminal plasma have been determined by many studies [80,81]. As for testosterone, the range of its concentration in seminal plasma from normal persons is very broad due to the methodology employed rather than to differences in sperm parameters [81]. In most of the earlier papers, no significant testosterone content differences were observed between normal and impaired spermatogenesis [82,83,84,85]. However, later refined studies discriminated the correlation between sperm abnormalities (oligo-, astheno-, and azoospermia) and hormone concentrations. Testosterone levels in infertile patients (oligozoospermia, obstructive azoospermia (OA), and nonobstructive azoospermia (NOA)) are significantly lower than those of normospermia men [86]. For varicocele, it is still controversial whether seminal testosterone concentration is altered or not. One study stated an observed slightly higher testosterone concentration in men with varicocele [87], but another reported no significant differences in varicocele patients from controls [88].
Alterations in SP hormone profiles are associated with elevated sperm abnormalities. Seminal testosterone and dihydrotestosterone concentrations were significantly lower in patients with abnormal sperm characteristics, such as sperm concentration and motility, than in men with normozoospermia [89]. The effect of hormone changes on other compositions in seminal plasma has been explored. In secondary male hypogonadism patients with significantly lower testosterone, estradiol (E2), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) in serum, 33 proteins were missing among the 61 proteins detected in the normogonadal men, which indicated the effect of hormones on the secretory function of male accessory glands. Further, 14 proteins reappeared after 6 months of testosterone replacement therapy (TRT), confirming testosterone regulation on seminal plasma constituents [90]. Another study on seminal plasma protein profiling of hypogonadotropic hypogonadism patients showed 11 proteins’ levels were decreased compared with controls, which were mainly involved in hydrolase activity and protein-binding activity, whereas the levels of six proteins were recovered after testosterone replacement therapy (TRT) [21]. Notably, the significantly reduced levels of prostatic-acid phosphatase, zinc, prostate-specific antigen, lactoferrin, and fructose in seminal plasma were observed after testosterone administration [91]. In addition, a single injection of testosterone enanthate (TE) in oligospermic men led to increased ornithine decarboxylase activity and higher levels of fructose and sialic acid [92].
Apart from testosterone, other hormones, such as estradiol, human chorionic gonadotropin, Anti-Müllerian hormone (AMH), and relaxin, also are presented in seminal plasma. For infertile men, the estradiol level was significantly increased [85]. Specifically, estradiol levels in OA patients are significantly higher than those in normospermic and NOA cases [86]. AMH is a glycoprotein produced by Sertoli cells that belongs to the transforming growth factor (TGF) beta superfamily. Thus, it is reasonable that seminal AMH is undetectable in all OA patients due to its origin, and besides, in NOA patients, seminal AMH concentration was also significantly lower and may serve as a seminal marker for spermatogenesis [93]. In addition, total AMH (pmol/ejaculate) content in seminal plasma was positively correlated with sperm concentration and sperm count [94,95]. For hCG, its free beta-subunit levels are significantly lower in infertile patients than those in controls and correlated with sperm count and sperm motility [96]. Relaxin is a hormone that contributes to pregnancy and parturition in females and can be detected in seminal plasma at a concentration of approximately 50 ng/mL, which is mainly produced by the prostate gland [97,98]. Relaxin can positively affect sperm functions related to fertilization ability [99]. However, there are disagreements regarding relaxin’s effects on sperm motility. Some groups indeed observed statistically significant effects of relaxin on human sperm motility [100,101], but other studies did not present effective data of relaxin’s effect on the motility of sperm [102,103].
5. SP Factors and Related Pathways
The interactions among SP factors composing signaling pathways make their functions more complicated; thus, it is challenging to dissect each factor and related pathways in the modulation of sperm function and interaction with the maternal reproductive tract. Several signaling pathways have been reported to implicate seminal plasma factors. For example, extracellular Zn2+ in seminal plasma binds to G protein-coupled receptor 39 (GPR39)-type Zn-receptor and activates the AC-cAMP-PKA-Src-EGFR signaling cascade, which is essential for hyperactivated motility (HAM) during sperm capacitation [104]. Besides, Fms-like tyrosine kinase 3 (FLT3) is a type III kinase enriched in seminal plasma and highly expressed in infertile men. Its inhibition can repress fertilization and early embryonic development through a PKA-dependent pathway, which indicates that FLT3 is a key factor for male fertility [105]. Alterations in distinct pathways can be induced by internal healthy state and external factors. Obesity negatively regulates proteins enriched in the intrinsic pathway of apoptosis, activation of immune and inflammatory responses, and antioxidant activity [25]. In bilateral varicocele patients, compared with healthy men, pathways involved in response to reactive oxygen species and oxidative stress and tissue homeostasis were affected [19]. Smoking influences inflammatory state-linked signaling pathways, such as antigen processing and presentation, protein kinase A signaling, complement activation, the cytokine-mediated signaling pathway regulation, and acute inflammatory response regulation, which indicates the inflammatory activity of seminal plasma in coping with cigarette toxicity [31].
6. SP Factors Function as Immunomodulators in the Maternal Reproductive Tract
It is well known that sperm is an auto-antigen and an alloantigen in both men and women, and immune factors also play an important role in infertility. However, due to a series of immune protection mechanisms, including a series of immunological mediators found in seminal plasma in recent years, this avoids the occurrence of the autoimmune phenomenon and fertilized egg rejection by affecting maternal immune cell function [106]. Cellular integration between the immune system and the reproductive system is a basis for normal male reproductive physiology. Cytokines are part of the male reproductive tract’s autocrine/paracrine network, which plays a vital role in testicular function and spermatogenesis. IL-6 is the core member of cytokines, which is produced by activated T lymphocytes, peripheral blood monocytes, macrophages, vascular endothelial cells, and smooth muscle cells, and participates in inflammation and immunity. Seshardi et al. and Camejo et al. reported an increase in pro-inflammatory IL-6 levels in SP in infertile patients [107,108]. Interestingly, Seshadri et al. found that the levels of IL-6 in mild and severe oligozoospermia, and IL-8 and IL-10 levels in asthenospermia, were significantly increased. In addition, Qian et al. found that the level of IL-11 in the semen of the infertility group, which was positively correlated with sperm viability, motility, survival rate, and normal sperm morphology but negatively correlated with IL-17 and IL-18 levels, was significantly lower than that of the normal sperm group [109].
Transforming growth factor-β is produced by seminal vesicles and the prostate and its content in seminal plasma is several times higher than in serum. The TGF-β family includes three polypeptides, TGF-β1, TGF-β2, and TGF-β3, all of which exist in human SP. TGF-β1 is the most abundant TGF-β [110]. It is reported that the levels of TGF-β1 and IL-18 in seminal plasma are related to the reproductive outcome of patients exposed to SP during in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) [111]. It can be seen that cytokines rarely play a role alone, rather they play a role in a complex network, directly or indirectly affecting sperm function. The relationship between cytokines and the reproductive system is mutual. All kinds of cells in the reproductive system can produce cytokines on their own and regulate the secretion of cytokines. If the secretion of cytokines is abnormal, it may lead to the damage of reproductive function, which may be due to the increase or decrease in suppressor T lymphocytes’ activity and the activation of helper T cells. At present, many studies have confirmed that seminal plasma immune factors are closely related to infertility and reproductive outcome. Future monitoring of more cytokines and other immune factors in seminal plasma will provide evidence for a better understanding of the mechanism of infertility (Figure 2).
