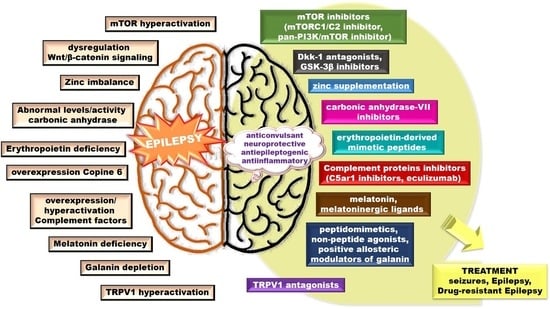
Insights into Potential Targets for Therapeutic Intervention in Epilepsy
Abstract
:1. Introduction
2. Wnt Signaling Pathway
2.1. Wnt/β-Catenin Pathway
2.2. Wnt/β-Catenin Pathway and Epilepsy
3. The Mammalian Target of Rapamycin (mTOR) Signaling Pathway
mTOR Signaling and Epilepsy
4. Zinc Signaling
Zinc, Seizures and Epilepsy
5. Carbonic Anhydrase
Carbonic Anhydrase, Seizures and Epilepsy
6. Erythropoietin
Erythropoietin and Epilepsy
7. Copines
Copine 6 and Epilepsy
8. The Complement System
8.1. Complement System Activation
8.2. Complement System and Epilepsy
9. Transient Receptor Potential Vanilloid Type 1 (TRPV1)
TRPV1 and Epilepsy
10. Galanin and Galanin Receptors
Galanin and Galanin-Receptors in Epilepsy
11. Melatonin and Melatonin-Receptors
Melatonin and Melatonin Receptors in Epilepsy
12. Other Potential Therapeutic Targets to Consider
12.1. G protein-Coupled Receptors
12.2. BDNF/TrkB Signaling
12.3. Pannexins
13. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TLE | temporal lobe epilepsy |
| AEDs | antiepileptic drugs |
| KA | kainic acid |
| SE | status epilepticus |
| PTZ | Pentylenetetrazole |
| BBB | blood–brain barrier |
| CNS | central nervous system |
References
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Prim. 2018, 4, 18024. [Google Scholar] [CrossRef]
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2009, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Engel, J. Mesial Temporal Lobe Epilepsy: What Have We Learned? Neuroscientist 2001, 7, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P. The natural history of epilepsy: An epidemiological view. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Strategies for antiepileptogenesis: Antiepileptic drugs versus novel approaches evaluated in post-status epilepticus models of temporal lobe epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Pitkänen, A.; Lukasiuk, K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011, 10, 173–186. [Google Scholar] [CrossRef]
- Nusse, R.; Brown, A.; Papkoff, J.; Scambler, P.; Shackleford, G.; McMahon, A.; Moon, R.; Varmus, H. A new nomenclature for int-1 and related genes: The Wnt gene family. Cell 1991, 64, 231. [Google Scholar] [CrossRef]
- Nusse, R.; Varmus, H. Three decades of Wnts: A personal perspective on how a scientific field developed. EMBO J. 2012, 31, 2670–2684. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [Green Version]
- Buechling, T.; Boutros, M. Wnt Signaling. Curr. Top. Dev. Biol. 2011, 97, 21–53. [Google Scholar] [CrossRef]
- Axelrod, J.D. Progress and challenges in understanding planar cell polarity signaling. Semin. Cell Dev. Biol. 2009, 20, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mlodzik, M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009, 19, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühl, M.; Sheldahl, L.C.; Park, M.; Miller, J.R.; Moon, R.T. The Wnt/Ca2+ pathway. Trends Genet. 2000, 16, 279–283. [Google Scholar] [CrossRef]
- Kohn, A.D.; Moon, R.T. Wnt and calcium signaling: β-Catenin-independent pathways. Cell Calcium 2005, 38, 439–446. [Google Scholar] [CrossRef]
- Aberle, H.; Bauer, A.; Stappert, J.; Kispert, A.; Kemler, R. β-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1997, 16, 3797–3804. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.-H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of β-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef] [Green Version]
- Stamos, J.L.; Weis, W.I. The -Catenin Destruction Complex. Cold Spring Harb. Perspect. Biol. 2012, 5, a007898. [Google Scholar] [CrossRef]
- Bilic, J.; Huang, Y.-L.; Davidson, G.; Zimmermann, T.; Cruciat, C.-M.; Bienz, M.; Niehrs, C. Wnt Induces LRP6 Signalosomes and Promotes Dishevelled-Dependent LRP6 Phosphorylation. Science 2007, 316, 1619–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikels, A.J.; Nusse, R. Wnts as ligands: Processing, secretion and reception. Oncogene 2006, 25, 7461–7468. [Google Scholar] [CrossRef] [Green Version]
- Arrázola, M.S.; Varela-Nallar, L.; Colombres, M.; Toledo, E.M.; Cruzat, F.; Pavez, L.; Assar, R.; Aravena, A.; Gonzalez, M.; Montecino, M.; et al. Calcium/calmodulin-dependent protein kinase type IV is a target gene of the Wnt/β-catenin signaling pathway. J. Cell. Physiol. 2009, 221, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Hödar, C.; Assar, R.; Colombres, M.; Aravena, A.; Pavez, L.; González, M.; Martínez, S.; Inestrosa, N.C.; Maass, A. Genome-wide identification of new Wnt/β-catenin target genes in the human genome using CART method. BMC Genom. 2010, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.C.; Colamarino, S.A.; Song, H.-J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nat. Cell Biol. 2005, 437, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.M.; Newton, S.S.; Eaton, M.E.; Russell, D.S.; Duman, R.S. Chronic electroconvulsive seizure up-regulates β-catenin expression in rat hippocampus: Role in adult neurogenesis. Biol. Psychiatry 2003, 54, 1006–1014. [Google Scholar] [CrossRef]
- Rubio, A.R.-A.C. Increase Signaling of Wnt/β-Catenin Pathway and Presence of Apoptosis in Cerebellum of Kindled Rats. CNS Neurol. Disord. Drug Targets 2017, 16, 1. [Google Scholar] [CrossRef]
