The Role of Neutrophils in Hypertension
Abstract
1. Introduction
2. Role of Immune System in Hypertension
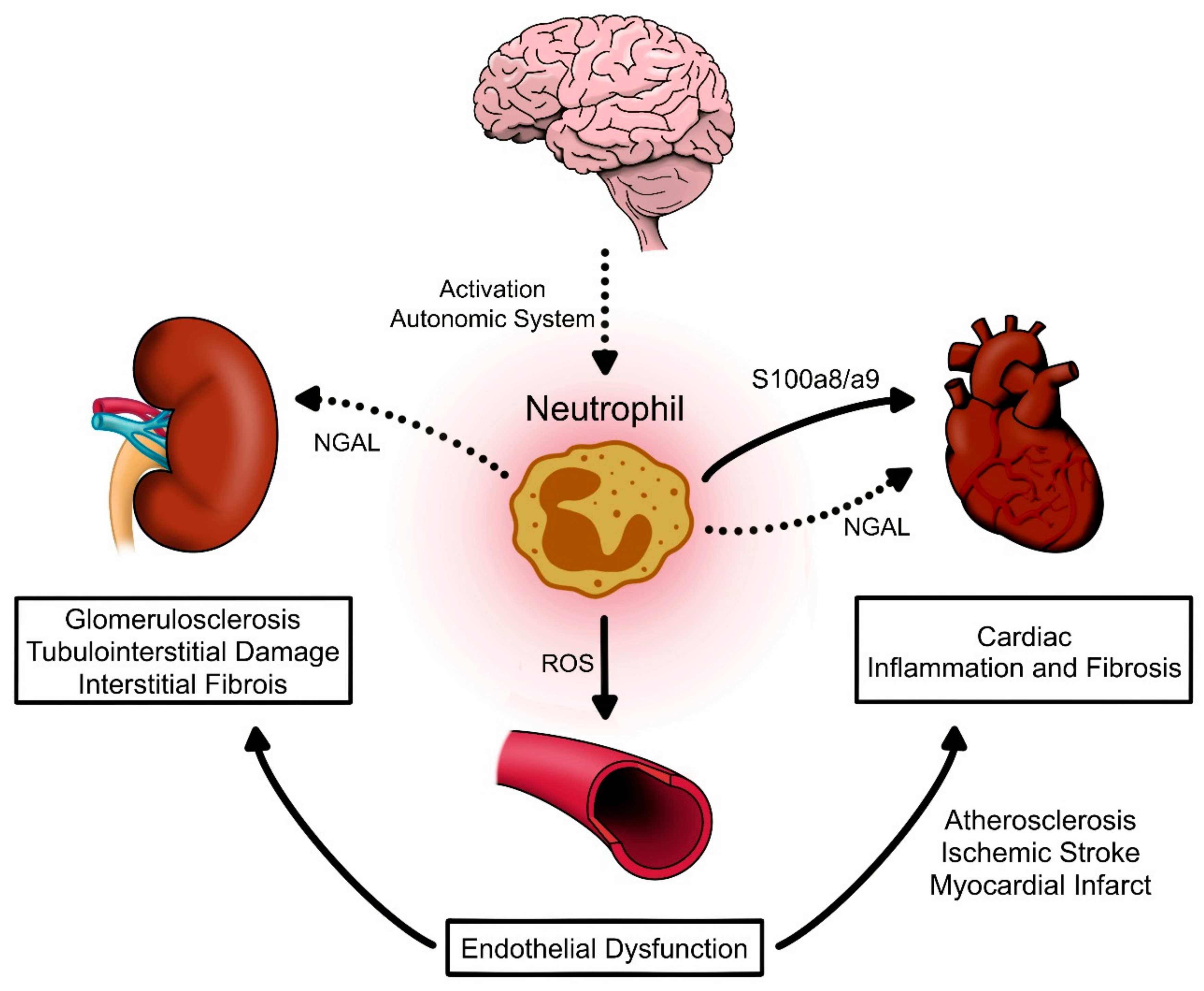
3. Neutrophils as Contributors of High Blood Pressure
3.1. Neutrophils Can Modulate Oxidative Stress and Vascular Response
3.2. Neutrophils Can Induce Tissue Inflammation and Fibrosis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.C.o.D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Xiao, L.; Harrison, D.G. Inflammation in Hypertension. Can. J. Cardiol. 2020, 36, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Koroskenyi, K.; Juba, F.; Vajda, G. Human vascular antigen complement consumption test of hypertensive patients (preliminary report). Experientia 1961, 17, 91–92. [Google Scholar] [CrossRef]
- Hilme, E.; Herlitz, H.; Soderstrom, T.; Hansson, L. Increased secretion of immunoglobulins in malignant hypertension. J. Hypertens. 1989, 7, 91–95. [Google Scholar] [CrossRef]
- Okuda, T.; Grollman, A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex. Rep. Biol. Med. 1967, 25, 257–264. [Google Scholar]
- Olsen, F. Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol. Microbiol. Scand. C 1980, 88, 1–5. [Google Scholar] [CrossRef]
- Svendsen, U.G. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol. Microbiol. Scand. A 1976, 84, 523–528. [Google Scholar] [CrossRef]
- Bataillard, A.; Freiche, J.C.; Vincent, M.; Sassard, J.; Touraine, J.L. Antihypertensive effect of neonatal thymectomy in the genetically hypertensive LH rat. Thymus 1986, 8, 321–330. [Google Scholar] [PubMed]
- Kenney, M.J.; Ganta, C.K. Autonomic nervous system and immune system interactions. Compr. Physiol. 2014, 4, 1177–1200. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, D.; Perrotta, M.; Pallante, F.; Fardella, V.; Iacobucci, R.; Fardella, S.; Carnevale, L.; Carnevale, R.; De Lucia, M.; Cifelli, G.; et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat. Commun. 2016, 7, 13035. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef]
- Fehrenbach, D.J.; Dasinger, J.H.; Lund, H.; Zemaj, J.; Mattson, D.L. Splenocyte transfer exacerbates salt-sensitive hypertension in rats. Exp. Physiol. 2020, 105, 864–875. [Google Scholar] [CrossRef]
- Crowley, S.D.; Song, Y.S.; Lin, E.E.; Griffiths, R.; Kim, H.S.; Ruiz, P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol 2010, 298, R1089–R1097. [Google Scholar] [CrossRef]
- Trott, D.W.; Thabet, S.R.; Kirabo, A.; Saleh, M.A.; Itani, H.; Norlander, A.E.; Wu, J.; Goldstein, A.; Arendshorst, W.J.; Madhur, M.S.; et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 2014, 64, 1108–1115. [Google Scholar] [CrossRef]
- Liu, Y.; Rafferty, T.M.; Rhee, S.W.; Webber, J.S.; Song, L.; Ko, B.; Hoover, R.S.; He, B.; Mu, S. CD8(+) T cells stimulate Na-Cl co-transporter NCC in distal convoluted tubules leading to salt-sensitive hypertension. Nat. Commun. 2017, 8, 14037. [Google Scholar] [CrossRef]