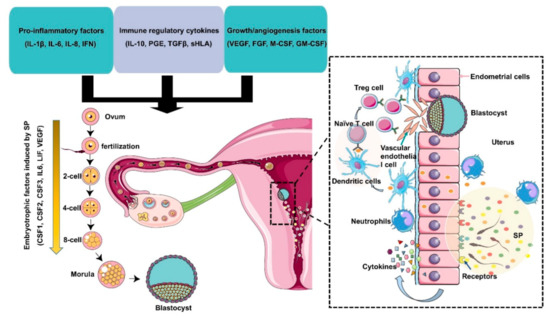
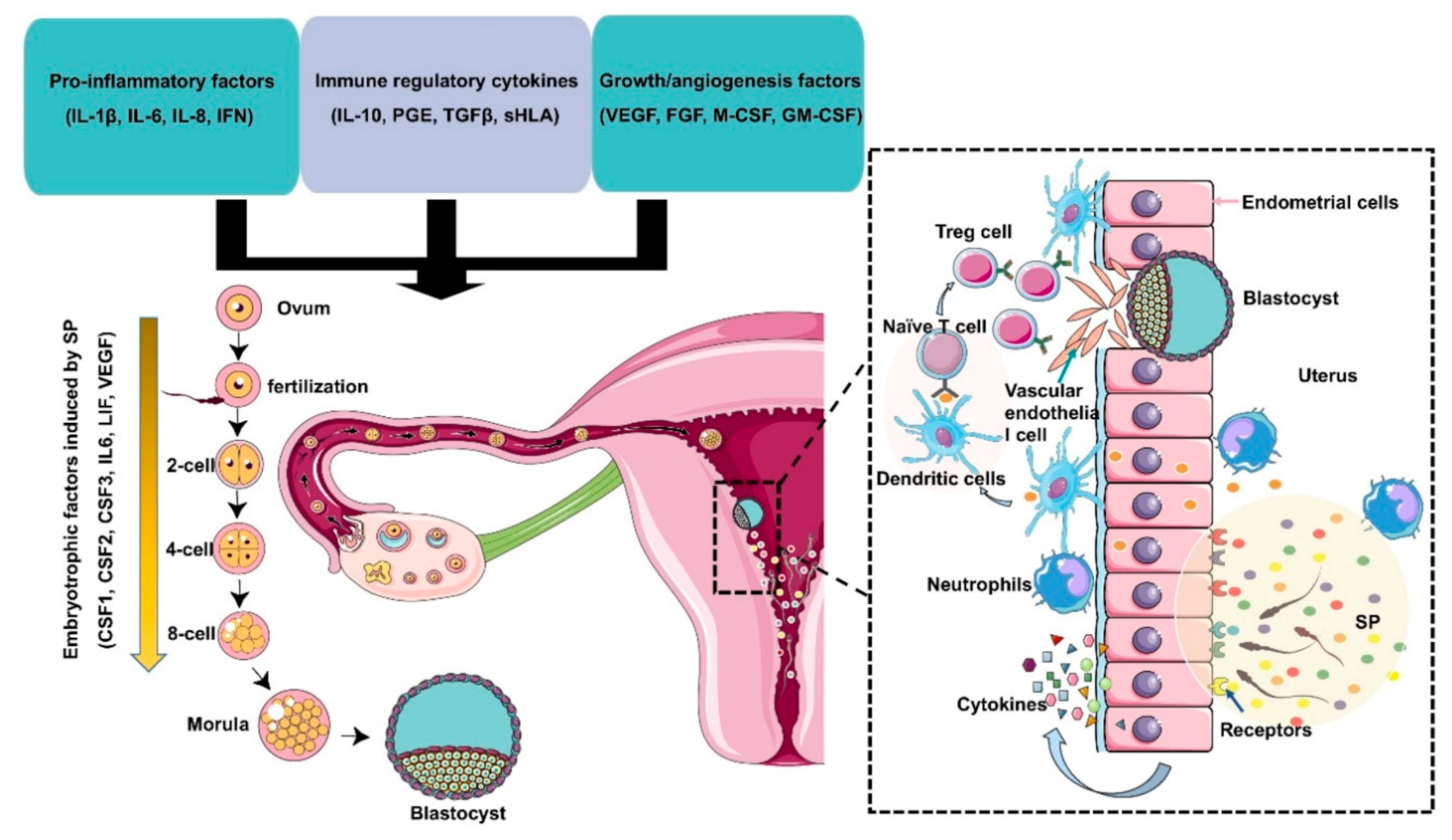
Figure 2.
Immunomodulation of SP factors in the maternal reproductive tract to facilitate early embryo development and implantation. Seminal plasma contains chemokines, cytokines, and prostaglandins produced by Leydig and Sertoli cells, seminal vesicles, leukocytes, and other immune cells present in the male reproductive tract. These factors potentially induce inflammation, immunotolerance, and angiogenesis to support embryo development and implantation through the upregulation of embryokines, chemokines, and cytokines from oviductal and uterine tissues. Binding of active moieties in SP to endothelial cells stimulates the recruitment of neutrophils into the uterine tissues, which can clear up excess sperm and seminal debris. Moreover, dendritic cells can recognize paternal major histocompatibility antigens and pass them onto naive T cells and generate an expanded T regulatory (Treg) cell pool in lymph nodes. Since the embryo presents the same paternal antigens in the SP, the response of Tregs to these antigens can be efficiently suppressed, which allows implantation of the semiallogenic embryo into the maternal uterine. On the other hand, Tregs also contribute to endometrial tissue remodeling and receptivity for implantation through the regulation of angiogenesis. SP also results in the production of cytokines and growth factors, such as colony-stimulating factors (CSFs), leukemia inhibitory factor (LIF), and interleukin 6 (IL-6), by the maternal reproductive tract, which are essential for promoting embryo development before implantation. The arrow indicates that embryotrophic factors (CSF1, CSF2, CSF3, IL6, VEGF) are induced by SP during the early embryonic development.
Under the factors of injury, infection, and obstruction, the abnormal opening and regulation of the blood–testis barrier lead to the contact of spermatocytes, spermatids, and sperm with the immune system of the body, resulting in autoimmune response and the formation of various types of anti-sperm antibodies (ASA). Sperm autoimmune reaction, which produces anti-sperm antibodies, is also one of the causes of male infertility, accounting for about 10–30% of infertile patients [112,113]. In normal spermatozoa, the sperm autoimmune response leads to a decline in fertility caused by the acrosome reaction (AR) and dysfunction of capacitation and DNA fragmentation. The damage of sperm quality in immune infertility is more obvious: decreased sperm concentration and motility, morphological changes, acrosome reaction damage, and DNA fragmentation. The possible pathogenesis of these damages is related to oxidative stress (OS) [114]. Researchers have found that the production of AsAb is closely related to the level of the cytokine TNF-α in infertile patients, and the levels of AsAb and TNF-α in seminal plasma are significantly higher than those in serum, which indicates that local immune activation and immune damage in reproductive tract play a vital role in the occurrence of male infertility [115]. ASA is also found in the seminal plasma of patients with varicocele. In evaluating fertility recovery after varicocelectomy, anti-sperm immune response reduces the effect of varicocelectomy on reproductive function recovery; the higher the proportion of ASA, the lower the grade of varicocele and the worse the prognosis [116]. A systematic review and meta-analysis showed that sperm anti-sperm antibody was not associated with pregnancy rate after IVF or ICSI, indicating that these two kinds of assisted reproductive technologies are still feasible options for infertile couples with anti-sperm antibodies [117].