- Theilhaber, J.; Rakhade, S.N.; Sudhalter, J.; Kothari, N.; Klein, P.; Pollard, J.; Jensen, F.E. Gene Expression Profiling of a Hypoxic Seizure Model of Epilepsy Suggests a Role for mTOR and Wnt Signaling in Epileptogenesis. PLoS ONE 2013, 8, e74428. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Wu, Y.; Zhao, B.; Liu, X.; Pan, Y.; Liu, Y.; Ding, Y.; Qiu, M.; Wang, Y.-Z.; et al. Wnt/β-catenin signaling mediates the seizure-facilitating effect of postischemic reactive astrocytes after pentylenetetrazole-kindling. Glia 2016, 64, 1083–1091. [Google Scholar] [CrossRef]
- Qu, Z.; Su, F.; Qi, X.; Sun, J.; Wang, H.; Qiao, Z.; Zhao, H.; Zhu, Y. Wnt/β-catenin signalling pathway mediated aberrant hippocampal neurogenesis in kainic acid-induced epilepsy. Cell Biochem. Funct. 2017, 35, 472–476. [Google Scholar] [CrossRef]
- Campos, V.E.; Du, M.; Li, Y. Increased seizure susceptibility and cortical malformation in β-catenin mutant mice. Biochem. Biophys. Res. Commun. 2004, 320, 606–614. [Google Scholar] [CrossRef]
- Gorter, J.A.; Iyer, A.M.; White, I.; Colzi, A.; Van Vliet, E.A.; Sisodiya, S.M.; Aronica, E. Hippocampal subregion-specific microRNA expression during epileptogenesis in experimental temporal lobe epilepsy. Neurobiol. Dis. 2014, 62, 508–520. [Google Scholar] [CrossRef]
- Pirone, A.; Alexander, J.; Lau, L.A.; Hampton, D.; Zayachkivsky, A.; Yee, A.; Yee, A.; Jacob, M.H.; Dulla, C.G. APC conditional knock-out mouse is a model of infantile spasms with elevated neuronal β-catenin levels, neonatal spasms, and chronic seizures. Neurobiol. Dis. 2017, 98, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paciorkowski, A.R.; Thio, L.L.; Dobyns, W.B. Genetic and Biologic Classification of Infantile Spasms. Pediatr. Neurol. 2011, 45, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Malenka, R.C. β-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 2003, 6, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Ehenríquez, J.P.; Salinas, P.C. Dual roles for Wnt signalling during the formation of the vertebrate neuromuscular junction. Acta Physiol. 2011, 204, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kimelman, D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J. Cell Sci. 2007, 120, 3337–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortress, A.M.; Frick, K.M. Hippocampal Wnt Signaling. Neuroscientist 2016, 22, 278–294. [Google Scholar] [CrossRef]
- Busceti, C.L.; Biagioni, F.; Aronica, E.; Riozzi, B.; Storto, M.; Battaglia, G.; Giorgi, F.S.; Gradini, R.; Fornai, F.; Caricasole, A.; et al. Induction of the Wnt Inhibitor, Dickkopf-1, Is Associated with Neurodegeneration Related to Temporal Lobe Epilepsy. Epilepsia 2007, 48, 694–705. [Google Scholar] [CrossRef]
- Zorn, A.M. Wnt signalling: Antagonistic Dickkopfs. Curr. Biol. 2001, 11, R592–R595. [Google Scholar] [CrossRef] [Green Version]
- Grotewold, L. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002, 21, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Grotewold, L.; Rüther, U. Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: The role of Dickkopf-1. Int. J. Dev. Biol. 2002, 46, 943–947. [Google Scholar]
- Caricasole, A.; Copani, A.; Caruso, A.; Caraci, F.; Iacovelli, L.; Sortino, M.A.; Terstappen, G.C.; Nicoletti, F. The Wnt pathway, cell-cycle activation and β-amyloid: Novel therapeutic strategies in Alzheimer’s disease? Trends Pharmacol. Sci. 2003, 24, 233–238. [Google Scholar] [CrossRef]
- Willert, K.; Nusse, R. β-catenin: A key mediator of Wnt signaling. Curr. Opin. Genet. Dev. 1998, 8, 95–102. [Google Scholar] [CrossRef]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, W.-J.; Ehrlich, A.K.; Chan, P.Y.; Teixeira, A.M.; Henegariu, O.; Hao, L.; Shin, J.H.; Park, J.-H.; Tang, W.H.; Kim, S.-T.; et al. The Wnt Antagonist Dickkopf-1 Promotes Pathological Type 2 Cell-Mediated Inflammation. Immunity 2016, 44, 246–258. [Google Scholar] [CrossRef] [Green Version]
- Scott, E.L.; Brann, D.W. Estrogen regulation of Dkk1 and Wnt/β-Catenin signaling in neurodegenerative disease. Brain Res. 2012, 1514, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Coghlan, M.P.; A Culbert, A.; Cross, D.A.; Corcoran, S.L.; Yates, J.W.; Pearce, N.J.; Rausch, O.L.; Murphy, G.J.; Carter, P.S.; Cox, L.R.; et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000, 7, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.; Mix, E.; Frahm, J.; Glass, Ä.; Müller, J.; Schmitt, O.; Schmöle, A.-C.; Klemm, K.; Ortinau, S.; Hübner, R.; et al. Small molecule GSK-3 inhibitors increase neurogenesis of human neural progenitor cells. Neurosci. Lett. 2011, 488, 36–40. [Google Scholar] [CrossRef]
- Atkinson, J.M.; Rank, K.B.; Zeng, Y.; Capen, A.; Yadav, V.; Manro, J.R.; Engler, T.A.; Chedid, M. Activating the Wnt/β-Catenin Pathway for the Treatment of Melanoma—Application of LY2090314, a Novel Selective Inhibitor of Glycogen Synthase Kinase-3. PLoS ONE 2015, 10, e0125028. [Google Scholar] [CrossRef] [Green Version]
- Morales-Garcia, J.A.; Luna-Medina, R.; Alonso-Gil, S.; Sanz-SanCristobal, M.; Palomo, V.; Gil, C.; Santos, A.; Martinez, A.; Perez-Castillo, A. Glycogen Synthase Kinase 3 Inhibition Promotes Adult Hippocampal Neurogenesis in Vitro and in Vivo. ACS Chem. Neurosci. 2012, 3, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Licht-Murava, A.; Paz, R.; Vaks, L.; Avrahami, L.; Plotkin, B.; Eisenstein, M.; Eldar-Finkelman, H. A unique type of GSK-3 inhibitor brings new opportunities to the clinic. Sci. Signal. 2016, 9, ra110. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling. Cold Spring Harb. Perspect. Biol. 2011, 4, a011593. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crino, P.B. The mTOR signalling cascade: Paving new roads to cure neurological disease. Nat. Rev. Neurol. 2016, 12, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Switon, K.; Kotulska, K.; Janusz-Kaminska, A.; Zmorzynska, J.; Jaworski, J. Molecular neurobiology of mTOR. Neuroscientist 2017, 341, 112–153. [Google Scholar] [CrossRef] [Green Version]