- Itani, H.A.; McMaster, W.G., Jr.; Saleh, M.A.; Nazarewicz, R.R.; Mikolajczyk, T.P.; Kaszuba, A.M.; Konior, A.; Prejbisz, A.; Januszewicz, A.; Norlander, A.E.; et al. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension 2016, 68, 123–132. [Google Scholar] [CrossRef]
- Ji, Q.; Cheng, G.; Ma, N.; Huang, Y.; Lin, Y.; Zhou, Q.; Que, B.; Dong, J.; Zhou, Y.; Nie, S. Circulating Th1, Th2, and Th17 Levels in Hypertensive Patients. Dis. Markers 2017, 2017, 7146290. [Google Scholar] [CrossRef]
- Norlander, A.E.; Madhur, M.S. Inflammatory cytokines regulate renal sodium transporters: How, where, and why? Am. J. Physiol. Ren. Physiol. 2017, 313, F141–F144. [Google Scholar] [CrossRef] [PubMed]
- Kamat, N.V.; Thabet, S.R.; Xiao, L.; Saleh, M.A.; Kirabo, A.; Madhur, M.S.; Delpire, E.; Harrison, D.G.; McDonough, A.A. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ-/- and interleukin-17A-/- mice. Hypertension 2015, 65, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Amador, C.A.; Barrientos, V.; Peña, J.; Herrada, A.A.; González, M.; Valdés, S.; Carrasco, L.; Alzamora, R.; Figueroa, F.; Kalergis, A.M.; et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 2014, 63, 797–803. [Google Scholar] [CrossRef]
- Kasal, D.A.; Barhoumi, T.; Li, M.W.; Yamamoto, N.; Zdanovich, E.; Rehman, A.; Neves, M.F.; Laurant, P.; Paradis, P.; Schiffrin, E.L. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 2012, 59, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Hirooka, Y.; Kishi, T.; Sunagawa, K. Decreased proportion of Foxp3+ CD4+ regulatory T cells contributes to the development of hypertension in genetically hypertensive rats. J. Hypertens. 2015, 33, 773–783, discussion 783. [Google Scholar] [CrossRef]
- Caillon, A.; Mian, M.O.R.; Fraulob-Aquino, J.C.; Huo, K.G.; Barhoumi, T.; Ouerd, S.; Sinnaeve, P.R.; Paradis, P.; Schiffrin, E.L. γδ T Cells Mediate Angiotensin II-Induced Hypertension and Vascular Injury. Circulation 2017, 135, 2155–2162. [Google Scholar] [CrossRef]
- Kirabo, A.; Fontana, V.; de Faria, A.P.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W.; et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef]
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020. [Google Scholar] [CrossRef]
- Van Beusecum, J.P.; Barbaro, N.R.; McDowell, Z.; Aden, L.A.; Xiao, L.; Pandey, A.K.; Itani, H.A.; Himmel, L.E.; Harrison, D.G.; Kirabo, A. High Salt Activates CD11c+ Antigen-presenting cells via SGK (Serum Glucocorticoid kinase) 1 to promote renal inflammation and Salt-Sensitive hypertension. Hypertension 2019, 74, 555–563. [Google Scholar] [CrossRef]
- Norlander, A.E.; Saleh, M.A.; Pandey, A.K.; Itani, H.A.; Wu, J.; Xiao, L.; Kang, J.; Dale, B.L.; Goleva, S.B.; Laroumanie, F.; et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Hevia, D.; Araos, P.; Prado, C.; Fuentes Luppichini, E.; Rojas, M.; Alzamora, R.; Cifuentes-Araneda, F.; Gonzalez, A.A.; Amador, C.A.; Pacheco, R.; et al. Myeloid CD11c+ Antigen-Presenting Cells Ablation Prevents Hypertension in Response to Angiotensin II Plus High-Salt Diet. Hypertension 2018, 71, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Araos, P.; Prado, C.; Lozano, M.; Figueroa, S.; Espinoza, A.; Berger, T.; Mak, T.W.; Jaisser, F.; Pacheco, R.; Michea, L.; et al. Dendritic cells are crucial for cardiovascular remodeling and modulate neutrophil gelatinase-associated lipocalin expression upon mineralocorticoid receptor activation. J. Hypertens. 