There is an immunosuppressive substance in seminal plasma, seminal plasma immunosuppressive factor (SPIF), which can block and change sperm membrane antigen and inhibit the immune system, such as lymphocytes, NK cells, macrophages, and complement systems [118,119,120]. SPIF is one of the crucial factors to protect sperm. Under normal circumstances, SPIF can protect sperm from the attack of immune cells and autoimmune responses and play an immunological role in the male reproductive system, ensuring the reproductive process’ smooth progress. The lack of immunosuppressive factors in male sperm can lead to the production of anti-sperm antibodies, resulting in infertility, or the spouse develops an allergic reaction or anti-sperm antibody after sexual intercourse [121,122,123]. At the same time, the existence of SPIF also promotes the occurrence, infection, and transmission of some diseases (such as AIDS). When the activity of SPIF is too high, the recognition and killing effect of immune factors on malignant tumors and pathogenic microorganisms is weakened and it is easy to spread sexually transmitted diseases (STDs); if the activity of SPIF is too low, it cannot effectively inhibit the killing effect of immune factors on sperm, resulting in the decrease in sperm motility and viability and the implantation ability of fertilized eggs. In some conditions, such as reproductive tract infection or trauma, SPIF can induce the body to produce anti-SPIF antibodies so that the amount of SPIF is relatively reduced, resulting in low SPIF activity. Vanage et al. found that SPIF antibody could induce sperm agglutination mediated by antibody, resulting in decreased fertility, and determined the positive rates of anti-SPIF-IgG and IgA; anti-SPIF-IgG levels in the seminal plasma of infertile men were slightly higher than those of IgA but there was no significant difference. This study suggested that there may be two sources of anti-SPIF antibodies in seminal plasma [120].
7. Potential Application of Seminal Plasma Factors as Bio-Markers
It is promising that seminal plasma proteins might be sources of biomarkers for the noninvasive diagnosis of male fertility disorders due to their relatively high levels and ease of collection. Aberrant protein concentration changes in seminal plasma can indicate pathological process stages and discriminate different types of male infertility. The verified and validated protein biomarkers are L-PGDS, ACRV1, ECM1, and TEX10. L-PGDS is a 26KDa enzyme and glycoprotein, mainly secreted by Sertoli cells, with unclear male fertility function. ELISA determined the seminal concentration of this enzyme ranging from 0.3 to 42 μg/mL, which was significantly lower in the oligozoospermic group (2.47 ± 0.51 μg/mL) than in the normozoospermic group (9.75 ± 1.49 μg/mL) [124]. A more detailed association study revealed that the concentration of seminal L-PGDS is significantly positively correlated with sperm concentration, sperm motility, and percentage of normal morphology and decreased progressive motility from normal to oligospermic patients [125]. Male infertility can be classified into oligozoospermia, asthenospermia, and azoospermia, based on the number and motility of sperm, among which azoospermia is the most severe of male infertility, with near absence of sperm in semen. SP-10 is an acrosomal matrix protein encoded by the acrosomal vesicle protein 1 (ACRV1) gene, which is specifically expressed in spermatids after meiosis and sperm [126]. ELISA using monoclonal antibody revealed a direct relationship between sperm count and seminal concentration of SP-10, which permits it to be a biomarker for oligozoospermic diagnosis. SpermCheck Fertility, a reliable and straightforward immunodiagnostic test based on the above idea, was developed, which quickly provides information for males on whether their sperm number is normal or not to evaluate their fertility [127], and the clinical and consumer trial of SpermCheck Fertility has been applied to detect extreme oligospermia or azoospermia [128].
To date, testicular biopsy is the only valuable method to detect whether spermatozoa are in the testis or not and to distinguish obstructive azoospermia (OA) and nonobstructive azoospermia (NOA). However, surgical exploration of random testicular tissue may not accurately reflect NOA histopathology because of the spatial distribution of spermatogenesis [129]. Searching for biomarkers may allow for avoiding the painful biopsy and deviation of the diagnosis results. Fortunately, some proteins can potentially differentiate normal, OA, and NOA patients. Seminal L-PGDS level could be applied as a biomarker for azoospermia, and high L-PGDS (more than 100 g/L) in men with azoospermia could be potentially diagnosed as nonobstructive azoospermia without biopsy, which contributes to infertility in almost 30% of these men [130]. The concentrations of epididymis-specific expressed ECM1 in OA patients are significantly lower than in control and NOA cases and, more importantly, could distinguish OA cases from normal ones with 100% specificity and sensitivity, and OAs from NOAs with 73% specificity, at 100% sensitivity at a cutoff of 2.3 mg/mL [129].
TEX101 is a membrane glycoprotein and is specifically expressed in germ cells without any expression in other tissues and cells, which can be cleaved from the spermatozoa surface and released into seminal plasma during sperm maturation into epididymis [131]. The notably decreased concentration in azoospermia makes it valuable as a biomarker to discriminate azoospermia from normal controls. Furthermore, the specific expression pattern allows it to differentiate various histopathological NOA subtypes, including hypospermatogenesis (HS), maturation arrest (MA), and Sertoli cell-only syndrome (SCO). In fact, the average concentration of seminal TEX101 from normal men is approximately 2 mg/mL, whereas there are low levels in SP (<120 ng/mL) in NOA cases with HS and MA, and it is not detectable in SCO without germ cells [129].
8. Conclusions and Perspective
To summarize, this paper reviewed the latest achievements of proteins and cell-free nucleic acids present in seminal plasma and the immunomodulation function in male infertility. Standard semen parameters are poor predictors of reproductive outcomes. Clinically employing the “gold-standard” semen analysis provides limited sperm information and cannot discriminate fertile from infertile men because of individual bias. Seminal factors contributing to men’s infertility have been explored over the past few decades, along with developing tools to evaluate and diagnose infertility. Although the emerging “omics” technologies promised to validate or discover known or novel factors, due to the complexity and variability of seminal plasma composition, it is still challenging to determine their origins from accessory sex glands and the proper functions contributing to sperm and to explain the underlying molecular mechanisms. Encouraging signs of progress have been made in the development of biomarkers in seminal plasma, for instance, the success of commercial TEX101- and ECM1-based immunodiagnostic assays with high specificity and sensitivity for azoospermia. Researchers will keep working on exploring and validating the function of SP factors to develop biomarkers for male infertility diagnosis and clinical consultation.