- Wullschleger, S.; Loewith, R.J.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.D.; Delgoffe, G.M. The Mammalian Target of Rapamycin: Linking T Cell Differentiation, Function, and Metabolism. Immunity 2010, 33, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef]
- Huang, K.; Fingar, D.C. Growing knowledge of the mTOR signaling network. Semin. Cell Dev. Biol. 2014, 36, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Baybis, M.; Yu, J.; Lee, A.; Golden, J.A.; Weiner, H.; McKhann, G.; Aronica, E.; Crino, P.B. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann. Neurol. 2004, 56, 478–487. [Google Scholar] [CrossRef]
- Holz, M.K. The role of S6K1 in ER-positive breast cancer. Cell Cycle 2012, 11, 3159–3165. [Google Scholar] [CrossRef] [Green Version]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, D.A.E.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nat. Cell Biol. 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Lozovaya, N.A.; Gataullina, S.; Tsintsadze, T.; Pallesipocachard, E.; Minlebaev, M.; Goriounova, N.A.; Buhler, E.M.; Watrin, F.; Shityakov, S.; Becker, A.J.; et al. Selective suppression of excessive GluN2C expression rescues early epilepsy in a tuberous sclerosis murine model. Nat. Commun. 2014, 5, 4563. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, B.; Kuchcinska, K.; Blazejczyk, M.; Jaworski, J.; Bartosz, T.; Kinga, K.; Magdalena, B.; Jacek, J. Pathological mTOR mutations impact cortical development. Hum. Mol. Genet. 2019, 28, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Ran, I.; Gkogkas, C.G.; Vasuta, C.; Tartas, M.; Khoutorsky, A.; Laplante, I.; Parsyan, A.; Nevarko, T.; Sonenberg, N.; Lacaille, J.-C. Selective Regulation of GluA Subunit Synthesis and AMPA Receptor-Mediated Synaptic Function and Plasticity by the Translation Repressor 4E-BP2 in Hippocampal Pyramidal Cells. J. Neurosci. 2013, 33, 1872–1886. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Zhang, M.-X.; Swank, M.W.; Kunz, J.; Wu, G.-Y. Regulation of Dendritic Morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK Signaling Pathways. J. Neurosci. 2005, 25, 11288–11299. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Wang, B.; Xiao, Z.; Gao, Y.; Zhao, Y.; Zhang, J.; Chen, B.; Wang, X.; Dai, J. Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol. Cell. Neurosci. 2008, 39, 118–124. [Google Scholar] [CrossRef]
- Fishwick, K.J.; Li, R.A.; Halley, P.; Deng, P.; Storey, K.G. Initiation of neuronal differentiation requires PI3-kinase/TOR signalling in the vertebrate neural tube. Dev. Biol. 2010, 338, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Garza-Lombó, C.; Schroder, A.; Reyes-Reyes, E.M.; Franco, R. mTOR/AMPK signaling in the brain: Cell metabolism, proteostasis and survival. Curr. Opin. Toxicol. 2018, 8, 102–110. [Google Scholar] [CrossRef]
- Pernet, V.; Schwab, M.E. Lost in the jungle: New hurdles for optic nerve axon regeneration. Trends Neurosci. 2014, 37, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Figlia, G.; Gerber, D.; Suter, U. Myelination and mTOR. Glia 2018, 66, 693–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, C.D.; Lisi, L.; Feinstein, U.L.; Navarra, P. mTOR kinase, a key player in the regulation of glial functions: Relevance for the therapy of multiple sclerosis. Glia 2012, 61, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Huang, C.-C.; Hsu, K.-S. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology 2011, 61, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Raab-Graham, K.F.; Haddick, P.C.G.; Jan, Y.N.; Jan, L.Y. Activity- and mTOR-Dependent Suppression of Kv1.1 Channel mRNA Translation in Dendrites. Science 2006, 314, 144–148. [Google Scholar] [CrossRef]
- Wang, Y.; Barbaro, M.F.; Baraban, S.C. A role for the mTOR pathway in surface expression of AMPA receptors. Neurosci. Lett. 2006, 401, 35–39. [Google Scholar] [CrossRef]
- Jaworski, J.; Sheng, M. The Growing Role of mTOR in Neuronal Development and Plasticity. Mol. Neurobiol. 2006, 34, 205–220. [Google Scholar] [CrossRef]
- Stoica, L.; Zhu, P.J.; Huang, W.; Zhou, H.; Kozma, S.C.; Costa-Mattioli, M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc. Natl. Acad. Sci. USA 2011, 108, 3791–3796. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhu, P.J.; Zhang, S.; Zhou, H.; Stoica, L.; Galiano, M.; Krnjević, K.; Roman, G.; Costa-Mattioli, M. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat. Neurosci. 2013, 16, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.R.; Urbanska, M.; Macias, M.; Skalecka, A.; Jaworski, J. Beyond control of protein translation: What we have learned about the non-canonical regulation and function of mammalian target of rapamycin (mTOR). Biochim. Biophys. Acta 2013, 1834, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.O.; Sahin, M. The Neurology of mTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleary, C.; Linde, J.; Hiscock, K.; Hadas, I.; Belmaker, R.; Agam, G.; Flaisher-Grinberg, S.; Einat, H. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res. Bull. 2008, 76, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, C.S.; Goswami, D.B.; Austin, M.C.; Iyo, A.H.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1774–1779. [Google Scholar] [CrossRef] [Green Version]
