An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization
Abstract
1. Introduction
2. The Development Stage of Osteoarthritis (OA)
- (a)
- The activity of osteoclasts in the joints increases, which disrupts the balance between bone formation and bone resorption, and eventually makes the thickness of subchondral bone significantly thinner than normal [15].
- (b)
- The depletion of phagocytic synovial lining cells causes a decrease in the influx of polymorphonuclear neutrophils, which inhibits proteoglycan degradation. Overall, the above changes lead to the death of articular cartilage cells (collagen-induced arthritis (CIA) model). Besides, the reduction of synovial macrophages attenuates the formation of joint osteophytes (in the OA mouse model) [16].
- (c)
- Elevated cholesterol promotes the formation of ectopic bone (CIA model) [17].
- (a)
- Cartilage is mostly degraded, accompanied by severe pain. At the mechanism level, pain is mainly caused by the following phenomenon: the synovium in the joints of advanced OA patients is infiltrated by macrophages. Subsequently, the activated macrophages release pro-inflammatory cytokines, causing chronic pain and inflammation. From a diagnostic point of view, the levels of three pro-inflammatory mediators in the synovial tissue are significantly up-regulated, which could be considered as a diagnostic marker for OA. The three types of pro-inflammatory mediators are (1) interleukin-1b (IL-1β), (2) IL-6, and (3) nerve growth factor (NGF). Therefore, these indicators may be promising clinical markers for monitoring the progression of OA [18].
- (b)
- The production of IRF5 (interferon regulatory factor 5, IRF5) in the synovial macrophages of OA patients is markedly enhanced, indicating that IRF5 is positively correlated with the severity of OA. Additionally, on the synovial macrophages of OA patients, the IRF5 level in Stage 4 OA patients is significantly higher than that in Stage 2 and Stage 3 [19].
- (c)
- The subchondral plate of OA patients becomes significantly thicker, and the thickness of the unmineralized articular cartilage is significantly thinner than average [15].
3. Macrophage Polarization
4. Macrophages Are an Emerging Target for OA Treatment
4.1. Physical Stimuli
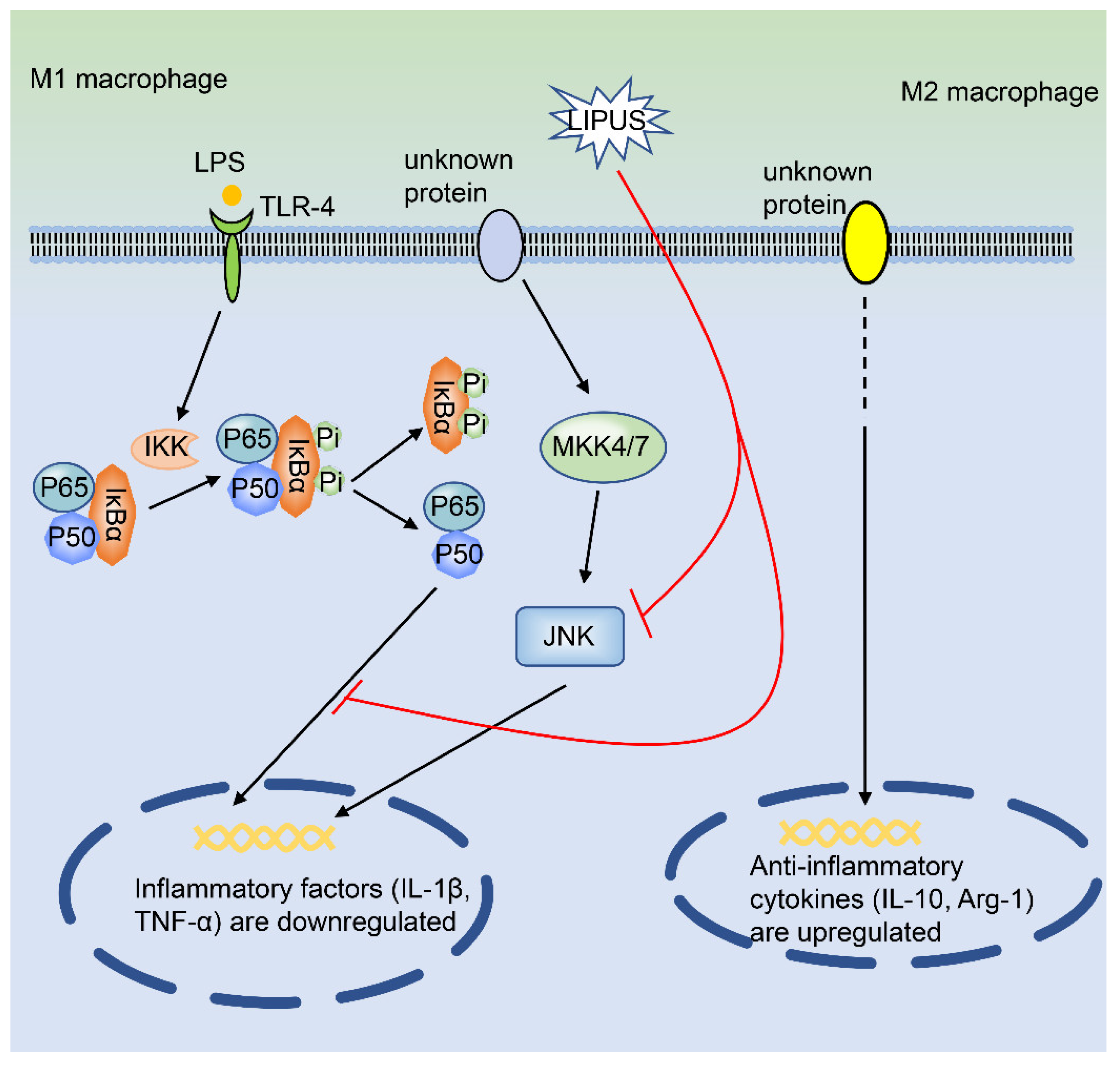
Low-Intensity Pulsed Ultrasound (LIPUS)
4.2. Chemical Compounds
4.2.1. Kinsenoside
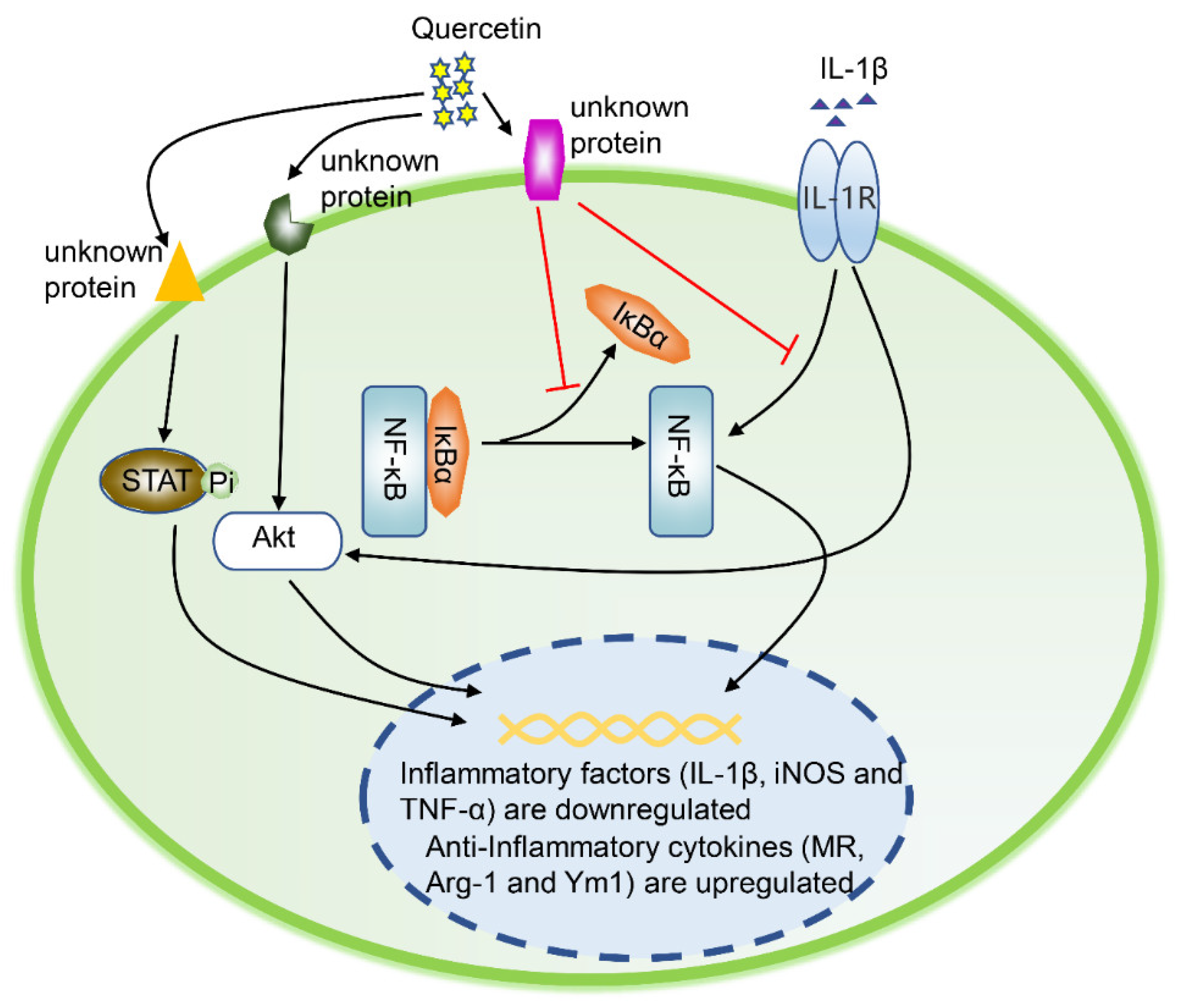
4.2.2. Quercetin
4.2.3. Dexamethasone
4.2.4. Pravastatin
4.2.5. Rapamycin
4.3. Biological Molecules
4.3.1. Cells
Mesenchymal Stem Cells
TissueGene-C
4.3.2. Proteins
R-Spondin-2
Interferon Regulatory Factor 5
Pro-Resolving Lipid Mediator
Lumican
Bone Morphogenetic Protein 7
Squid Type II Collagen
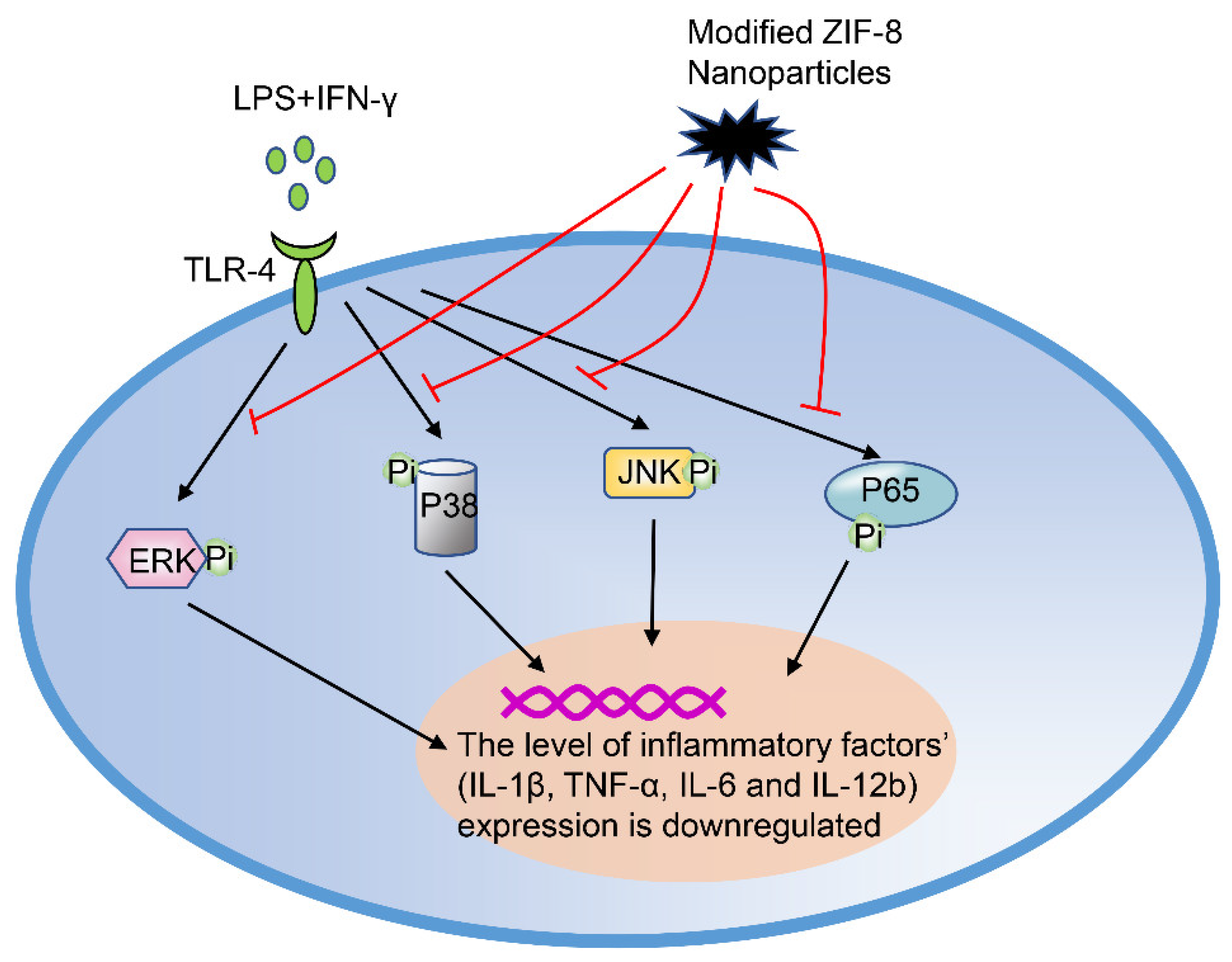