Author Contributions
F.W., W.Y. and S.O. reviewed the literature and wrote the manuscript. S.Y. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work supported by grants from the National Natural Science Foundation of China (31900511 to F.L.) and the Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology (No. 2019kfyXJJS089 to F.L.).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DEP | Differentially expressed proteins |
| LC-MS/MS | Liquid chromatography (LC)-tandem mass spectrometry (MS) |
| HPLC/MS | High Performance Liquid Chromatography coupled mass spectrometry |
| CID LC-MS/MS | Collision-induced dissociation, Liquid chromatography (LC)-tandem mass spectrometry |
| SELDI-TOF-MS | Surface-Enhanced Laser Desorption Ionization Time-of-Flight mass spectrometry |
| 2D SDS-PAGE | Two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| iTRAQ | Isobaric tag for relative and absolute quantification |
| 1D-PAGE | One dimensional (1D)-polyacrylamide gel electrophoresis (PAGE) |
| DEPs | Differentially expressed proteins |
| ESI-QTOF MS/MS | Electrospray ionization-quadrupole/time-of-flight tandem mass spectrometry |
| 2D DIGE | Two-dimensional differential gel electrophoresis |
References
- Gurunath, S.; Pandian, Z.; Anderson, R.A.; Bhattacharya, S. Defining infertility—A systematic review of prevalence studies. Hum. Reprod. Update 2011, 17, 575–588. [Google Scholar] [CrossRef]
- Sullivan, E.A.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; De Mouzon, J.; Nygren, K.G.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: Assisted reproductive technology 2004. Hum. Reprod. 2013, 28, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Thonneau, P.; Marchand, S.; Tallec, A.; Ferial, M.L.; Ducot, B.; Lansac, J.; Lopes, P.; Tabaste, J.M.; Spira, A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum. Reprod. 1991, 6, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gilany, K.; Minai-Tehrani, A.; Savadi-Shiraz, E.; Rezadoost, H.; Lakpour, N. Exploring the human seminal plasma proteome: An unexplored gold mine of biomarker for male infertility and male reproduction disorder. J. Reprod. Infertil. 2015, 16, 61–71. [Google Scholar] [PubMed]
- World Health Organization. WHO Laboratory Manual for The Examination And Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Swizerland, 2010. [Google Scholar]
- Hamada, A.; Esteves, S.C.; Nizza, M.; Agarwal, A. Unexplained male infertility: Diagnosis and management. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2012, 38, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Szczykutowicz, J.; Kaluza, A.; Kazmierowska-Niemczuk, M.; Ferens-Sieczkowska, M. The Potential Role of Seminal Plasma in the Fertilization Outcomes. Biomed. Res. Int. 2019, 2019, 5397804. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, P.; Bergeron, A.; Lefebvre, J.; Fan, J. Seminal plasma proteins: Functions and interaction with protective agents during semen preservation. Soc. Reprod. Fertil. Suppl. 2007, 65, 217–228. [Google Scholar] [PubMed]
- Moon, K.H.; Bunge, R.G. Observations on the biochemistry of human semen: 5. Protein. Fertil. Steril. 1970, 21, 220–221. [Google Scholar] [CrossRef]
- Robert, M.; Gagnon, C. Sperm motility inhibitor from human seminal plasma: Presence of a precursor molecule in seminal vesicle fluid and its molecular processing after ejaculation. Int. J. Androl. 1994, 17, 232–240. [Google Scholar] [CrossRef]
- Batruch, I.; Lecker, I.; Kagedan, D.; Smith, C.R.; Mullen, B.J.; Grober, E.; Lo, K.C.; Diamandis, E.P.; Jarvi, K.A. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J. Proteome Res. 2011, 10, 941–953. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Messana, I.; Pontecorvi, A.; De Marinis, L.; Castagnola, M.; Marana, R. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil. Steril. 2012, 97, 67–73.e1. [Google Scholar] [CrossRef]
- Utleg, A.G.; Yi, E.C.; Xie, T.; Shannon, P.; White, J.T.; Goodlett, D.R.; Hood, L.; Lin, B. Proteomic analysis of human prostasomes. Prostate 2003, 56, 150–161. [Google Scholar] [CrossRef]
- Thimon, V.; Frenette, G.; Saez, F.; Thabet, M.; Sullivan, R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: A proteomic and genomic approach. Hum. Reprod. 2008, 23, 1698–1707. [Google Scholar] [CrossRef]
- Edwards, J.J.; Tollaksen, S.L.; Anderson, N.G. Proteins of human semen. I. Two-dimensional mapping of human seminal fluid. Clin. Chem. 1981, 27, 1335–1340. [Google Scholar] [CrossRef]
- Martinez-Heredia, J.; De Mateo, S.; Vidal-Taboada, J.M.; Ballesca, J.L.; Oliva, R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum. Reprod. 2008, 23, 783–791. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Baskaran, S. Proteomic analysis of seminal plasma from bilateral varicocele patients indicates an oxidative state and increased inflammatory response. Asian J. 2019, 21, 544–550. [Google Scholar]
- Wu, Y.; Yuan, Y.; Chen, L.; Wang, M.; Yang, Y.; Wang, Y.; Quan, C.; Chen, D.; Chen, Y.; Huang, X.; et al. Quantitative Proteomic Analysis of Human Seminal Plasma from Normozoospermic and Asthenozoospermic Individuals. Biomed. Res. Int. 2019, 2019, 2735038. [Google Scholar] [CrossRef]
- Grande, G.; Vincenzoni, F.; Mancini, F.; Barrachina, F.; Giampietro, A.; Castagnola, M.; Urbani, A.; Oliva, R.; Milardi, D.; Pontecorvi, A. Quantitative Analysis of the Seminal Plasma Proteome in Secondary Hypogonadism. J. Clin. Med. 2019, 8, 2128. [Google Scholar] [CrossRef]
- Kanannejad, Z.; Gharesi-Fard, B. Difference in the seminal plasma protein expression in unexplained infertile men with successful and unsuccessful in vitro fertilisation outcome. Andrologia 2019, 51, e13158. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, A.; He, Y.; Li, Z.; Li, Z.; Wang, G.; Sun, F. Proteomic analysis of seminal extracellular vesicle proteins involved in asthenozoospermia by iTRAQ. Mol. Reprod. Dev. 2019, 86, 1094–1105. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Sharma, R.; Samanta, L.; Gupta, S.; Dias, T.R.; Martins, A.D. Protein Fingerprinting of Seminal Plasma Reveals Dysregulation of Exosome-Associated Proteins in Infertile Men with Unilateral Varicocele. World J. Mens Health 2019, 37. [Google Scholar] [CrossRef] [PubMed]