- Siuta, M.A.; Robertson, S.D.; Kocalis, H.; Saunders, C.; Gresch, P.J.; Khatri, V.; Shiota, C.; Kennedy, J.P.; Lindsley, C.W.; Daws, L.C.; et al. Dysregulation of the Norepinephrine Transporter Sustains Cortical Hypodopaminergia and Schizophrenia-Like Behaviors in Neuronal Rictor Null Mice. PLoS Biol. 2010, 8, e1000393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, K.R.; Law, A.J. Neurodevelopmental concepts of schizophrenia in the genome-wide association era: AKT/mTOR signaling as a pathological mediator of genetic and environmental programming during development. Schizophr. Res. 2020, 217, 95–104. [Google Scholar] [CrossRef]
- Steinmetz, A.B.; Stern, S.A.; Kohtz, A.S.; Descalzi, G.; Alberini, C.M. Insulin-Like Growth Factor II Targets the mTOR Pathway to Reverse Autism-Like Phenotypes in Mice. J. Neurosci. 2018, 38, 1015–1029. [Google Scholar] [CrossRef]
- Rosina, E.; Battan, B.; Siracusano, M.; Di Criscio, L.; Hollis, F.; Pacini, L.; Curatolo, P.; Bagni, C. Disruption of mTOR and MAPK pathways correlates with severity in idiopathic autism. Transl. Psychiatry 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Spencer, B.; Potkar, R.; Trejo, M.; Rockenstein, E.; Patrick, C.; Gindi, R.; Adame, A.; Wyss-Coray, T.; Masliah, E. Beclin 1 Gene Transfer Activates Autophagy and Ameliorates the Neurodegenerative Pathology in -Synuclein Models of Parkinson’s and Lewy Body Diseases. J. Neurosci. 2009, 29, 13578–13588. [Google Scholar] [CrossRef] [Green Version]
- Lan, A.-P.; Chen, J.; Zhao, Y.; Chai, Z.; Hu, Y. mTOR Signaling in Parkinson’s Disease. NeuroMol. Med. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Spilman, P.; Podlutskaya, N.; Hart, M.J.; Debnath, J.; Gorostiza, O.; Bredesen, D.; Richardson, A.; Strong, R.; Galvan, V. Inhibition of mTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-β Levels in a Mouse Model of Alzheimer’s Disease. PLoS ONE 2010, 5, e9979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citraro, R.; Leo, A.; Constanti, A.; Russo, E.; De Sarro, G. mTOR pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis. Pharmacol. Res. 2016, 107, 333–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, S.L.; Lugo, J.N. Therapeutic role of targeting mTOR signaling and neuroinflammation in epilepsy. Epilepsy Res. 2020, 161, 106282. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Chiang, A.C.Y.; Vinters, H.V. Insulin signaling pathways in cortical dysplasia and TSC-tubers: Tissue microarray analysis. Ann. Neurol. 2004, 56, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Chu-Shore, C.J.; Major, P.; Camposano, S.; Muzykewicz, D.; Thiele, E.A. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 2009, 51, 1236–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blümcke, I.; Spreafico, R.; Haaker, G.; Coras, R.; Kobow, K.; Bien, C.G.; Pfäfflin, M.; Elger, C.; Widman, G.; Schramm, J.; et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017, 377, 1648–1656. [Google Scholar] [CrossRef] [Green Version]
- Wong, M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia 2010, 51, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Sadowski, K.; Kotulska-Jóźwiak, K.; Jóźwiak, S. Role of mTOR inhibitors in epilepsy treatment. Pharmacol. Rep. 2015, 67, 636–646. [Google Scholar] [CrossRef]
- Zeng, L.-H.; Xu, L.; Gutmann, D.H.; Wong, M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. Neurol. 2008, 63, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Buckmaster, P.S.; Ingram, E.A.; Wen, X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J. Neurosci. 2009, 29, 8259–8269. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.-H.; Rensing, N.R.; Wong, M. The Mammalian Target of Rapamycin Signaling Pathway Mediates Epileptogenesis in a Model of Temporal Lobe Epilepsy. J. Neurosci. 2009, 29, 6964–6972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macias, M.; Blazejczyk, M.; Kazmierska, P.; Caban, B.; Skalecka, A.; Tarkowski, B.; Rodo, A.; Konopacki, J.; Jaworski, J. Spatiotemporal Characterization of mTOR Kinase Activity Following Kainic Acid Induced Status Epilepticus and Analysis of Rat Brain Response to Chronic Rapamycin Treatment. PLoS ONE 2013, 8, e64455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shima, A.; Nitta, N.; Suzuki, F.; Laharie, A.-M.; Nozaki, K.; Depaulis, A. Activation of mTOR signaling pathway is secondary to neuronal excitability in a mouse model of mesio-temporal lobe epilepsy. Eur. J. Neurosci. 2015, 41, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wong, M. Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia 2012, 53, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vliet, E.A.; Forte, G.; Holtman, L.; Burger, J.C.G.D.; Sinjewel, A.; De Vries, H.E.; Aronica, E.; Gorter, J.A. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia 2012, 53, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, M.Y.; Lee, N.H.; Jeon, B.T.; Roh, G.S.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S. Decreased interaction between FoxO3a and Akt correlates with seizure-induced neuronal death. Epilepsy Res. 2014, 108, 367–378. [Google Scholar] [CrossRef]
