Efficiency of Hydroxycinnamic Phenolic Acids to Inhibit the Production of Ochratoxin A by Aspergillus westerdijkiae and Penicillium verrucosum
Abstract
1. Introduction
2. Results
2.1. Search for Suitable Culture Conditions to Investigate the Effects of Hydroxycinnamic Acids on Fungal Growth and OTA Production by A. westerdijkiae and P. verrucosum
2.1.1. Comparison of Fungal Biomass in the Five Tested Media under Static and Agitated Conditions
2.1.2. OTA Production by A. westerdijkiae and P. verrucosum in the Five Tested Media under Static and Agitated Conditions
2.1.3. Evolution of pH in the Inoculated Media
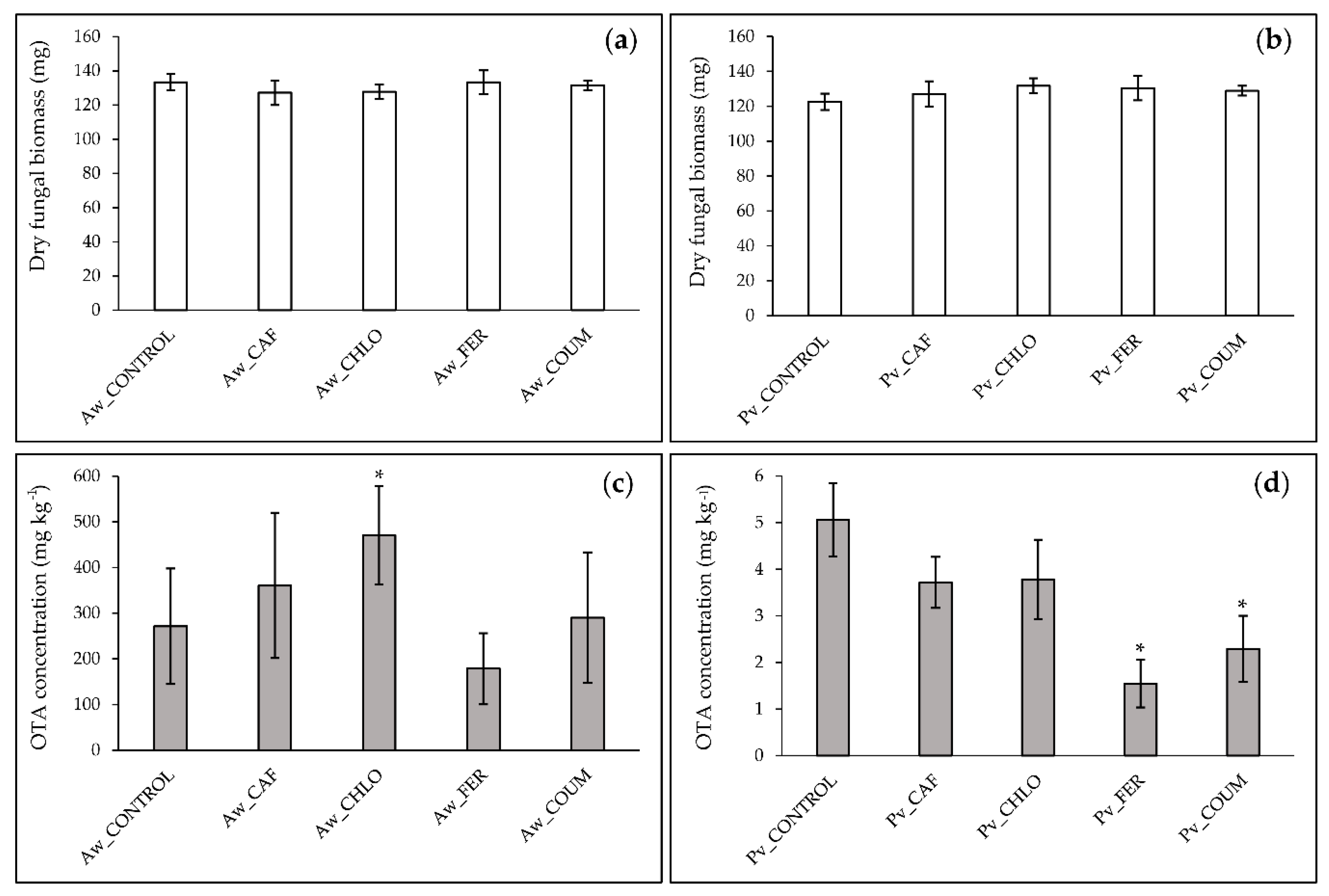
2.2. In Vitro Effect of Hydroxycinnamic Acids on Fungal Growth and OTA Production by A. westerdijkiae and P. verrucosum
2.3. Kinetic Study of Fungal Growth, OTA Production, and Fate of Phenolic Acids in P. verrucosum Broths Supplemented with FER and COUM
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fungal Species
4.3. Media and Culture Conditions
- AM medium: glucose 50 g, yeast extract 3 g, (NH4)2 SO4 3 g, KH2PO4 10 g, MgSO4.7H2O 2 g, distilled water 1000 mL.