- Ferigolo, P.C.; Ribeiro de Andrade, M.B.; Camargo, M.; Carvalho, V.M.; Cardozo, K.H.M.; Bertolla, R.P.; Fraietta, R. Sperm functional aspects and enriched proteomic pathways of seminal plasma of adult men with obesity. Andrology 2019, 7, 341–349. [Google Scholar] [CrossRef]
- Grande, G.; Vincenzoni, F.; Mancini, F.; Baroni, S.; Luca, G.; Calafiore, R.; Marana, R.; Castagnola, M.; Pontecorvi, A.; Milardi, D. Semen Proteomics Reveals the Impact of Enterococcus faecalis on male Fertility. Protein Pept. Lett. 2018, 25, 472–477. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Zhu, P.; Wang, J.; Wang, Y.; Wang, X.; Liu, J.; Li, N.; Wang, X.; Lin, C.; et al. In-depth quantitative proteome analysis of seminal plasma from men with oligoasthenozoospermia and normozoospermia. Reprod. Biomed. Online 2018, 37, 467–479. [Google Scholar] [CrossRef]
- Yang, C.; Guo, W.B.; Zhang, W.S.; Bian, J.; Yang, J.K.; Zhou, Q.Z.; Chen, M.K.; Peng, W.; Qi, T.; Wang, C.Y.; et al. Comprehensive proteomics analysis of exosomes derived from human seminal plasma. Andrology 2017, 5, 1007–1015. [Google Scholar] [CrossRef]
- Del Giudice, P.T.; Belardin, L.B.; Camargo, M.; Zylbersztejn, D.S.; Carvalho, V.M.; Cardozo, K.H.; Bertolla, R.P.; Cedenho, A.P. Determination of testicular function in adolescents with varicocoele-A proteomics approach. Andrology 2016, 4, 447–455. [Google Scholar] [CrossRef]
- Saraswat, M.; Joenvaara, S.; Tomar, A.K.; Singh, S.; Yadav, S.; Renkonen, R. N-Glycoproteomics of Human Seminal Plasma Glycoproteins. J. Proteome Res. 2016, 15, 991–1001. [Google Scholar] [CrossRef]
- Antoniassi, M.P.; Intasqui, P.; Camargo, M.; Zylbersztejn, D.S.; Carvalho, V.M.; Cardozo, K.H.; Bertolla, R.P. Analysis of the functional aspects and seminal plasma proteomic profile of sperm from smokers. BJU Int. 2016, 118, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, F.; Yan, Y.; Zhou, T.; Guo, Y.; Sun, G.; Zhou, Z.; Zhang, W.; Guo, X.; Sha, J. Proteomic analysis of N-glycosylation of human seminal plasma. Proteomics 2015, 15, 1255–1258. [Google Scholar] [CrossRef]
- Intasqui, P.; Antoniassi, M.P.; Camargo, M.; Nichi, M.; Carvalho, V.M.; Cardozo, K.H.; Zylbersztejn, D.S.; Bertolla, R.P. Differences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parameters. Fertil. Steril. 2015, 104, 292–301. [Google Scholar] [CrossRef]
- Cadavid, J.A.; Alvarez, A.; Markert, U.R.; Cardona Maya, W. Differential protein expression in seminal plasma from fertile and infertile males. J. Hum. Reprod. Sci. 2014, 7, 206–211. [Google Scholar]
- Sharma, R.; Agarwal, A.; Mohanty, G.; Du Plessis, S.S.; Gopalan, B.; Willard, B.; Yadav, S.P.; Sabanegh, E. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod. Biol. Endocrinol. 2013, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.F.; Souza, G.H.; Lo Turco, E.G.; Del Giudice, P.T.; Soler, T.B.; Spaine, D.M.; Borrelli Junior, M.; Gozzo, F.C.; Pilau, E.J.; Garcia, J.S.; et al. Differential seminal plasma proteome according to semen retrieval in men with spinal cord injury. Fertil. Steril. 2013, 100, 959–969. [Google Scholar] [CrossRef]
- Del Giudice, P.T.; da Silva, B.F.; Lo Turco, E.G.; Fraietta, R.; Spaine, D.M.; Santos, L.F.; Pilau, E.J.; Gozzo, F.C.; Cedenho, A.P.; Bertolla, R.P. Changes in the seminal plasma proteome of adolescents before and after varicocelectomy. Fertil. Steril. 2013, 100, 667–672. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui Lopes, P.; Del Giudice, P.T.; Carvalho, V.M.; Cardozo, K.H.; Andreoni, C.; Fraietta, R.; Bertolla, R.P. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Hum. Reprod. 2013, 28, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Davalieva, K.; Kiprijanovska, S.; Noveski, P.; Plaseski, T.; Kocevska, B.; Broussard, C.; Plaseska-Karanfilska, D. Proteomic analysis of seminal plasma in men with different spermatogenic impairment. Andrologia 2012, 44, 256–264. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Agarwal, A.; Ayaz, A.; Samanta, L.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Sabanegh, E. Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clin. Proteom. 2015, 12, 23. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A.; Mohanty, G.; Jesudasan, R.; Gopalan, B.; Willard, B.; Yadav, S.P.; Sabanegh, E. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reprod. Biol. Endocrinol. 2013, 11, 38. [Google Scholar] [CrossRef]
- Martins, A.D.; Panner Selvam, M.K.; Agarwal, A.; Alves, M.G.; Baskaran, S. Alterations in seminal plasma proteomic profile in men with primary and secondary infertility. Sci. Rep. 2020, 10, 7539. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Belardin, L.B.; Antoniassi, M.P.; Cardozo, K.H.M.; Carvalho, V.M.; Fraietta, R.; Bertolla, R.P. Molecular pathways of varicocele and its repair—A paired labelled shotgun proteomics approach. J. Proteom. 2019, 196, 22–32. [Google Scholar] [CrossRef]
- Kagedan, D.; Lecker, I.; Batruch, I.; Smith, C.; Kaploun, I.; Lo, K.; Grober, E.; Diamandis, E.P.; Jarvi, K.A. Characterization of the seminal plasma proteome in men with prostatitis by mass spectrometry. Clin. Proteom. 2012, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Antoniassi, M.P.; Belardin, L.B.; Camargo, M.; Intasqui, P.; Carvalho, V.M.; Cardozo, K.H.M.; Bertolla, R.P. Seminal plasma protein networks and enriched functions in varicocele: Effect of smoking. Andrologia 2020, 52, e13562. [Google Scholar] [CrossRef]
- Koistinen, H.; Koistinen, R.; Kamarainen, M.; Salo, J.; Seppala, M. Multiple forms of messenger ribonucleic acid encoding glycodelin in male genital tract. Lab. Investig. 1997, 76, 683–690. [Google Scholar]
- Chiu, P.C.; Chung, M.K.; Tsang, H.Y.; Koistinen, R.; Koistinen, H.; Seppala, M.; Lee, K.F.; Yeung, W.S. Glycodelin-S in human seminal plasma reduces cholesterol efflux and inhibits capacitation of spermatozoa. J. Biol. Chem. 2005, 280, 25580–25589. [Google Scholar] [CrossRef]
- Chou, J.S.; Jacobson, J.D.; Patton, W.C.; King, A.; Chan, P.J. Modified isocratic capillary electrophoresis detection of cell-free DNA in semen. J. Assist. Reprod. Genet. 2004, 21, 397–400. [Google Scholar] [CrossRef]