- Bhowmik, M.; Khanam, R.; Saini, N.; Vohora, D. Activation of AKT/GSK3β pathway by TDZD-8 attenuates kainic acid induced neurodegeneration but not seizures in mice. Neuro Toxicol. 2015, 46, 44–52. [Google Scholar] [CrossRef]
- Talos, D.M.; Bs, L.M.J.; Gourmaud, S.; Ba, C.A.C.; Sun, H.; Lim, K.-C.; Lucas, T.H.; Davis, K.A.; Martinez-Lage, M.; Jensen, F.E. Mechanistic target of rapamycin complex 1 and 2 in human temporal lobe epilepsy. Ann. Neurol. 2018, 83, 311–327. [Google Scholar] [CrossRef]
- Goto, E.M.; Silva, M.D.P.; Perosa, S.R.; Argañaraz, G.A.; Pesquero, J.B.; Cavalheiro, É.A.; Naffah-Mazzacoratti, M.G.; Teixeira, V.P.C.; Silva, J.A. Akt pathway activation and increased neuropeptide Y mRNA expression in the rat hippocampus: Implications for seizure blockade. Neuropeptides 2010, 44, 169–176. [Google Scholar] [CrossRef]
- Brewster, A.L.; Lugo, J.N.; Patil, V.V.; Lee, W.L.; Qian, Y.; Vanegas, F.; Anderson, A.E. Rapamycin Reverses Status Epilepticus-Induced Memory Deficits and Dendritic Damage. PLoS ONE 2013, 8, e57808. [Google Scholar] [CrossRef]
- McDaniel, S.S.; Wong, M. Therapeutic role of mammalian target of rapamycin (mTOR) inhibition in preventing epileptogenesis. Neurosci. Lett. 2011, 497, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckmaster, P.S.; Lew, F. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J. Neurosci. 2011, 31, 2337–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; McMahon, J.; Yang, J.; Shin, D.; Huang, Y. Rapamycin down-regulates KCC2 expression and increases seizure susceptibility to convulsants in immature rats. Neuroscience 2012, 219, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, A.L.; Santos, P.; Dolce, A.; Hardwick, J.M. The mTOR Inhibitor Rapamycin Has Limited Acute Anticonvulsant Effects in Mice. PLoS ONE 2012, 7, e45156. [Google Scholar] [CrossRef] [Green Version]
- Heng, K.; Haney, M.M.; Buckmaster, P.S. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia 2013, 54, 1535–1541. [Google Scholar] [CrossRef]
- Sliwa, A.; Plucinska, G.; Bednarczyk, J.; Lukasiuk, K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci. Lett. 2012, 509, 105–109. [Google Scholar] [CrossRef]
- Muncy, J.; Butler, I.J.; Koenig, M.K. Rapamycin reduces seizure frequency in tuberous sclerosis complex. J. Child Neurol. 2009, 24, 477. [Google Scholar] [CrossRef] [Green Version]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for Subependymal Giant-Cell Astrocytomas in Tuberous Sclerosis. New Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef]
- Krueger, D.A.; Wilfong, A.A.; Holland-Bouley, K.; Anderson, A.E.; Agricola, K.; Tudor, C.; Mays, M.; Lopez, C.M.; Kim, M.-O.; Franz, D.N. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann. Neurol. 2013, 74, 679–687. [Google Scholar] [CrossRef]
- Perek-Polnik, M.; Jóźwiak, S.; Jurkiewicz, E.; Perek, D.; Kotulska-Jóźwiak, K. Effective everolimus treatment of inoperable, life-threatening subependymal giant cell astrocytoma and intractable epilepsy in a patient with tuberous sclerosis complex. Eur. J. Paediatr. Neurol. 2012, 16, 83–85. [Google Scholar] [CrossRef]
- Cardamone, M.; Flanagan, D.; Mowat, D.; Kennedy, S.E.; Chopra, M.; Lawson, J.A. Mammalian Target of Rapamycin Inhibitors for Intractable Epilepsy and Subependymal Giant Cell Astrocytomas in Tuberous Sclerosis Complex. J. Pediatr. 2014, 164, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Meikle, L.; Pollizzi, K.; Egnor, A.; Kramvis, I.; A Lane, H.; Sahin, M.; Kwiatkowski, D.J. Response of a Neuronal Model of Tuberous Sclerosis to Mammalian Target of Rapamycin (mTOR) Inhibitors: Effects on mTORC1 and Akt Signaling Lead to Improved Survival and Function. J. Neurosci. 2008, 28, 5422–5432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klawitter, J.; Nashan, B.; Christians, U. Everolimus and sirolimus in transplantation-related but different. Expert Opin. Drug Saf. 2015, 14, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Hillmann, P.; Noack, A.; Römermann, K.; Öhler, L.A.; Rageot, D.; Beaufils, F.; Melone, A.; Sele, A.M.; Wymann, M.P.; et al. The novel, catalytic mTORC1/2 inhibitor PQR620 and the PI3K/mTORC1/2 inhibitor PQR530 effectively cross the blood-brain barrier and increase seizure threshold in a mouse model of chronic epilepsy. Neuropharmacology 2018, 140, 107–120. [Google Scholar] [CrossRef]
- Rageot, D.; Bohnacker, T.; Melone, A.; Langlois, J.-B.; Borsari, C.; Hillmann, P.; Sele, A.M.; Beaufils, F.; Zvelebil, M.; Hebeisen, P.; et al. Discovery and Preclinical Characterization of 5-[4,6-Bis({3-oxa-8-azabicyclo[3.2.1]octan-8-yl})-1,3,5-triazin-2-yl]-4-(difluoromethyl)pyridin-2-amine (PQR620), a Highly Potent and Selective mTORC1/2 Inhibitor for Cancer and Neurological Disorders. J. Med. Chem. 2018, 61, 10084–10105. [Google Scholar] [CrossRef]
- Rageot, D.; Bohnacker, T.; Keles, E.; McPhail, J.A.; Hoffmann, R.M.; Melone, A.; Borsari, C.; SriRamaratnam, R.; Sele, A.M.; Beaufils, F.; et al. (S)-4-(Difluoromethyl)-5-(4-(3-methylmorpholino)-6-morpholino-1,3,5-triazin-2-yl)pyridin-2-amine (PQR530), a Potent, Orally Bioavailable, and Brain-Penetrable Dual Inhibitor of Class I PI3K and mTOR Kinase. J. Med. Chem. 2019, 62, 6241–6261. [Google Scholar] [CrossRef] [Green Version]
- Gericke, B.; Brandt, C.; Theilmann, W.; Welzel, L.; Schidlitzki, A.; Twele, F.; Kaczmarek, E.; Anjum, M.; Hillmann, P.; Löscher, W. Selective inhibition of mTORC1/2 or PI3K/mTORC1/2 signaling does not prevent or modify epilepsy in the intrahippocampal kainate mouse model. Neuropharmacology 2020, 162, 107817. [Google Scholar] [CrossRef]