2019, 37, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Justin Rucker, A.; Crowley, S.D. The role of macrophages in hypertension and its complications. Pflug. Arch. 2017, 469, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef]
- Sag, C.M.; Schnelle, M.; Zhang, J.; Murdoch, C.E.; Kossmann, S.; Protti, A.; Santos, C.X.C.; Sawyer, G.; Zhang, X.; Mongue-Din, H.; et al. Distinct Regulatory Effects of Myeloid Cell and Endothelial Cell NAPDH Oxidase 2 on Blood Pressure. Circulation 2017, 135, 2163–2177. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Tatsukawa, Y.; Hsu, W.L.; Yamada, M.; Cologne, J.B.; Suzuki, G.; Yamamoto, H.; Yamane, K.; Akahoshi, M.; Fujiwara, S.; Kohno, N. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens. Res. 2008, 31, 1391–1397. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Wu, H.; Du, H.; Liu, L.; Shi, H.; Wang, C.; Xia, Y.; Guo, X.; Li, C.; et al. Blood Neutrophil to Lymphocyte Ratio as a Predictor of Hypertension. Am. J. Hypertens. 2015, 28, 1339–1346. [Google Scholar] [CrossRef]
- Sunbul, M.; Gerin, F.; Durmus, E.; Kivrak, T.; Sari, I.; Tigen, K.; Cincin, A. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin. Exp. Hypertens. 2014, 36, 217–221. [Google Scholar] [CrossRef]
- Belen, E.; Sungur, A.; Sungur, M.A.; Erdogan, G. Increased Neutrophil to Lymphocyte Ratio in Patients With Resistant Hypertension. J. Clin. Hypertens. (Greenwich) 2015, 17, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Fici, F.; Celik, T.; Balta, S.; Iyisoy, A.; Unlu, M.; Demitkol, S.; Yaman, H.; Brambilla, G.; Kardesoglu, E.; Kilic, S.; et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J. Cardiovasc. Pharm. 2013, 62, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Balta, S.; Seyit Ahmet, A.Y.; Cakar, M.; Naharci, I.; Demirkol, S.; Celik, T.; Arslan, Z.; Kurt, O.; Kocak, N.; et al. The comparative effects of valsartan and amlodipine on vWf levels and N/L ratio in patients with newly diagnosed hypertension. Clin. Exp. Hypertens. 2013, 35, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Kristal, B.; Shurtz-Swirski, R.; Chezar, J.; Manaster, J.; Levy, R.; Shapiro, G.; Weissman, I.; Shasha, S.M.; Sela, S. Participation of peripheral polymorphonuclear leukocytes in the oxidative stress and inflammation in patients with essential hypertension. Am. J. Hypertens. 1998, 11, 921–928. [Google Scholar] [CrossRef]
- Tsukimori, K.; Fukushima, K.; Tsushima, A.; Nakano, H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension 2005, 46, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Tsukimori, K.; Nakano, H.; Wake, N. Difference in neutrophil superoxide generation during pregnancy between preeclampsia and essential hypertension. Hypertension 2007, 49, 1436–1441. [Google Scholar] [CrossRef]