- Li, H.G.; Huang, S.Y.; Zhou, H.; Liao, A.H.; Xiong, C.L. Quick recovery and characterization of cell-free DNA in seminal plasma of normozoospermia and azoospermia: Implications for non-invasive genetic utilities. Asian J. 2009, 11, 703–709. [Google Scholar] [CrossRef]
- Wu, C.; Ding, X.; Tan, H.; Li, H.; Xiong, C. Alterations of testis-specific promoter methylation in cell-free seminal deoxyribonucleic acid of idiopathic nonobstructive azoospermic men with different testicular phenotypes. Fertil. Steril. 2016, 106, 1331–1337. [Google Scholar] [CrossRef]
- Chen, Y.; Liao, T.; Zhu, L.; Lin, X.; Wu, R.; Jin, L. Seminal plasma cell-free mitochondrial DNA copy number is associated with human semen quality. Eur. J. Obs. Gynecol. Reprod. Biol. 2018, 231, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef]
- McIver, S.C.; Roman, S.D.; Nixon, B.; McLaughlin, E.A. miRNA and mammalian male germ cells. Hum. Reprod. Update 2012, 18, 44–59. [Google Scholar] [CrossRef]
- Li, H.; Huang, S.; Guo, C.; Guan, H.; Xiong, C. Cell-free seminal mRNA and microRNA exist in different forms. PLoS ONE 2012, 7, e34566. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, Y.; Xiao, Q.; Zou, S.S.; Zhu, Y.C.; Ping, P.; Chen, X.F. Seminal exosomal miR-210-3p as a potential marker of Sertoli cell damage in varicocoele. Andrology 2020. [Google Scholar] [CrossRef]
- Eikmans, M.; DH Anholts, J.; Blijleven, L.; Meuleman, T.; van Beelen, E.; Van Der Hoorn, M.P.; Claas, F.H.J. Optimization of microRNA Acquirement from Seminal Plasma and Identification of Diminished Seminal microRNA-34b as Indicator of Low Semen Concentration. Int. J. Mol. Sci. 2020, 21, 4089. [Google Scholar] [CrossRef]
- Che, Q.; Wang, W.; Duan, P.; Fang, F.; Liu, C.; Zhou, T.; Li, H.; Xiong, C.; Zhao, K. Downregulation of miR-322 promotes apoptosis of GC-2 cell by targeting Ddx3x. Reprod. Biol. Endocrinol. 2019, 17, 63. [Google Scholar] [CrossRef]
- Radtke, A.; Dieckmann, K.P.; Grobelny, F.; Salzbrunn, A.; Oing, C.; Schulze, W.; Belge, G. Expression of miRNA-371a-3p in seminal plasma and ejaculate is associated with sperm concentration. Andrology 2019, 7, 469–474. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Yang, Y.; Liu, X.; Chen, Y. Seminal plasma miR-210-3p is a biomarker for screening dyszoospermia caused by varicocele. Andrologia 2019, 51, e13244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, Y.; Du, G.; Han, L.; Zheng, S.; Liang, J.; Huang, X.; Qin, Y.; Wu, W.; Chen, M.; et al. Down-regulated let-7b-5p represses glycolysis metabolism by targeting AURKB in asthenozoospermia. Gene 2018, 663, 83–87. [Google Scholar] [CrossRef]
- Zhi, E.L.; Liang, G.Q.; Li, P.; Chen, H.X.; Tian, R.H.; Xu, P.; Li, Z. Seminal plasma miR-192a: A biomarker predicting successful resolution of nonobstructive azoospermia following varicocele repair. Asian J. 2018, 20, 396–399. [Google Scholar]
- Zhang, X.; Wei, R.; Lou, J.; Zhou, J. [Seminal plasma miR-122-3p and miR-141-5p stability and its diagnosis value for idiopathic asthenospermia]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2016, 33, 320–323. [Google Scholar] [PubMed]
- Zhou, R.; Wang, R.; Qin, Y.; Ji, J.; Xu, M.; Wu, W.; Chen, M.; Wu, D.; Song, L.; Shen, H.; et al. Mitochondria-related miR-151a-5p reduces cellular ATP production by targeting CYTB in asthenozoospermia. Sci. Rep. 2015, 5, 17743. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, Y.; Yang, R.; Bai, Y.; Li, C.; Li, H.; Xiong, C. miR-424/322 is downregulated in the semen of patients with severe DNA damage and may regulate sperm DNA damage. Reprod. Fertil. Dev. 2015, 28, 1598–1607. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Chen, X.; Yao, B.; Yang, C.; Zhu, C.; Li, L.; Wang, J.; Li, X.; Shao, Y.; et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin. Chem. 2011, 57, 1722–1731. [Google Scholar] [CrossRef]
- Wu, W.; Hu, Z.; Qin, Y.; Dong, J.; Dai, J.; Lu, C.; Zhang, W.; Shen, H.; Xia, Y.; Wang, X. Seminal plasma microRNAs: Potential biomarkers for spermatogenesis status. Mol. Hum. Reprod. 2012, 18, 489–497. [Google Scholar] [CrossRef]
- Wu, W.; Qin, Y.; Li, Z.; Dong, J.; Dai, J.; Lu, C.; Guo, X.; Zhao, Y.; Zhu, Y.; Zhang, W.; et al. Genome-wide microRNA expression profiling in idiopathic non-obstructive azoospermia: Significant up-regulation of miR-141, miR-429 and miR-7-1-3p. Hum. Reprod. 2013, 28, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Belleannee, C.; Legare, C.; Calvo, E.; Thimon, V.; Sullivan, R. microRNA signature is altered in both human epididymis and seminal microvesicles following vasectomy. Hum. Reprod. 2013, 28, 1455–1467. [Google Scholar] [CrossRef]
- Mostafa, T.; Rashed, L.A.; Nabil, N.I.; Osman, I.; Mostafa, R.; Farag, M. Seminal miRNA Relationship with Apoptotic Markers and Oxidative Stress in Infertile Men with Varicocele. Biomed. Res. Int. 2016, 2016, 4302754. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Ludwig, N.; Hart, M.; Leidinger, P.; Backes, C.; Keller, A.; Hammadeh, M.; Meese, E. Altered micro-ribonucleic acid expression profiles of extracellular microvesicles in the seminal plasma of patients with oligoasthenozoospermia. Fertil. Steril. 2016, 106, 1061–1069.e3. [Google Scholar] [CrossRef]
- Barcelo, M.; Mata, A.; Bassas, L.; Larriba, S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Hum. Reprod. 2018, 33, 1087–1098. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Tian, Y.; Hu, M.; Le, F.; Wang, L.; Liu, X.; Jin, F. Differential microRNAs expression in seminal plasma of normospermic patients with different sperm DNA fragmentation indexes. Reprod. Toxicol. 2020, 94, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Bonasio, R.; Shiekhattar, R. Regulation of transcription by long noncoding RNAs. Annu. Rev. Genet. 2014, 48, 433–455. [Google Scholar] [CrossRef]
- Sun, Q.; Hao, Q.; Prasanth, K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2018, 34, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, D.; Wang, P.; Sun, W.; Xue, X.; Hu, Y.; Xie, C.; Ma, Y. RNA-sequencing and bioinformatics analysis of long noncoding RNAs and mRNAs in the asthenozoospermia. Biosci. Rep. 2020, 40, 4041. [Google Scholar] [CrossRef]