- CYA medium: sucrose 30 g, yeast extract 5 g, NaNO3 0.5 g, KCl 0.05 g, MgSO4.7H2O 0.5 g, FeSO4.7H2O 0.01 g, K2HPO4 1.3 g, CuSO4.5H2O 0.005 g, ZnSO4.7H2O 0.01 g, distilled water 1000 mL.
- ME medium: sucrose 30 g, malt extract 40 g, peptone 1 g, CuSO4.5H2O 0.005 g, ZnSO4.7H2O 0.01 g, distilled water 1000 mL.
- YES_A medium: sucrose 150 g, yeast extract 20 g, MgSO4.7H2O 0.5 g, CuSO4.5H2O 0.005 g, ZnSO4.7H2O 0.01 g, distilled water 1000 mL.
- YES_B medium: sucrose 150 g, yeast extract 10 g, MgSO4.7H2O 2 g, CuSO4.5H2O 0.005 g, ZnSO4.7H2O 0.01 g, distilled water 1000 mL.
4.4. OTA Extraction and Measurement
4.5. Analysis of Phenolic Acids and Their Biotransformation Products
4.6. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OTA | Ochratoxin A |
| Pv | Penicillium verrucosum |
| Aw | Aspergillus westerdijkiae |
| HBENZ | p-Hydroxybenzoic acid |
| CAF | Caffeic acid |
| CHLO | Chlorogenic acid |
| COUM | p-Coumaric acid |
| FER | Ferulic acid |
| VAN | Vanillic acid |
| LC/DAD | Liquid chromatography coupled with a Diode Array Detector |
| LC/FLD | Liquid chromatography coupled with a fluorescence detector |
| ANOVA | Analysis of variance |
| FAO | Food and Agriculture Organization |
| IARC | International Agency for Research on Cancer |
| EC | European Commission |
| AM medium | Adye and Mateles medium |
| CYA medium | Czapek Yeast Autolysate medium |
| ME medium | Malt Extract medium |
| YES_A medium | Yeast Extract Sucrose_A medium |
| YES_B medium | Yeast Extract Sucrose_B medium |
References
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; HajšLová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Stoll, D.; Schmidt-Heydt, M.; Geisen, R. Differences in the Regulation of Ochratoxin A by the HOG Pathway in Penicillium and Aspergillus in Response to High Osmolar Environments. Toxins 2013, 5, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Mulè, G.; Susca, A.; Battilani, P.; Pietri, A.; Logrieco, A. Ochratoxin A Production and Amplified Fragment Length Polymorphism Analysis of Aspergillus carbonarius, Aspergillus tubingensis, and Aspergillus niger Strains Isolated from Grapes in Italy. Appl. Environ. Microbiol. 2006, 72, 680–685. [Google Scholar] [CrossRef]
- Duarte, S.; Pena, A.; Lino, C. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef]
- Haubeck, H.D.; Lorkowski, G.; Kölsch, E.; Röschenthaler, R. Immunosuppression by ochratoxin A and its prevention by phenylalanine. Appl. Environ. Microbiol. 1981, 41, 1040–1042. [Google Scholar] [CrossRef]
- Petzinger, E.; Ziegler, K. Ochratoxin A from a toxicological perspective. J. Veter-Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef]