- Alba, G.; El Bekay, R.; Chacon, P.; Reyes, M.E.; Ramos, E.; Olivan, J.; Jimenez, J.; Lopez, J.M.; Martin-Nieto, J.; Pintado, E.; et al. Heme oxygenase-1 expression is down-regulated by angiotensin II and under hypertension in human neutrophils. J. Leukoc. Biol. 2008, 84, 397–405. [Google Scholar] [CrossRef]
- Bozduman, F.; Yildirim, E.; Cicek, G. Biomarkers of nondipper hypertension in prehypertensive and hypertensive patients. Biomark. Med. 2019, 13, 371–378. [Google Scholar] [CrossRef]
- Aydin, M.; Yuksel, M.; Yildiz, A.; Polat, N.; Bilik, M.Z.; Akil, M.A.; Acet, H.; Demir, M.; Inci, U.; Toprak, N. Association between the neutrophil to lymphocyte ratio and prehypertension. Bratisl. Lek. Listy 2015, 116, 475–479. [Google Scholar] [CrossRef]
- Sun, X.; Luo, L.; Zhao, X.; Ye, P.; Du, R. The neutrophil-to-lymphocyte ratio on admission is a good predictor for all-cause mortality in hypertensive patients over 80 years of age. BMC Cardiovasc. Disord. 2017, 17, 167. [Google Scholar] [CrossRef]
- Morton, J.; Coles, B.; Wright, K.; Gallimore, A.; Morrow, J.D.; Terry, E.S.; Anning, P.B.; Morgan, B.P.; Dioszeghy, V.; Kuhn, H.; et al. Circulating neutrophils maintain physiological blood pressure by suppressing bacteria and IFNgamma-dependent iNOS expression in the vasculature of healthy mice. Blood 2008, 111, 5187–5194. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Saluja, R.; Tewari, S.; Barthwal, M.K.; Goel, S.K.; Dikshit, M. Augmented nitric oxide generation in neutrophils: Oxidative and pro-inflammatory implications in hypertension. Free Radic Res. 2009, 43, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zhang, C.; Wang, Y.; Cui, W.; Li, H.; Du, J. S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II-Induced cardiac inflammation and injury. Hypertension 2014, 63, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Ahadzadeh, E.; Rosendahl, A.; Czesla, D.; Steffens, P.; Prussner, L.; Meyer-Schwesinger, C.; Wanner, N.; Paust, H.J.; Huber, T.B.; Stahl, R.A.K.; et al. The chemokine receptor CX3CR1 reduces renal injury in mice with angiotensin II-induced hypertension. Am. J. Physiol. Ren. Physiol. 2018, 315, F1526–F1535. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xue, K.Y.; Xin, J.J.; Yan, X.; Li, R.L.; Wang, X.X.; Wang, X.L.; Tong, M.M.; Gan, L.; Li, H.; et al. 5-Lipoxagenase deficiency attenuates L-NAME-induced hypertension and vascular remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2379–2392. [Google Scholar] [CrossRef]
- Siedlinski, M.; Jozefczuk, E.; Xu, X.; Teumer, A.; Evangelou, E.; Schnabel, R.B.; Welsh, P.; Maffia, P.; Erdmann, J.; Tomaszewski, M.; et al. White Blood Cells and Blood Pressure: A Mendelian Randomization Study. Circulation 2020, 141, 1307–1317. [Google Scholar] [CrossRef]