Modified ZIF-8 Nanoparticles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| ACLT | Anterior cruciate ligament transection |
| ADAMTS-4 | A disintegrin and metalloproteinase with thrombospondin motifs 4 |
| Akt | Protein kinase B |
| Arg-1 | Arginase-1 |
| BMP-7 | Bone morphogenetic protein 7 |
| CIA | Collagen-induced arthritis |
| CIOA | Collagenase-induced osteoarthritis |
| CL | Clodronate-loaded liposomes |
| CM | Conditioned medium |
| COX2 | Cyclooxygenase 2 |
| DMOADs | Disease-modifying osteoarthritis drugs |
| DXMS | Dexamethasone |
| ERK | Extracellular regulated protein kinase |
| H2O2 | Hydrogen peroxide |
| IFN-γ | Interferon-γ |
| IL-1 | Interleukin-1 |
| IL-1β | Interleukin-1β |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-13 | Interleukin-13 |
| IL-23 | Interleukin-23 |
| iNOS | Inducible nitric oxide synthase |
| IRF5 | Interferon regulatory factor 5 |
| IκBα | Inhibitor of NF-κB |
| JNK | C-Jun N-terminal kinase |
| Kin | Kinsenoside |
| LIPUS | Low-intensity pulsed ultrasound |
| LPS | Lipopolysaccharide |
| LUM | Lumican |
| MAPK | Mitogen-activated protein kinase |
| MIA | Monosodium iodoacetate |
| MMP-13 | Matrix metalloproteinase-13 |
| MR | Mannose receptor |
| MSCs | Mesenchymal stem cells |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa B |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| O2 | Oxygen |
| OA | Osteoarthritis |
| PTOA | Post-traumatic osteoarthritis |
| ROS | Reactive oxygen species |
| Rspo2 | R-spondin-2 |
| RvD1 | Retinoid D1 |
| SCII | Squid type II collagen |
| STAT6 | Signal transducer and activator of transcription 6 |
| TAM | Tumor-associated macrophages |
| TCR+ | T-cell receptor-positive |
| TGF-β | Transforming growth factor-beta |
| TGF-β1 | Transforming growth factor-beta 1 |
| TGF-β3 | Transforming growth factor-beta 3 |
| TLR | Toll-like receptor |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor α |
| YM1 | Chitinase-like protein |
| ZIF-8 | Zeolitic imidazolate framework-8 |
| ZIF-8 NPs | ZIF-8 nanoparticles |
Appendix A
| Type | Name | OA Sample—Cells | OA Sample—Animal | Signaling Pathway | Function | Research Stage/Application Schedule | Mechanism | Refs. | |
|---|---|---|---|---|---|---|---|---|---|
| Physical stimuli | Low-intensity pulsed ultrasound (LIPUS) | THP-1 cells; RAW 264.7; synovial macrophages | Mouse medial meniscus instability (DMM) arthritis | JNK NF-κB | Inhibit the expression of related genes in M1 macrophages and promote the expression of related genes in M2 macrophages | Preclinical stage/NA | LIPUS regulates the polarization of synovial macrophages, thus inhibiting osteoarthritis. | [27] | |