- Stiborová, M.; Arlt, V.M.; Schmeiser, H.H. Balkan endemic nephropathy: An update on its aetiology. Arch. Toxicol. 2016, 90, 2595–2615. [Google Scholar] [CrossRef]
- Sun, J.; Janisiewicz, W.J.; Nichols, B.; Ii, W.M.J.; Chen, P. Composition of phenolic compounds in wild apple with multiple resistance mechanisms against postharvest blue mold decay. Postharvest Biol. Technol. 2017, 127, 68–75. [Google Scholar] [CrossRef]
- Telles, A.C.; Kupski, L.; Badiale-Furlong, E. Phenolic compound in beans as protection against mycotoxins. Food Chem. 2017, 214, 293–299. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Bernillon, S.; Marchegay, G.; Lornac, A.; Pinson-Gadais, L.; Ponts, N.; Zehraoui, E.; Barreau, C.; Richard-Forget, F. Bioguided Isolation, Characterization, and Biotransformation by Fusarium verticillioides of Maize Kernel Compounds That Inhibit Fumonisin Production. Mol. Plant-Microbe Interact. 2014, 27, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, E.; Weinert, C.H.; Egert, B.; Trierweiler, B.; Schmidt-Heydt, M.; Horneburg, B.; Graeff-Hönninger, S.; Kulling, S.E.; Geisen, R. Chlorogenic acid, a metabolite identified by untargeted metabolome analysis in resistant tomatoes, inhibits the colonization by Alternaria alternata by inhibiting alternariol biosynthesis. Eur. J. Plant Pathol. 2014, 139, 735–747. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Atanasova-Penichon, V.; Ebarreau, C.; Erichard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’Keeffe, T.L.; Mahoney, N.E. Inhibition of ochratoxin A production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathology 2007, 164, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.; Gsponer, N.; Etcheverry, M. Natural Maize Phenolic Acids for Control of Aflatoxigenic Fungi on Maize. J. Food Sci. 2007, 72, M180–M185. [Google Scholar] [CrossRef]
- Ferrara, M.; Gallo, A.; Scalzo, R.L.; Haidukowski, M.; Picchi, V.; Perrone, G.; Haidukowski, M. Inhibition of ochratoxin A production in Aspergillus carbonarius by hydroxycinnamic acids from grapes. World Mycotoxin J. 2015, 8, 283–289. [Google Scholar] [CrossRef]
- Romero, S.M.; Alberto, M.R.; De Nadra, M.C.M.; Vaamonde, G. Inhibition of growth and ochratoxin A biosynthesis in Aspergillus carbonarius by flavonoid and nonflavonoid compounds. Mycotoxin Res. 2009, 25, 165–170. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.; Schieber, A.; Gänzle, M. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Almajano, M.; Carbó, R.; Delgado, M.; Gordon, M.H. Effect of pH on the Antimicrobial Activity and Oxidative Stability of Oil-in-Water Emulsions Containing Caffeic Acid. J. Food Sci. 2007, 72, C258–C263. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.O.; Svendsen, A.; Smedsgaard, J. Biochemical Characterization of Ochratoxin A-Producing Strains of the Genus Penicillium. Appl. Environ. Microbiol. 2001, 67, 3630–3635. [Google Scholar] [CrossRef]