- Yu, S.; Arima, H.; Bertmar, C.; Clarke, S.; Herkes, G.; Krause, M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J. Neurol. Sci. 2018, 387, 115–118. [Google Scholar] [CrossRef]
- Derya, M.A.; Demir, V.; Ede, H. Relationship between neutrophil/lymphocyte ratio and epicardial fat tissue thickness in patients with newly diagnosed hypertension. J. Int. Med. Res. 2018, 46, 940–950. [Google Scholar] [CrossRef]
- Balta, S.; Celik, T.; Mikhailidis, D.P.; Ozturk, C.; Demirkol, S.; Aparci, M.; Iyisoy, A. The Relation Between Atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin. Appl. Thromb. Hemost. 2016, 22, 405–411. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Schiffrin, E.L. How Structure, Mechanics, and Function of the Vasculature Contribute to Blood Pressure Elevation in Hypertension. Can. J. Cardiol. 2020, 36, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Milic, N.M.; Milin-Lazovic, J.S.; Garovic, V.D. Impaired Flow-Mediated Dilation Before, During, and After Preeclampsia: A Systematic Review and Meta-Analysis. Hypertension 2016, 67, 415–423. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Chatterjee, V.; Meegan, J.E.; Beard, R.S., Jr.; Yuan, S.Y. Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability. Front. Immunol. 2019, 10, 1037. [Google Scholar] [CrossRef]
- Kanashiro, A.; Hiroki, C.H.; da Fonseca, D.M.; Birbrair, A.; Ferreira, R.G.; Bassi, G.S.; Fonseca, M.D.; Kusuda, R.; Cebinelli, G.C.M.; da Silva, K.P.; et al. The role of neutrophils in neuro-immune modulation. Pharm. Res. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Nicholls, A.J.; Wen, S.W.; Hall, P.; Hickey, M.J.; Wong, C.H.Y. Activation of the sympathetic nervous system modulates neutrophil function. J. Leukoc. Biol. 2018, 103, 295–309. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharm. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Amador, C.A.; Bertocchio, J.P.; Andre-Gregoire, G.; Placier, S.; Duong Van Huyen, J.P.; El Moghrabi, S.; Berger, S.; Warnock, D.G.; Chatziantoniou, C.; Jaffe, I.Z.; et al. Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int. 2016, 89, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Provost, P.; Lam, J.Y.; Lacoste, L.; Merhi, Y.; Waters, D. Endothelium-derived nitric oxide attenuates neutrophil adhesion to endothelium under arterial flow conditions. Arter. Thromb. 1994, 14, 331–335. [Google Scholar] [CrossRef]
- Hossain, M.; Qadri, S.M.; Liu, L. Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J. Inflamm. (Lond.) 2012, 9, 28. [Google Scholar] [CrossRef]
- Banick, P.D.; Chen, Q.; Xu, Y.A.; Thom, S.R. Nitric oxide inhibits neutrophil beta 2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J. Cell Physiol. 1997, 172, 12–24. [Google Scholar] [CrossRef]
- Fraticelli, A.; Serrano, C.V., Jr.; Bochner, B.S.; Capogrossi, M.C.; Zweier, J.L. Hydrogen peroxide and superoxide modulate leukocyte adhesion molecule expression and leukocyte endothelial adhesion. Biochim. Biophys. Acta 1996, 1310, 251–259. [Google Scholar] [CrossRef]