| Chemical compounds | Kinsenoside (Kin) | RAW264.7 | Anterior cruciate ligament transection (ACLT) mouse model | NF-κB JNK MAPK ERK | Transform M1 macrophages into M2 macrophages; reduce the infiltration of M1 in mouse joints and promote the infiltration of M2 in mouse synovitis | Preclinical stage/NA | Kinsenoside relieves the symptoms of osteoarthritis by inactivating NF-κB/MAPK signaling and promoting macrophage repolarization. | [15] | |
| Quercetin | RAW264.7 | IL-1β-induced rat osteoarthritis model | Akt/NF-κB | Promote the formation of M2 macrophages | Preclinical stage/NA | Quercetin repairs damaged cartilage by promoting the conversion of synovial macrophages into M2 macrophages, and finally alleviates OA symptoms. | [34] | ||
| Dexamethasone (DXMS) | Synovial macrophages | Patients with osteoarthritis | NA | Inhibit the activity of M1 macrophages and promote the formation of M2 macrophages | Preclinical stage/NA | DXMS promotes the conversion of macrophages into M2 macrophages to relieve OA symptoms. | [16] | ||
| Pravastatin | Primary human monocytes | Patients with osteoarthritis | NA | Promote the formation of M2 macrophages | Preclinical stage/NA | DXMS promotes the conversion of macrophages into M2 macrophages to relieve OA symptoms. | [16] | ||
| Rapamycin | Primary human monocytes | Patients with osteoarthritis | mTOR | Enhance the function of M1 macrophages and inhibit the activity of M2 macrophages | Preclinical stage/NA | DXMS promotes the conversion of macrophages into M2 macrophages to relieve OA symptoms. | [16,47] | ||
| Biological molecules | Cell | Mesenchymal stem cell (MSC) | Synovial macrophages of OA patients | In vitro co-culture OA model consisting of patient-matched cartilage and macrophages | NA | Inhibit the activity of M1 macrophages and induce polarization of M2 macrophages | Preclinical stage/NA | MSCs promote the formation of M2 macrophages, thereby relieving OA symptoms | [46,51] |
| TissueGene-C | Human joint macrophages | Patients with osteoarthritis | NA | Promote the formation of M2 macrophages | Phase III of a clinical trial/NA | TissueGene-C induces the formation of M2 macrophages, thereby relieving pain in patients with OA. | [52,53] | ||
| Protein | R-spondin-2 | NA | NA | Wnt/β-catenin | NA | Preclinical stage/NA | The formation of M1 macrophages promoted the secretion of rspo2 in chondrocytes, thereby exacerbating the symptoms of experimental OA. | [47] | |
| Interferon regulatory factor 5 (IRF5) | Synovial macrophages | Patients with osteoarthritis | NA | Promote the formation of M1 macrophages | Preclinical stage/NA | M1 macrophage-secreted IRF5 is positively correlated with the severity of OA symptoms. | [19,58] | ||
| Pro-resolving lipid mediator | Synovial macrophages | Obesity-induced osteoarthritis | NA | Reduce the number of M1 macrophages and increase the formation of M2 macrophages | Preclinical stage/NA | CL attenuates obesity-induced OA by regulating the polarization of macrophages. | [59] | ||