- Coleman, J.E. Zinc Proteins: Enzymes, Storage Proteins, Transcription Factors, and Replication Proteins. Annu. Rev. Biochem. 1992, 61, 897–946. [Google Scholar] [CrossRef]
- Takeda, A. Movement of zinc and its functional significance in the brain. Brain Res. Rev. 2000, 34, 137–148. [Google Scholar] [CrossRef]
- Klug, A. The Discovery of Zinc Fingers and Their Applications in Gene Regulation and Genome Manipulation. Annu. Rev. Biochem. 2010, 79, 213–231. [Google Scholar] [CrossRef] [Green Version]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals” . Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Tepaamorndech, S. The SLC30 family of zinc transporters – A review of current understanding of their biological and pathophysiological roles. Mol. Asp. Med. 2013, 34, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, H.J.; Cole, T.B.; Born, D.E.; Schwartzkroin, P.A.; Palmiter, R.D. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA 1997, 94, 12676–12681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef] [Green Version]
- Palmiter, R.; Findley, S. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [CrossRef]
- Palmiter, R.D. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc. Natl. Acad. Sci. USA 2004, 101, 4918–4923. [Google Scholar] [CrossRef] [Green Version]
- Sekler, I.; Moran, A.; Hershfinkel, M.; Dori, A.; Margulis, A.; Birenzweig, N.; Nitzan, Y.; Silverman, W.F. Distribution of the zinc transporter ZnT-1 in comparison with chelatable zinc in the mouse brain. J. Comp. Neurol. 2002, 447, 201–209. [Google Scholar] [CrossRef]
- Rafalo-Ulinska, A.; Piotrowska, J.; Kryczyk, A.; Opoka, W.; Sowa-Kucma, M.; Misztak, P.; Rajkowska, G.; Stockmeier, C.A.; Datka, W.; Nowak, G.; et al. Zinc transporters protein level in postmortem brain of depressed subjects and suicide victims. J. Psychiatr. Res. 2016, 83, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Rafalo, A.; Zadrozna, M.; Nowak, B.; Kotarska, K.; Wiatrowska, K.; Pochwat, B.; Sowa-Kucma, M.; Misztak, P.; Nowak, G.; Szewczyk, B. The level of the zinc homeostasis regulating proteins in the brain of rats subjected to olfactory bulbectomy model of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 72, 36–48. [Google Scholar] [CrossRef]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian Zinc Transport, Trafficking, and Signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, J.P.; Cousins, R.J. Mammalian zinc transporters. Annu. Rev. Nutr. 2004, 24, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Schweigel-Röntgen, M. The Families of Zinc (SLC30 and SLC39) and Copper (SLC31) Transporters. Co-Transp. Syst. 2014, 73, 321–355. [Google Scholar] [CrossRef]
- Krężel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Masters, B.A.; Quaife, C.J.; Erickson, J.C.; Kelly, E.J.; Froelick, G.J.; Zambrowicz, B.P.; Brinster, R.L.; Palmiter, R.D. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J. Neurosci. 1994, 14, 5844–5857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.; Yokoyama, M.; Koh, J. Zinc neurotoxicity in cortical cell culture. Neuroscience 1988, 24, 67–79. [Google Scholar] [CrossRef]
- Perry, D.K.; Smyth, M.J.; Stennicke, H.R.; Salvesen, G.S.; Duriez, P.J.; Poirier, G.G.; Hannun, Y.A. Zinc Is a Potent Inhibitor of the Apoptotic Protease, Caspase-3. J. Biol. Chem. 1997, 272, 18530–18533. [Google Scholar] [CrossRef] [Green Version]
- Côté, A.; Chiasson, M.; Peralta, M.R.; LaFortune, K.; Pellegrini, L.; Tóth, K. Cell type-specific action of seizure-induced intracellular zinc accumulation in the rat hippocampus. J. Physiol. 2005, 566, 821–837. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Hosie, A.M.; Dunne, E.L.; Harvey, R.J.; Smart, T.G. Zinc-mediated inhibition of GABAA receptors: Discrete binding sites underlie subtype specificity. Nat. Neurosci. 2003, 6, 362–369. [Google Scholar] [CrossRef]
- Paoletti, P.; Vergnano, A.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Assaf, S.Y.; Chung, S.-H. Release of endogenous Zn2+ from brain tissue during activity. Nat. Cell Biol. 1984, 308, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Noebels, J.L. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J. Physiol. 2005, 566, 747–758. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of Zinc in the Central Nervous System: The Zinc-Containing Neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef]
- Kay, A.R.; Toth, K. Is Zinc a Neuromodulator? Sci. Signal. 2008, 1, re3. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Koh, J.-Y.; Aizenman, E.; Bush, A.I.; Hershfinkel, M. The Neurophysiology and Pathology of Brain Zinc. J. Neurosci. 2011, 31, 16076–16085. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.-Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Tóth, K. Zinc in Neurotransmission. Annu. Rev. Nutr. 2011, 31, 139–153. [Google Scholar] [CrossRef]
- Marger, L.; Schubert, C.; Bertrand, D. Zinc: An underappreciated modulatory factor of brain function. Biochem. Pharmacol. 2014, 91, 426–435. [Google Scholar] [CrossRef]