- Hofman, F.M.; Chen, P.; Jeyaseelan, R.; Incardona, F.; Fisher, M.; Zidovetzki, R. Endothelin-1 induces production of the neutrophil chemotactic factor interleukin-8 by human brain-derived endothelial cells. Blood 1998, 92, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Lopez Farre, A.; Riesco, A.; Espinosa, G.; Digiuni, E.; Cernadas, M.R.; Alvarez, V.; Monton, M.; Rivas, F.; Gallego, M.J.; Egido, J.; et al. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation 1993, 88, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Zouki, C.; Baron, C.; Fournier, A.; Filep, J.G. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: Role of ET(A) receptors and platelet-activating factor. Br. J. Pharm. 1999, 127, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Scanzano, A.; Schembri, L.; Rasini, E.; Luini, A.; Dallatorre, J.; Legnaro, M.; Bombelli, R.; Congiu, T.; Cosentino, M.; Marino, F. Adrenergic modulation of migration, CD11b and CD18 expression, ROS and interleukin-8 production by human polymorphonuclear leukocytes. Inflamm. Res. 2015, 64, 127–135. [Google Scholar] [CrossRef]
- Averill, M.M.; Kerkhoff, C.; Bornfeldt, K.E. S100A8 and S100A9 in cardiovascular biology and disease. Arter. Thromb. Vasc. Biol. 2012, 32, 223–229. [Google Scholar] [CrossRef]
- Kerkhoff, C.; Nacken, W.; Benedyk, M.; Dagher, M.C.; Sopalla, C.; Doussiere, J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005, 19, 467–469. [Google Scholar] [CrossRef]
- Viemann, D.; Strey, A.; Janning, A.; Jurk, K.; Klimmek, K.; Vogl, T.; Hirono, K.; Ichida, F.; Foell, D.; Kehrel, B.; et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 2005, 105, 2955–2962. [Google Scholar] [CrossRef]
- Bennouna, S.; Bliss, S.K.; Curiel, T.J.; Denkers, E.Y. Cross-talk in the innate immune system: Neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J. Immunol. 2003, 171, 6052–6058. [Google Scholar] [CrossRef]
- Martynowicz, H.; Janus, A.; Nowacki, D.; Mazur, G. The role of chemokines in hypertension. Adv. Clin. Exp. Med. 2014, 23, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Paragas, N.; Qiu, A.; Zhang, Q.; Samstein, B.; Deng, S.X.; Schmidt-Ott, K.M.; Viltard, M.; Yu, W.; Forster, C.S.; Gong, G.; et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 2011, 17, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Latouche, C.; El Moghrabi, S.; Messaoudi, S.; Nguyen Dinh Cat, A.; Hernandez-Diaz, I.; Alvarez de la Rosa, D.; Perret, C.; Lopez Andres, N.; Rossignol, P.; Zannad, F.; et al. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension 2012, 59, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [PubMed]
- Buonafine, M.; Martinez-Martinez, E.; Amador, C.; Gravez, B.; Ibarrola, J.; Fernandez-Celis, A.; El Moghrabi, S.; Rossignol, P.; Lopez-Andres, N.; Jaisser, F. Neutrophil Gelatinase-Associated Lipocalin from immune cells is mandatory for aldosterone-induced cardiac remodeling and inflammation. J. Mol. Cell Cardiol. 2018, 115, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Park, C.G.; Choi, K.M. Lipocalin-2, A-FABP and inflammatory markers in relation to flow-mediated vasodilatation in patients with essential hypertension. Clin. Exp. Hypertens. 2014, 36, 478–483. [Google Scholar] [CrossRef]