| Lumican (LUM) | Human primary chondrocytes and macrophages | NA | NF-κB | LUM+LPS induces the differentiation of macrophages into M1 type | Preclinical stage/NA | In the TLR4-mediated OA model, the expression of LUM is up-regulated, which promotes the formation of M1 macrophages and aggravates OA symptoms. | [23,61,62,63,64] | ||
| Bone morphogenetic protein 7 (BMP-7) | Primary human monocytes | Patients with osteoarthritis | NA | NA | Preclinical stage/NA | BMP-7 regulates the inflammatory state of joints by changing the phenotype of OA synovium (polarizing monocytes into M2 type). | [16] | ||
| Squid type II collagen (SCII) | RAW264.7 cells; mouse chondrocytes | Mouse OA model induced by anterior cruciate ligament transection (ACLT) and partial meniscus resection (pMMx) | STAT6 | Promote the conversion of M1 macrophages into M2 macrophages | Preclinical stage/NA | SCII improves the symptoms of OA by promoting the polarization of M2 macrophages. | [68] | ||
| Modified ZIF-8 nanoparticles | RAW264.7 cells | A mouse model of OA with anterior cruciate ligament transection (ACLT). | MAPK NF-κB JNK | Promote the transformation of M1 macrophages into M2 macrophages | Preclinical stage/NA | Modified ZIF-8 nanoparticles transform macrophages from M0 to M2 for OA treatment. | [73] | ||
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Thomas, E.; Peat, G.; Croft, P. Defining and mapping the person with osteoarthritis for population studies and public health. Rheumatology 2014, 53, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.H.; David, N.; Campisi, J.; Elisseeff, J.H. Senescent cells and osteoarthritis: A painful connection. J. Clin. Investig. 2018, 128, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Deyle, G.D.; Allen, C.S.; Allison, S.C.; Gill, N.W.; Hando, B.R.; Petersen, E.J.; Dusenberry, D.I.; Rhon, D.I. Physical Therapy versus Glucocorticoid Injection for Osteoarthritis of the Knee. N. Engl. J. Med. 2020, 382, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K.P. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef]
- Sacitharan, P.K. Ageing and Osteoarthritis. Sub Cell. Biochem. 2019, 91, 123–159. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Wu, C.L.; Harasymowicz, N.S.; Klimak, M.A.; Collins, K.H.; Guilak, F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr. Cartil. 2020, 10.1016/j.joca.2019.12.007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 10.1016/j.joca.2020.01.007. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Brandt, K.D.; Radin, E.L.; Dieppe, P.A.; van de Putte, L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann. Rheum. Dis. 2006, 65, 1261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Mei, J.; Han, X.; Li, H.; Yang, S.; Wang, M.; Chu, L.; Qiao, H.; Tang, T. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-kappaB/MAPK signaling and protecting chondrocytes. Acta Pharm. Sin. B 2019, 9, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Utomo, L.; van Osch, G.J.; Bayon, Y.; Verhaar, J.A.; Bastiaansen-Jenniskens, Y.M. Guiding synovial inflammation by macrophage phenotype modulation: An in vitro study towards a therapy for osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1629–1638. [Google Scholar] [CrossRef]
- Sun, A.R.; Panchal, S.K.; Friis, T.; Sekar, S.; Crawford, R.; Brown, L.; Xiao, Y.; Prasadam, I. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS ONE 2017, 12, e0183693. [Google Scholar] [CrossRef]
- Sakurai, Y.; Fujita, M.; Kawasaki, S.; Sanaki, T.; Yoshioka, T.; Higashino, K.; Tofukuji, S.; Yoneda, S.; Takahashi, T.; Koda, K.; et al. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019, 160, 895–907. [Google Scholar] [CrossRef]
- Ni, Z.; Zhao, X.; Dai, X.; Zhao, L.; Xia, J. The Role of Interferon Regulatory Factor 5 in Macrophage Inflammation During Osteoarthritis. Inflammation 2019, 42, 1821–1829. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Loh, J.Z.; Yang, T.H.; Huang, K.T.; Wu, C.T.; Guan, S.S.; Liu, S.H.; Hung, K.Y. Prevention of acute kidney injury by low intensity pulsed ultrasound via anti-inflammation and anti-apoptosis. Sci. Rep. 2020, 10, 14317. [Google Scholar] [CrossRef]
- Maylia, E.; Nokes, L.D. The use of ultrasonics in orthopaedics—A review. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 1999, 7, 1–28. [Google Scholar] [CrossRef]
- Zhang, B.; Ni, Z.H.; Yang, P.; Kuang, L.; OuYang, J.J.; Xie, Y.L.; Jiang, W.L.; Liu, M.; Du, X.L.; Chen, L. Low-intensity pulsed ultrasound suppresses synovitis by modulating polarization of synovial macrophages in mice with osteoarthritis. J. Third Mil. Med Univ. 2019, 41, 747–756. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, T.; Tan, W.; Ren, H.; Li, H.; Liu, J.; Cao, H.; Cheng, Q.; Liu, X.; Zhu, H.; et al. Effects of kinsenoside, a potential immunosuppressive drug for autoimmune hepatitis, on dendritic cells/CD8(+) T cells communication in mice. Hepatology 2016, 64, 2135–2150. [Google Scholar] [CrossRef]
- Wu, J.B.; Chuang, H.R.; Yang, L.C.; Lin, W.C. A standardized aqueous extract of Anoectochilus formosanus ameliorated thioacetamide-induced liver fibrosis in mice: The role of Kupffer cells. Biosci. Biotechnol. Biochem. 2010, 74, 781–787. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Liu, L.; Ruan, Q.; Zhang, X.; Hong, W.; Wu, S.; Jin, G.; Bai, Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 2018, 154, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 2018, 9, 17937. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef]