- Xie, Y.; Yao, J.; Zhang, X.; Chen, J.; Gao, Y.; Zhang, C.; Chen, H.; Wang, Z.; Zhao, Z.; Chen, W.; et al. A panel of extracellular vesicle long noncoding RNAs in seminal plasma for predicting testicular spermatozoa in nonobstructive azoospermia patients. Hum. Reprod. 2020, 35, 2413–2427. [Google Scholar] [CrossRef]
- Shirai, M.; Matsuda, S.; Mitsukawa, S.; Nakamura, M.; Yonezawa, K. FSH, LH and testosterone levels in human seminal plasma. Tohoku J. Exp. Med. 1975, 116, 201–202. [Google Scholar] [CrossRef][Green Version]
- Hampl, R.; Kubatova, J.; Sobotka, V.; Heracek, J. Steroids in semen, their role in spermatogenesis, and the possible impact of endocrine disruptors. Horm. Mol. Biol Clin. Investig. 2013, 13, 1–5. [Google Scholar] [CrossRef]
- Adamopoulos, D.A.; Lawrence, D.M.; Swyer, G.I. Determinantion of testosterone concentration in semen of men with normal or subnormal sperm counts and after vasectomy. Acta Eur. Fertil. 1976, 7, 219–225. [Google Scholar]
- Adamopoulos, D.; Lawrence, D.M.; Vassilopoulos, P.; Kapolla, N.; Kontogeorgos, L.; McGarrigle, H.H. Hormone levels in the reproductive system of normospermic men and patients with oligospermia and varicocele. J. Clin. Endocrinol. Metab. 1984, 59, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.; Sagiv, M.; Bruchis, S.; Barnet, M.; Kaufman, H.; Servadio, C. Total and free testosterone and estradiol in human semen. Int. J. Fertil. 1987, 32, 145–148. [Google Scholar]
- Bujan, L.; Mieusset, R.; Audran, F.; Lumbroso, S.; Sultan, C. Increased oestradiol level in seminal plasma in infertile men. Hum. Reprod. 1993, 8, 74–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Q.; Bai, Q.; Yuan, Y.; Liu, P.; Qiao, J. Assessment of seminal estradiol and testosterone levels as predictors of human spermatogenesis. J. Androl. 2010, 31, 215–220. [Google Scholar] [CrossRef]
- Redmon, J.B.; Drobnis, E.Z.; Sparks, A.; Wang, C.; Swan, S.H. Semen and reproductive hormone parameters in fertile men with and without varicocele. Andrologia 2019, 51, e13407. [Google Scholar] [CrossRef]
- Ando, S.; Giacchetto, C.; Beraldi, E.; Panno, M.L.; Carpino, A.; Sposato, G.; Lombardi, A. Testosterone and dihydrotestosterone seminal plasma levels in varicocele patients. Andrologia 1983, 15, 374–379. [Google Scholar] [CrossRef]
- Zalata, A.; Hafez, T.; Verdonck, L.; Vermeulen, L.; Comhaire, F. Androgens in seminal plasma: Markers of the surface epithelium of the male reproductive tract. Int. J. 1995, 18, 271–277. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Giampietro, A.; Messana, I.; Castagnola, M.; Marana, R.; De Marinis, L.; Pontecorvi, A. Novel biomarkers of androgen deficiency from seminal plasma profiling using high-resolution mass spectrometry. J. Clin. Endocrinol. Metab. 2014, 99, 2813–2820. [Google Scholar] [CrossRef][Green Version]
- Carpino, A.; Sisci, D.; Aquila, S.; Beraldi, E.; Sessa, M.T.; Siciliano, L.; De Luca, G.; Ando, S. Effects of short-term high-dose testosterone propionate administration on medium molecular-weight proteins of human seminal plasma. Andrologia 1994, 26, 241–245. [Google Scholar] [CrossRef]
- Sheth, A.R.; Jayatilak, P.G.; Thakur, A.N.; Mugatwala, P.; Pardanani, D.S. Effect of administration of a single dose of testosterone oenanthate on constituents of human seminal plasma and serum gonadotropins. Andrologia 1976, 8, 259–264. [Google Scholar] [CrossRef]
- Fenichel, P.; Rey, R.; Poggioli, S.; Donzeau, M.; Chevallier, D.; Pointis, G. Anti-Mullerian hormone as a seminal marker for spermatogenesis in non-obstructive azoospermia. Hum. Reprod. 1999, 14, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.M.; Herning, H.; Witczak, O.; Haugen, T.B. Anti-Mullerian hormone in seminal plasma and serum: Association with sperm count and sperm motility. Hum. Reprod. 2016, 31, 1662–1667. [Google Scholar] [CrossRef]
- Fujisawa, M.; Yamasaki, T.; Okada, H.; Kamidono, S. The significance of anti-Mullerian hormone concentration in seminal plasma for spermatogenesis. Hum. Reprod. 2002, 17, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Caroppo, E.; Niederberger, C.; Iacovazzi, P.A.; Correale, M.; Palagiano, A.; D’Amato, G. Human chorionic gonadotropin free beta-subunit in the human seminal plasma: A new marker for spermatogenesis? Eur. J. Obs. Gynecol. Reprod. Biol. 2003, 106, 165–169. [Google Scholar] [CrossRef]
- Weiss, G.; Goldsmith, L.T.; Schoenfeld, C.; D’Eletto, R. Partial purification of relaxin from human seminal plasma. Am. J. Obs. Gynecol. 1986, 154, 749–755. [Google Scholar] [CrossRef]
- Essig, M.; Schoenfeld, C.; D’Eletto, R.T.; Amelar, R.; Steinetz, B.G.; O’Byrne, E.M.; Weiss, G. Relaxin in human seminal plasma. Ann. N. Y. Acad. Sci. 1982, 380, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Menegazzo, M.; Gianesello, L.; Selice, R.; Foresta, C. Effect of relaxin on human sperm functions. J. Androl. 2012, 33, 474–482. [Google Scholar] [CrossRef]
- Essig, M.; Schoenfeld, C.; Amelar, R.D.; Dubin, L.; Weiss, G. Stimulation of human sperm motility by relaxin. Fertil. Steril. 1982, 38, 339–343. [Google Scholar] [CrossRef]
- Lessing, J.B.; Brenner, S.H.; Schoenfeld, C.; Goldsmith, L.T.; Amelar, R.D.; Dubin, L.; Weiss, G. The effect of relaxin on the motility of sperm in freshly thawed human semen. Fertil. Steril. 1985, 44, 406–409. [Google Scholar] [CrossRef]
- Brenner, S.H.; Lesing, J.B.; Schoenfeld, C.; Goldsmith, L.T.; Amelar, R.; Dubin, L.; Weiss, G. Human semen relaxin and its correlation with the parameters of semen analysis. Fertil. Steril. 1987, 47, 714–716. [Google Scholar] [CrossRef]
- Neuwinger, J.; Jockenhovel, F.; Nieschlag, E. The influence of relaxin on motility of human sperm in vitro. Andrologia 1990, 22, 335–339. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Bakhshi, D.; Breitbart, H. Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn(2). Mol. Reprod. Dev. 2019, 86, 502–515. [Google Scholar] [CrossRef]
- Kwon, W.S.; Kim, Y.J.; Ryu, D.Y.; Kwon, K.J.; Song, W.H.; Rahman, M.S.; Pang, M.G. Fms-like tyrosine kinase 3 is a key factor of male fertility. Theriogenology 2019, 126, 145–152. [Google Scholar] [CrossRef]