- Rao, V.K.; Ramana, M.V.; Girisham, S.; Reddy, S.M. Culture Media and Factors Influencing Ochratoxin A Production by Two Species of Penicillium Isolated from Poultry Feeds. Natl. Acad. Sci. Lett. 2013, 36, 101–110. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Pons, S.; Pinson-Gadais, L.; Picot, A.; Marchegay, G.; Bonnin-Verdal, M.-N.; Ducos, C.; Barreau, C.; Roucolle, J.; Sehabiague, P.; et al. Chlorogenic Acid and Maize Ear Rot Resistance: A Dynamic Study Investigating Fusarium graminearum Development, Deoxynivalenol Production, and Phenolic Acid Accumulation. Mol. Plant-Microbe Interact. 2012, 25, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Lima, J.D.P. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Boz, H. Ferulic acid in cereals—A review. Czech J. Food Sci. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Mahoney, N.; Molyneux, R.J.; Kim, J.H.; Campbell, B.C.; Waiss, A.C.; Hagerman, A.E. Aflatoxigenesis induced in Aspergillus flavus by oxidative stress and reduction by phenolic antioxidants from tree nuts. World Mycotoxin J. 2010, 3, 49–57. [Google Scholar] [CrossRef]
- El Khoury, R.; Atoui, A.; Mathieu, F.; Kawtharani, H.; El Khoury, A.; Maroun, R.G.; El Khoury, A. Antifungal and Antiochratoxigenic Activities of Essential Oils and Total Phenolic Extracts: A Comparative Study. Antioxidants 2017, 6, 44. [Google Scholar] [CrossRef]
- Ricelli, A.; De Angelis, M.; Primitivo, L.; Righi, G.; Sappino, C.; Antonioletti, R. Role of Some Food-Grade Synthesized Flavonoids on the Control of Ochratoxin A in Aspergillus carbonarius. Molecules 2019, 24, 2553. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Legoahec, L.; Bernillon, S.; Deborde, C.; Maucourt, M.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Ponts, N.; Moing, A.; Richard-Forget, F. Mycotoxin Biosynthesis and Central Metabolism Are Two Interlinked Pathways in Fusarium graminearum, as Demonstrated by the Extensive Metabolic Changes Induced by Caffeic Acid Exposure. Appl. Environ. Microbiol. 2018, 84, e01705-17. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Genetic regulation of aflatoxin, ochratoxin A, trichothecene, and fumonisin biosynthesis: A review. Int. Microbiol. 2019, 23, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Montibus, M.; Ducos, C.; Bonnin-Verdal, M.