- Stilund, M.; Reuschlein, A.-K.; Christensen, T.; Møller, H.J.; Rasmussen, P.V.; Petersen, T. Soluble CD163 as a Marker of Macrophage Activity in Newly Diagnosed Patients with Multiple Sclerosis. PLoS ONE 2014, 9, e98588. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 2015, 1, e000014. [Google Scholar] [CrossRef]
- Aktas, E.; Sener, E.; Gocun, P.U. Mechanically induced experimental knee osteoarthritis benefits from anti-inflammatory and immunomodulatory properties of simvastatin via inhibition of matrix metalloproteinase-3. J. Orthop. Traumatol. 2011, 12, 145–151. [Google Scholar] [CrossRef]
- Zhang, O.; Zhang, J. Atorvastatin promotes human monocyte differentiation toward alternative M2 macrophages through p38 mitogen-activated protein kinase-dependent peroxisome proliferator-activated receptor γ activation. Int. Immunopharmacol. 2015, 26, 58–64. [Google Scholar] [CrossRef]
- Ma, W.; Liu, Y.; Wang, C.; Zhang, L.; Crocker, L.; Shen, J. Atorvastatin inhibits CXCR7 induction to reduce macrophage migration. Biochem. Pharmacol. 2014, 89, 99–108. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Revilla-Nuin, B.; Ramirez, P.; Pons, J.A. Immunosuppressive potency of mechanistic target of rapamycin inhibitors in solid-organ transplantation. World J. Transpl. 2016, 6, 183–192. [Google Scholar] [CrossRef]
- Ballou, L.M.; Lin, R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Byles, V.; Covarrubias, A.J.; Ben-Sahra, I.; Lamming, D.W.; Sabatini, D.M.; Manning, B.D.; Horng, T. The TSC-mTOR pathway regulates macrophage polarization. Nat. Commun. 2013, 4, 2834. [Google Scholar] [CrossRef] [PubMed]
- De Vita, V. Altered mTORC1 signalling may contribute to macrophage dysregulation in hidradenitis suppurativa. Inflamm. Res. 2018, 67, 207–208. [Google Scholar] [CrossRef]
- Oh, M.H.; Collins, S.L.; Sun, I.H.; Tam, A.J.; Patel, C.H.; Arwood, M.L.; Chan-Li, Y.; Powell, J.D.; Horton, M.R. mTORC2 Signaling Selectively Regulates the Generation and Function of Tissue-Resident Peritoneal Macrophages. Cell Rep. 2017, 20, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Zeng, C.; Yan, B.; Ouyang, J.; Liu, X.; Sun, Q.; Zhao, C.; Fang, H.; Pan, J.; et al. mTORC1 activation downregulates FGFR3 and PTH/PTHrP receptor in articular chondrocytes to initiate osteoarthritis. Osteoarthr. Cartil. 2017, 25, 952–963. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012, 2012, 461718. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Liang, S.; Chen, Z.; Zhang, Y.; Qian, A.; Hu, L. The Application of MSCs-Derived Extracellular Vesicles in Bone Disorders: Novel Cell-Free Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 619. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Hamilton, A.M.; Cheung, W.Y.; Gómez-Aristizábal, A.; Sharma, A.; Nakamura, S.; Chaboureau, A.; Bhatt, S.; Rabani, R.; Kapoor, M.; Foster, P.J.; et al. Iron nanoparticle-labeled murine mesenchymal stromal cells in an osteoarthritic model persists and suggests anti-inflammatory mechanism of action. PLoS ONE 2019, 14, e0214107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am. J. Transl. Res. 2020, 12, 261–268. [Google Scholar] [PubMed]
- Xie, J.; Huang, Z.; Yu, X.; Zhou, L.; Pei, F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 2019, 46, 36–44. [Google Scholar] [CrossRef]
- Seshagiri, S.; Stawiski, E.W.; Durinck, S.; Modrusan, Z.; Storm, E.E.; Conboy, C.B.; Chaudhuri, S.; Guan, Y.; Janakiraman, V.; Jaiswal, B.S.; et al. Recurrent R-spondin fusions in colon cancer. Nature 2012, 488, 660–664. [Google Scholar] [CrossRef]
- Takegami, Y.; Ohkawara, B.; Ito, M.; Masuda, A.; Nakashima, H.; Ishiguro, N.; Ohno, K. R-spondin 2 facilitates differentiation of proliferating chondrocytes into hypertrophic chondrocytes by enhancing Wnt/β-catenin signaling in endochondral ossification. Biochem. Biophys. Res. Commun. 2016, 473, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zheng, X.F.; Yang, Y.H.; Li, B.; Wang, Y.R.; Jiang, S.D.; Jiang, L.S. LGR4 acts as a key receptor for R-spondin 2 to promote osteogenesis through Wnt signaling pathway. Cell. Signal. 2016, 28, 989–1000. [Google Scholar] [CrossRef]
- Almuttaqi, H.; Udalova, I.A. Advances and challenges in targeting IRF5, a key regulator of inflammation. FEBS J. 2019, 286, 1624–1637. [Google Scholar] [CrossRef]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef]
- Sun, A.R.; Wu, X.; Liu, B.; Chen, Y.; Armitage, C.W.; Kollipara, A.; Crawford, R.; Beagley, K.W.; Mao, X.; Xiao, Y.; et al. Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation. Sci. Rep. 2019, 9, 426. [Google Scholar] [CrossRef]
- Hultgårdh-Nilsson, A.; Borén, J.; Chakravarti, S. The small leucine-rich repeat proteoglycans in tissue repair and atherosclerosis. J. Intern Med. 2015, 278, 447–461. [Google Scholar] [CrossRef]
- Young, A.A.; Smith, M.M.; Smith, S.M.; Cake, M.A.; Ghosh, P.; Read, R.A.; Melrose, J.; Sonnabend, D.H.; Roughley, P.J.; Little, C.B. Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res. 2005, 7, R852–R861. [Google Scholar] [CrossRef]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis--finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2015, 11, 159–170. [Google Scholar] [CrossRef]
- Barreto, G.; Senturk, B.; Colombo, L.; Bruck, O.; Neidenbach, P.; Salzmann, G.; Zenobi-Wong, M.; Rottmar, M. Lumican is upregulated in osteoarthritis and contributes to TLR4-induced pro-inflammatory activation of cartilage degradation and macrophage polarization. Osteoarthr. Cartil. 2020, 28, 92–101. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Stabler, T.; Pei, F.X.; Kraus, V.B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775. [Google Scholar] [CrossRef]