- Ong, K.L.; Tso, A.W.; Cherny, S.S.; Sham, P.C.; Lam, T.H.; Lam, K.S.; Cheung, B.M. Investigators of the Hong Kong Cardiovascular Risk Factor Prevalence, S. Role of genetic variants in the gene encoding lipocalin-2 in the development of elevated blood pressure. Clin. Exp. Hypertens. 2011, 33, 484–491. [Google Scholar] [CrossRef]
- Tarjus, A.; Martinez-Martinez, E.; Amador, C.; Latouche, C.; El Moghrabi, S.; Berger, T.; Mak, T.W.; Fay, R.; Farman, N.; Rossignol, P.; et al. Neutrophil Gelatinase-Associated Lipocalin, a Novel Mineralocorticoid Biotarget, Mediates Vascular Profibrotic Effects of Mineralocorticoids. Hypertension 2015, 66, 158–166. [Google Scholar] [CrossRef]
- Sreejit, G.; Abdel-Latif, A.; Athmanathan, B.; Annabathula, R.; Dhyani, A.; Noothi, S.K.; Quaife-Ryan, G.A.; Al-Sharea, A.; Pernes, G.; Dragoljevic, D.; et al. Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction. Circulation 2020, 141, 1080–1094. [Google Scholar] [CrossRef]
- Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F.; Luscher, T.F.; Camici, G.G.; Liberale, L. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc. Res. 2019, 115, 1266–1285. [Google Scholar] [CrossRef] [PubMed]
- Sickinger, S.; Maier, H.; Konig, S.; Vallant, N.; Kofler, M.; Schumpp, P.; Schwelberger, H.; Hermann, M.; Obrist, P.; Schneeberger, S.; et al. Lipocalin-2 as mediator of chemokine expression and granulocyte infiltration during ischemia and reperfusion. Transpl. Int. 2013, 26, 761–769. [Google Scholar] [CrossRef]
- Doring, Y.; Libby, P.; Soehnlein, O. Neutrophil Extracellular Traps Participate in Cardiovascular Diseases: Recent Experimental and Clinical Insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Pena-Martinez, C.; Duran-Laforet, V.; Garcia-Culebras, A.; Ostos, F.; Hernandez-Jimenez, M.; Bravo-Ferrer, I.; Perez-Ruiz, A.; Ballenilla, F.; Diaz-Guzman, J.; Pradillo, J.M.; et al. Pharmacological Modulation of Neutrophil Extracellular Traps Reverses Thrombotic Stroke tPA (Tissue-Type Plasminogen Activator) Resistance. Stroke 2019, 50, 3228–3237. [Google Scholar] [CrossRef]
- Lim, H.H.; Jeong, I.H.; An, G.D.; Woo, K.S.; Kim, K.H.; Kim, J.M.; Yun, S.H.; Park, J.I.; Cha, J.K.; Kim, M.H.; et al. Evaluation of neutrophil extracellular traps as the circulating marker for patients with acute coronary syndrome and acute ischemic stroke. J. Clin. Lab. Anal. 2020, 34, e23190. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Camara, N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharm. 2019, 10, 1192. [Google Scholar] [CrossRef]

| Experimental Model | Main Findings Related to Neutrophils and Blood Pressure | Reference |
|---|---|---|
| Studies in Patients or Human Samples | ||