- Noh, S.; Lee, S.R.; Jeong, Y.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Han, J. The direct modulatory activity of zinc toward ion channels. Integr. Med. Res. 2015, 4, 142–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besser, L.; Chorin, E.; Sekler, I.; Silverman, W.F.; Atkin, S.; Russell, J.T.; Hershfinkel, M. Synaptically Released Zinc Triggers Metabotropic Signaling via a Zinc-Sensing Receptor in the Hippocampus. J. Neurosci. 2009, 29, 2890–2901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorin, E.; Vinograd, O.; Fleidervish, I.A.; Gilad, D.; Herrmann, S.; Sekler, I.; Aizenman, E.; Hershfinkel, M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 2011, 31, 12916–12926. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, Z.; Schacht, T.; Herrmann, A.-K.; Albrecht, P.; Lefkimmiatis, K.; Methner, A. Protein kinase inhibitor β enhances the constitutive activity of G-protein-coupled zinc receptor GPR39. Biochem. J. 2014, 462, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jackson, V.R.; Nothacker, H.-P.; Civelli, O.O. GPR39 receptor expression in the mouse brain. Neuro Rep. 2006, 17, 813–816. [Google Scholar] [CrossRef]
- Hershfinkel, M. The Zinc Sensing Receptor, ZnR/GPR39, in Health and Disease. Int. J. Mol. Sci. 2018, 19, 439. [Google Scholar] [CrossRef] [Green Version]
- Peixoto-Santos, J.E.; Galvis-Alonso, O.Y.; Velasco, T.R.; Kandratavicius, L.; Assirati, J.A.; Carlotti, C.G.; Scandiuzzi, R.C.; Neder Serafini, L.; Leite, J. Increased Metallothionein I/II Expression in Patients with Temporal Lobe Epilepsy. PLoS ONE 2012, 7, e44709. [Google Scholar] [CrossRef]
- Mollah, M.A.H.; Rakshit, S.C.; Anwar, K.S.; Arslan, M.I.; Saha, N.; Ahmed, S.; Azad, K.; Hassan, T. Zinc concentration in serum and cerebrospinal fluid simultaneously decrease in children with febrile seizure: Findings from a prospective study in Bangladesh. Acta Paediatr. 2008, 97, 1707–1711. [Google Scholar] [CrossRef]
- Farahani, H.N.; Ashthiani, A.R.; Masihi, M.S. Study on serum zinc and selenium levels in epileptic patients. Neuroscience 2013, 18, 138–142. [Google Scholar]
- Seven, M.; Basaran, S.Y.; Cengiz, M.; Unal, S.; Yuksel, A. Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res. 2013, 104, 35–39. [Google Scholar] [CrossRef]
- Ni, H.; Jiang, Y.; Xiao, Z.-J.; Tao, L.-Y.; Jin, M.-F.; Wu, X.-R. Dynamic pattern of gene expression of ZnT-1, ZnT-3 and PRG-1 in rat brain following flurothyl-induced recurrent neonatal seizures. Toxicol. Lett. 2010, 194, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Feng, X.; Xiao, Z.-J.; Tao, L.-Y.; Jin, M.-F. Dynamic Pattern of Gene Expression of ZnT-4, Caspase-3, LC3, and PRG-3 in Rat Cerebral Cortex Following Flurothyl-Induced Recurrent Neonatal Seizures. Biol. Trace Element Res. 2011, 143, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Feng, X.; Gong, Y.; Tao, L.-Y.; Wu, X.-R. Acute Phase Expression Pattern of ZnTs, LC3, and Beclin-1 in Rat Hippocampus and Its Regulation by 3-Methyladenine Following Recurrent Neonatal Seizures. Biol. Trace Element Res. 2010, 143, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Ni, H.; Sun, B.-L. Neurobehavioral Deficits in a Rat Model of Recurrent Neonatal Seizures Are Prevented by a Ketogenic Diet and Correlate with Hippocampal Zinc/Lipid Transporter Signals. Biol. Trace Element Res. 2015, 167, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; A Robbins, C.; Wenzel, H.; Schwartzkroin, P.; Palmiter, R.D. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000, 39, 153–169. [Google Scholar] [CrossRef]
- McAllister, B.B.; Dyck, R.H. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 2017, 80, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Gilad, D.; Shorer, S.; Ketzef, M.; Friedman, A.; Sekler, I.; Aizenman, E.; Hershfinkel, M. Homeostatic regulation of KCC2 activity by the zinc receptor mZnR/GPR39 during seizures. Neurobiol. Dis. 2015, 81, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.P.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nat. Cell Biol. 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Zhu, L.; Lovinger, D.; Delpire, E. Cortical Neurons Lacking KCC2 Expression Show Impaired Regulation of Intracellular Chloride. J. Neurophysiol. 2005, 93, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- Woo, N.-S.; Lu, J.; England, R.; McClellan, R.; Dufour, S.; Mount, D.B.; Deutch, A.Y.; Lovinger, D.M.; Delpire, E. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus 2002, 12, 258–268. [Google Scholar] [CrossRef]
- Zhu, L.; Polley, N.; Mathews, G.C.; Delpire, E. NKCC1 and KCC2 prevent hyperexcitability in the mouse hippocampus. Epilepsy Res. 2008, 79, 201–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velíšková, J.; Claudio, O.I.; Galanopoulou, A.S.; Lado, F.A.; Ravizza, T.; Velíšek, L.; Moshé, S.L. Seizures in the Developing Brain. Epilepsia 2004, 45, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Huberfeld, G.; Wittner, L.; Clemenceau, S.; Baulac, M.; Kaila, K.; Miles, R.; Rivera, C. Perturbed Chloride Homeostasis and GABAergic Signaling in Human Temporal Lobe Epilepsy. J. Neurosci. 2007, 27, 9866–9873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puskarjov, M.; Seja, P.; E Heron, S.; Williams, T.C.; Ahmad, F.; Iona, X.; Oliver, K.L.; Grinton, B.E.; Vutskits, L.; Scheffer, I.E.; et al. A variant of KCC 2 from patients with febrile seizures impairs neuronal Cl−extrusion and dendritic spine formation. EMBO Rep. 2014, 15, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puskarjov, M.; Kahle, K.T.; Ruusuvuori, E.; Kaila, K. Pharmacotherapeutic targeting of cation-chloride cotransporters in neonatal seizures. Epilepsia 2014, 55, 806–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cristo, G.; Awad, P.N.; Hamidi, S.; Avoli, M. KCC2, epileptiform synchronization, and epileptic disorders. Prog. Neurobiol. 2018, 162, 1–16. [Google Scholar] [CrossRef]