- Politch, J.A.; Tucker, L.; Bowman, F.P.; Anderson, D.J. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 2007, 22, 2928–2935. [Google Scholar] [CrossRef]
- Seshadri, S.; Bates, M.; Vince, G.; Jones, D.I. The role of cytokine expression in different subgroups of subfertile men. Am. J. Reprod. Immunol. 2009, 62, 275–282. [Google Scholar] [CrossRef]
- Camejo, M.I. Relation between immunosuppressive PGE(2) and IL-10 to pro-inflammatory IL-6 in seminal plasma of infertile and fertile men. Arch. Androl. 2003, 49, 111–116. [Google Scholar] [CrossRef]
- Qian, L. Decreased interleukin-11 levels in the semen of infertile males. Cytokine 2018, 108, 57–59. [Google Scholar] [CrossRef]
- Sharkey, D.J.; Macpherson, A.M.; Tremellen, K.P.; Mottershead, D.G.; Gilchrist, R.B.; Robertson, S.A. TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J. Immunol. 2012, 189, 1024–1035. [Google Scholar] [CrossRef]
- Nikolaeva, M.A.; Babayan, A.A.; Stepanova, E.O.; Smolnikova, V.Y.; Kalinina, E.A.; Fernandez, N.; Krechetova, L.V.; Vanko, L.V.; Sukhikh, G.T. The relationship of seminal transforming growth factor-beta1 and interleukin-18 with reproductive success in women exposed to seminal plasma during IVF/ICSI treatment. J. Reprod. Immunol. 2016, 117, 45–51. [Google Scholar] [CrossRef]
- Saji, F.; Ohashi, K.; Kato, M.; Negoro, T.; Tanizawa, O. Clinical evaluation of the enzyme-linked immunosorbent assay (ELISA) kit for antisperm antibodies. Fertil. Steril. 1988, 50, 644–647. [Google Scholar] [CrossRef]
- Naz, R.K.; Menge, A.C. Antisperm antibodies: Origin, regulation, and sperm reactivity in human infertility. Fertil. Steril. 1994, 61, 1001–1013. [Google Scholar] [CrossRef]
- Bozhedomov, V.A.; Nikolaeva, M.A.; Ushakova, I.V.; Lipatova, N.A.; Bozhedomova, G.E.; Sukhikh, G.T. Functional deficit of sperm and fertility impairment in men with antisperm antibodies. J. Reprod. Immunol. 2015, 112, 95–101. [Google Scholar] [CrossRef]
- Budnik, L.T.; Jahner, D.; Mukhopadhyay, A.K. Inhibitory effects of TNF alpha on mouse tumor Leydig cells: Possible role of ceramide in the mechanism of action. Mol. Cell. Endocrinol. 1999, 150, 39–46. [Google Scholar] [CrossRef]
- Bozhedomov, V.A.; Lipatova, N.A.; Alexeev, R.A.; Alexandrova, L.M.; Nikolaeva, M.A.; Sukhikh, G.T. The role of the antisperm antibodies in male infertility assessment after microsurgical varicocelectomy. Andrology 2014, 2, 847–855. [Google Scholar] [CrossRef]
- Zini, A.; Fahmy, N.; Belzile, E.; Ciampi, A.; Al-Hathal, N.; Kotb, A. Antisperm antibodies are not associated with pregnancy rates after IVF and ICSI: Systematic review and meta-analysis. Hum. Reprod. 2011, 26, 1288–1295. [Google Scholar] [CrossRef]
- Saxena, S.; Jha, P.; Farooq, A. Purification and characterisation of an immunosuppressive factor from normal human seminal plasma. J. Reprod. Immunol. 1988, 13, 133–146. [Google Scholar] [CrossRef]
- Bandivdekar, A.H.; Moodbidri, S.B.; Sheth, A.R.; Joshi, D.S.; Sundaram, K. Flow cytometric analysis of human spermatozoa treated with antiserum to human seminal inhibin. Int. J. Fertil. 1989, 34, 74–77. [Google Scholar] [PubMed]
- Vanage, G.R.; Gopalkrishnan, K.; Sheth, A.R. Effect of antibodies to human seminal plasma inhibin on spermatogenesis and sperm agglutination in adult male rats. Mol. Reprod. Dev. 1990, 25, 227–236. [Google Scholar] [CrossRef]
- Ziyyat, A.; Lassalle, B.; Testart, J.; Briot, P.; Amar, E.; Finaz, C.; Lefevre, A. Flow cytometry isolation and reverse transcriptase-polymerase chain reaction characterization of human round spermatids in infertile patients. Hum. Reprod. 1999, 14, 379–387. [Google Scholar] [CrossRef]
- Robert, M.; Gagnon, C. Semenogelin I: A coagulum forming, multifunctional seminal vesicle protein. Cell Mol. Life Sci 1999, 55, 944–960. [Google Scholar] [CrossRef]
- Murakami, J.; Yoshiike, M.; Satoh, M.; Furuichi, Y.; Iwamoto, T. Characterization of recombinant precursor proteins of the human seminal plasma sperm motility inhibitor synthesized in insect cells. Int. J. Mol. Med. 1998, 2, 693–700. [Google Scholar] [CrossRef]
- Tokugawa, Y.; Kunishige, I.; Kubota, Y.; Shimoya, K.; Nobunaga, T.; Kimura, T.; Saji, F.; Murata, Y.; Eguchi, N.; Oda, H.; et al. Lipocalin-type prostaglandin D synthase in human male reproductive organs and seminal plasma. Biol. Reprod. 1998, 58, 600–607. [Google Scholar] [CrossRef]
- Diamandis, E.P.; Arnett, W.P.; Foussias, G.; Pappas, H.; Ghandi, S.; Melegos, D.N.; Mullen, B.; Yu, H.; Srigley, J.; Jarvi, K. Seminal plasma biochemical markers and their association with semen analysis findings. Urology 1999, 53, 596–603. [Google Scholar] [CrossRef]
- Kurth, B.E.; Wright, R.M.; Flickinger, C.J.; Herr, J.C. Stage-specific detection of mRNA for the sperm antigen SP-10 in human testes. Anat. Rec. 1993, 236, 619–625. [Google Scholar] [CrossRef]
- Coppola, M.A.; Klotz, K.L.; Kim, K.A.; Cho, H.Y.; Kang, J.; Shetty, J.; Howards, S.S.; Flickinger, C.J.; Herr, J.C. SpermCheck Fertility, an immunodiagnostic home test that detects normozoospermia and severe oligozoospermia. Hum. Reprod. 2010, 25, 853–861. [Google Scholar] [CrossRef]
- Klotz, K.L.; Coppola, M.A.; Labrecque, M.; Brugh, V.M., 3rd; Ramsey, K.; Kim, K.A.; Conaway, M.R.; Howards, S.S.; Flickinger, C.J.; Herr, J.C. Clinical and consumer trial performance of a sensitive immunodiagnostic home test that qualitatively detects low concentrations of sperm following vasectomy. J. Urol. 2008, 180, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Drabovich, A.P.; Dimitromanolakis, A.; Saraon, P.; Soosaipillai, A.; Batruch, I.; Mullen, B.; Jarvi, K.; Diamandis, E.P. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci. Transl. Med. 2013, 5, 212ra160. [Google Scholar] [CrossRef]
- Heshmat, S.M.; Mullen, J.B.; Jarvi, K.A.; Soosaipillai, A.; Diamandis, E.P.; Hamilton, R.J.; Lo, K.C. Seminal plasma lipocalin-type prostaglandin D synthase: A potential new marker for the diagnosis of obstructive azoospermia. J. Urol. 2008, 179, 1077–1080. [Google Scholar] [CrossRef]
- Fujihara, Y.; Tokuhiro, K.; Muro, Y.; Kondoh, G.; Araki, Y.; Ikawa, M.; Okabe, M. Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proc. Natl. Acad. Sci. USA 2013, 110, 8111–8116. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).