| Blood neutrophils from AH patients N = 37; case-control study | Superoxide production was increased in neutrophils from AH patients | [44] |
| Blood neutrophils from women with preeclampsia N = 34; case-control study | Superoxide production increased in neutrophils from women with preeclampsia | [45] [46] |
| Blood neutrophils from AH patients N = 9,383; cohort study N = 28,850; cohort study N = not indicate | Neutrophils were associated with incidence of AH and correlated with more risk of AH | [38] [39] |
| Reduction of HO-1 and Nrf2 in neutrophils from AH | [47] | |
| Blood neutrophils from AH patients N = 72; double-blind randomized prospective study N = 46; randomized study | Nebivolol and Valsartan decreased NLR ratio in AH patients | [42] [43] |
| N = 166; cross-sectional study N = 409; single-center retrospective study | NLR and neutrophil count were increased in AH patients with non-dipper pattern | [40] [48] |
| N = 150; observational study N = 33; cross-sectional study | NLR and neutrophil count were increased in RHT patients and in patients with ’normal-high’ AH grade | [41] [49] |
| Blood neutrophils from elderly patients with AH N = 341 single-center observational study | NLR was linked to a high probability of mortality in elderly patients with AH | [50] |
| Studies in Experimental Animals | ||
| Neutrophil depletion in normotensive mice | BP reduction in normotensive WT mice; iNOS or IFNγ ablation reversed this effect | [51] |
| SHR | iNOS, MPO activity and IL-1β increased in circulating neutrophils | [52] |
| AngII infused mice | Circulating and aortic wall neutrophils increased in AH mice | |
| Depletion of LysM+ cells prevented AH and increased circulating neutrophils | ||
| Adoptive transfer of neutrophils did not reestablish AH | [34] | |
| AngII infused mice | Early induction of S100a8/a9 in circulating neutrophils | [53] |
| The anti-S100a9 suppressed heart infiltration of neutrophils, with no effect on BP | ||
| Nephrectomy-AngII-salt mice | CX3CR1 ablation induced high renal damage with increased neutrophils infiltration on kidney | [54] |
| L-NAME hypertensive mice | Increased leukotrienes in neutrophils supernatants isolated from L-NAME mice | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araos, P.; Figueroa, S.; Amador, C.A. The Role of Neutrophils in Hypertension. Int. J. Mol. Sci. 2020, 21, 8536. https://doi.org/10.3390/ijms21228536
Araos P, Figueroa S, Amador CA. The Role of Neutrophils in Hypertension. International Journal of Molecular Sciences. 2020; 21(22):8536. https://doi.org/10.3390/ijms21228536
Chicago/Turabian StyleAraos, Patricio, Stefanny Figueroa, and Cristián A. Amador. 2020. "The Role of Neutrophils in Hypertension" International Journal of Molecular Sciences 21, no. 22: 8536. https://doi.org/10.3390/ijms21228536
APA StyleAraos, P., Figueroa, S., & Amador, C. A. (2020). The Role of Neutrophils in Hypertension. International Journal of Molecular Sciences, 21(22), 8536. https://doi.org/10.3390/ijms21228536