-N.; Bormann, J.; Ponts, N.; Richard-Forget, F.; Barreau, C. The bZIP Transcription Factor Fgap1 Mediates Oxidative Stress Response and Trichothecene Biosynthesis But Not Virulence in Fusarium graminearum. PLoS ONE 2013, 8, e83377. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Gazzetti, K.; Punelli, F.; Scarpari, M.; Zjalic, S.; Ricelli, A.; Fabbri, A.A.; Fanelli, C. Aoyap1 regulates OTA synthesis by controlling cell redox balance in Aspergillus ochraceus. Appl. Microbiol. Biotechnol. 2012, 95, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Sempere, A.; Selma-Lázaro, C.; Palumbo, J.D.; González-Candelas, L.; Martínez-Culebras, P.V. Effect of oxidant stressors and phenolic antioxidants on the ochratoxigenic fungus Aspergillus carbonarius. J. Sci. Food Agric. 2015, 96, 169–177. [Google Scholar] [CrossRef]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem. Rev. 2009, 9, 147–170. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Stoll, D.; Schütz, P.; Geisen, R. Oxidative stress induces the biosynthesis of citrinin by Penicillium verrucosum at the expense of ochratoxin. Int. J. Food Microbiol. 2015, 192, 1–6. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.-N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef]
- Ferruz, E.; Atanasova-Penichon, V.; Bonnin-Verdal, M.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Lorán, S.; Ariño, A.; Barreau, C.; Richard-Forget, F. Effects of Phenolic Acids on the Growth and Production of T-2 and HT-2 Toxins by Fusarium langsethiae and F. sporotrichioides. Molecules 2016, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, J.; Liu, X.; Wang, Y.; Liu, Y.; Liu, Y.; Xing, F. Effect of Cinnamaldehyde on Morphological Alterations of Aspergillus ochraceus and Expression of Key Genes Involved in Ochratoxin a Biosynthesis. Toxins 2018, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Baqueiro-Peña, I.; Rodríguez-Serrano, G.; González-Zamora, E.; Augur, C.; Loera, O.; Saucedo-Castañeda, J.G. Biotransformation of ferulic acid to 4-vinylguaiacol by a wild and a diploid strain of Aspergillus niger. Bioresour. Technol. 2010, 101, 4721–4724. [Google Scholar] [CrossRef] [PubMed]
- Nazila, M.; Ismail, M.; Forough, N. Bioconversion of ferulic acid to vanillin by combined action of Aspergillus niger K8 and Phanerochaete crysosporium ATCC 24725. Afr. J. Biotechnol. 2013, 12, 6618–6624. [Google Scholar] [CrossRef][Green Version]
- Falconnier, B.; Lapierre, C.; Lesage-Meessen, L.; Yonnet, G.; Brunerie, P.; Colonna-Ceccaldi, B.; Corrieu, G.; Asther, M. Vanillin as a product of ferulic acid biotransformation by the white-rot fungus Pycnoporus cinnabarinus I-937: Identification of metabolic pathways. J. Biotechnol. 1994, 37, 123–132. [Google Scholar] [CrossRef]