- Zou, G.L.; Zuo, S.; Lu, S.; Hu, R.H.; Lu, Y.Y.; Yang, J.; Deng, K.S.; Wu, Y.T.; Mu, M.; Zhu, J.J.; et al. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-beta/Smad signaling pathway. World J. Gastroenterol. 2019, 25, 4222–4234. [Google Scholar] [CrossRef]
- Bishop, G.B.; Einhorn, T.A. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int. Orthop. 2007, 31, 721–727. [Google Scholar] [CrossRef]
- Dai, M.; Liu, X.; Wang, N.; Sun, J. Squid type II collagen as a novel biomaterial: Isolation, characterization, immunogenicity and relieving effect on degenerative osteoarthritis via inhibiting STAT1 signaling in pro-inflammatory macrophages. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 283–294. [Google Scholar] [CrossRef]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Gong, M.; Zhuo, X.; Ma, A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med. Sci. Monit. Basic Res. 2017, 23, 240–249. [Google Scholar] [CrossRef]
- De Marchi, S.; Vázquez-Iglesias, L.; Bodelón, G.; Pérez-Juste, I.; Fernández, L.Á.; Pérez-Juste, J.; Pastoriza-Santos, I. Programmable Modular Assembly of Functional Proteins on Raman-Encoded Zeolitic Imidazolate Framework-8 (ZIF-8) Nanoparticles as SERS Tags. Chem. Mater. 2020, 32, 5739–5749. [Google Scholar] [CrossRef]
- Proenza, Y.G.; Longo, R.L. Simulation of the Adsorption and Release of Large Drugs by ZIF-8. J. Chem. Inf. Modeling 2020, 60, 644–652. [Google Scholar] [CrossRef]
- Zhou, F.; Mei, J.; Yang, S.; Han, X.; Li, H.; Yu, Z.; Qiao, H.; Tang, T. Modified ZIF-8 Nanoparticles Attenuate Osteoarthritis by Reprogramming the Metabolic Pathway of Synovial Macrophages. ACS Appl. Mater. Interfaces 2020, 12, 2009–2022. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zuo, Z.; Kuang, Y. An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization. Int. J. Mol. Sci. 2020, 21, 8513. https://doi.org/10.3390/ijms21228513
Sun Y, Zuo Z, Kuang Y. An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization. International Journal of Molecular Sciences. 2020; 21(22):8513. https://doi.org/10.3390/ijms21228513
Chicago/Turabian StyleSun, Yulong, Zhuo Zuo, and Yuanyuan Kuang. 2020. "An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization" International Journal of Molecular Sciences 21, no. 22: 8513. https://doi.org/10.3390/ijms21228513
APA StyleSun, Y., Zuo, Z., & Kuang, Y. (2020). An Emerging Target in the Battle against Osteoarthritis: Macrophage Polarization. International Journal of Molecular Sciences, 21(22), 8513. https://doi.org/10.3390/ijms21228513