- Tsujiyama, S.-I.; Ueno, M. Formation of 4-Vinyl Guaiacol as an Intermediate in Bioconversion of Ferulic Acid by Schizophyllum commune. Biosci. Biotechnol. Biochem. 2008, 72, 212–215. [Google Scholar] [CrossRef]
- Sachan, S.G.; Ghosh, S.; Mitra, A. Biotransformation of p-coumaric acid by Paecilomyces variotii. Lett. Appl. Microbiol. 2006, 42, 35–41. [Google Scholar] [CrossRef]
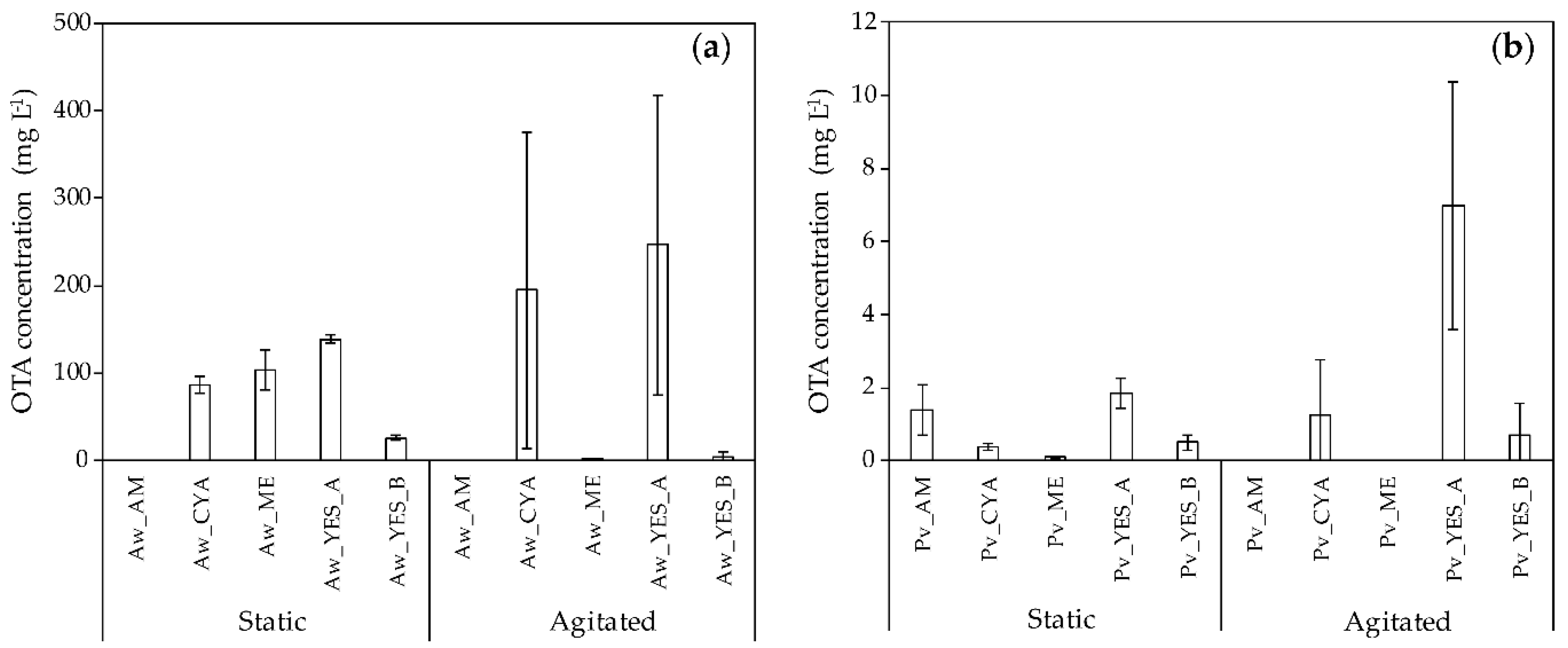


| Media | Initial pH | A. westerdijkiae | P. verrucosum | ||
|---|---|---|---|---|---|
| Final pH | Dry Fungal Biomass 1, mg | Final pH | Dry Fungal Biomass 1, mg | ||
| Static Condition | |||||
| AM | 5.1 | 2.0 | 202.37 ± 3.37 | 3.0 | 234.33 ± 29.40 |
| CYA | 7.2 | 8.0 | 170.33 ± 5.01 | 7.0 | 127.27 ± 1.72 |
| ME | 4.2 | 5.5 | 264.87 ± 3.89 | 5.0 | 235.93 ± 5.03 |
| YES_A | 6.6 | 6.0 | 506.50 ± 6.08 | 6.0 | 372.77 ± 7.06 |
| YES_B | 6.7 | 6.0 | 521.00 ± 53.66 | 4.5 | 426.67 ± 14.88 |
| Agitated Condition | |||||
| AM | 5.1 | 4.0 | 324.33 ± 33.01 | 4.5 | 432.37 ± 114.87 |
| CYA | 7.2 | 7.0 | 340.80 ± 25.64 | 6.0 | 383.63 ± 71.83 |
| ME | 4.2 | 4.0 | 609.70 ± 303.83 | 4.0 | 381.83 ± 57.87 |
| YES_A | 6.6 | 6.0 | 1484.33 ± 103.01 | 5.5 | 1135.37 ± 96.56 |
| YES_B | 6.7 | 4.0 | 1057.87 ± 43.37 | 5.5 | 880.77 ± 155.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonmee, S.; Atanasova, V.; Chéreau, S.; Marchegay, G.; Hyde, K.D.; Richard-Forget, F. Efficiency of Hydroxycinnamic Phenolic Acids to Inhibit the Production of Ochratoxin A by Aspergillus westerdijkiae and Penicillium verrucosum. Int. J. Mol. Sci. 2020, 21, 8548. https://doi.org/10.3390/ijms21228548
Boonmee S, Atanasova V, Chéreau S, Marchegay G, Hyde KD, Richard-Forget F. Efficiency of Hydroxycinnamic Phenolic Acids to Inhibit the Production of Ochratoxin A by Aspergillus westerdijkiae and Penicillium verrucosum. International Journal of Molecular Sciences. 2020; 21(22):8548. https://doi.org/10.3390/ijms21228548
Chicago/Turabian StyleBoonmee, Saranyaphat, Vessela Atanasova, Sylvain Chéreau, Gisèle Marchegay, Kevin D. Hyde, and Florence Richard-Forget. 2020. "Efficiency of Hydroxycinnamic Phenolic Acids to Inhibit the Production of Ochratoxin A by Aspergillus westerdijkiae and Penicillium verrucosum" International Journal of Molecular Sciences 21, no. 22: 8548. https://doi.org/10.3390/ijms21228548
APA StyleBoonmee, S., Atanasova, V., Chéreau, S., Marchegay, G., Hyde, K. D., & Richard-Forget, F. (2020). Efficiency of Hydroxycinnamic Phenolic Acids to Inhibit the Production of Ochratoxin A by Aspergillus westerdijkiae and Penicillium verrucosum. International Journal of Molecular Sciences, 21(22), 8548. https://doi.org/10.3390/ijms21228548