- Kelley, M.R.; Cardarelli, R.A.; Smalley, J.L.; Ollerhead, T.A.; Andrew, P.M.; Brandon, N.J.; Deeb, T.Z.; Moss, S.J. Locally Reducing KCC2 Activity in the Hippocampus is Sufficient to Induce Temporal Lobe Epilepsy. EBioMedicine 2018, 32, 62–71. [Google Scholar] [CrossRef]
- Saadi, R.A.; He, K.; Hartnett, K.A.; Kandler, K.; Hershfinkel, M.; Aizenman, E. SNARE-dependent upregulation of potassium chloride co-transporter 2 activity after metabotropic zinc receptor activation in rat cortical neurons in vitro. Neuroscience 2012, 210, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.Z. A possible significant role of zinc and GPR39 zinc sensing receptor in Alzheimer disease and epilepsy. Biomed. Pharmacother. 2016, 79, 263–272. [Google Scholar] [CrossRef]
- Kasarskis, E.J.; Forrester, T.M.; Slevin, J.T. Amygdalar kindling is associated with elevated zinc concentration in the cortex and hippocampus of rats. Epilepsy Res. 1987, 1, 227–233. [Google Scholar] [CrossRef]
- Foresti, M.L.; Arisi, G.M.; Fernandes, A.; Tilelli, C.Q.; Garcia-Cairasco, N. Chelatable zinc modulates excitability and seizure duration in the amygdala rapid kindling model. Epilepsy Res. 2008, 79, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Elsas, S.-M.; Hazany, S.; Gregory, W.L.; Mody, I. Hippocampal zinc infusion delays the development of afterdischarges and seizures in a kindling model of epilepsy. Epilepsia 2009, 50, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Sterman, M.B.; Shouse, M.N.; Fairchild, M.; Belsito, O. Kindled seizure induction alters and is altered by zinc absorption. Brain Res. 1986, 383, 382–386. [Google Scholar] [CrossRef]
- Fukahori, M.; Itoh, M. Effects of dietary zinc status on seizure susceptibility and hippocampal zinc content in theEl (epilepsy) mouse. Brain Res. 1990, 529, 16–22. [Google Scholar] [CrossRef]
- Takeda, A.; Hirate, M.; Tamano, H.; Nisibaba, D.; Oku, N. Susceptibility to kainate-induced seizures under dietary zinc deficiency. J. Neurochem. 2003, 85, 1575–1580. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H.; Oku, N. Involvement of unusual glutamate release in kainate-induced seizures in zinc-deficient adult rats. Epilepsy Res. 2005, 66, 137–143. [Google Scholar] [CrossRef]
- Takeda, A.; Itoh, H.; Hirate, M.; Oku, N. Region-specific loss of zinc in the brain in pentylentetrazole-induced seizures and seizure susceptibility in zinc deficiency. Epilepsy Res. 2006, 70, 41–48. [Google Scholar] [CrossRef]
- Takeda, A.; Iida, M.; Ando, M.; Nakamura, M.; Tamano, H.; Oku, N. Enhanced Susceptibility to Spontaneous Seizures of Noda Epileptic Rats by Loss of Synaptic Zn2+. PLoS ONE 2013, 8, e71372. [Google Scholar] [CrossRef]
- Kumar, H.; Katyal, J.; Gupta, Y.K. Low Dose Zinc Supplementation Beneficially Affects Seizure Development in Experimental Seizure Models in Rats. Biol. Trace Element Res. 2014, 163, 208–216. [Google Scholar] [CrossRef]
- Khanna, N.; Garg, A.; Sharma, K.K.; Khosla, R. Modulation of convulsive threshold of pentylene tetrazole by zinc. Indian J. Clin. Biochem. 1997, 12, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Ji, X.-J.; Wang, H.-D.; Pan, H.; Chen, M.; Lu, T.-J. Zinc neurotoxicity to hippocampal neurons in vitro induces ubiquitin conjugation that requires p38 activation. Brain Res. 2012, 1438, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Baraka, A.M.; El Nabi, W.H.; El Ghotni, S. Investigating the Role of Zinc in a Rat Model of Epilepsy. CNS Neurosci. Ther. 2011, 18, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Boillat, A.; Huang, D.; Liang, C.; Peers, C.; Gamper, N. Intracellular zinc activates KCNQ channels by reducing their dependence on phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. USA 2017, 114, E6410–E6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alterio, V.; Pan, P.; Parkkila, S.; Buonanno, M.; Supuran, C.T.; Monti, S.M.; De Simone, G. The structural comparison between membrane-associated human carbonic anhydrases provides insights into drug design of selective inhibitors. Biopolymers 2014, 101, 769–778. [Google Scholar] [CrossRef]
- Boone, C.D.; Pinard, M.; McKenna, R.; Silverman, D. Catalytic Mechanism of α-Class Carbonic Anhydrases: CO2 Hydration and Proton Transfer. Subcell. Biochem. 2013, 75, 31–52. [Google Scholar] [CrossRef]
- Bruno, E.; Buemi, M.R.; De Luca, L.; Ferro, S.; Monforte, A.-M.; Supuran, C.T.; Vullo, D.; De Sarro, G.; Russo, E.; Gitto, R. In Vivo Evaluation of Selective Carbonic Anhydrase Inhibitors as Potential Anticonvulsant Agents. ChemMedChem 2016, 11, 1812–1818. [Google Scholar] [CrossRef]
- Lomelino, C.L.; Supuran, C.T.; McKenna, R. Non-Classical Inhibition of Carbonic Anhydrase. Int. J. Mol. Sci. 2016, 17, 1150. [Google Scholar] [CrossRef] [Green Version]
- Ghandour, M.S.; Parkkila, A.-K.; Parkkila, S.; Waheed, A.; Sly, W.S. Mitochondrial Carbonic Anhydrase in the Nervous System. J. Neurochem. 2002, 75, 2212–2220. [Google Scholar] [CrossRef]
- Tong, C.-K.; Brion, L.P.; Suárez, C.; Chesler, M. Interstitial Carbonic Anhydrase (CA) Activity in Brain Is Attributable to Membrane-Bound CA Type IV. J. Neurosci. 2000, 20, 8247–8253. [Google Scholar] [CrossRef] [Green Version]
- Ruusuvuori, E.; Li, H.; Huttu, K.; Palva, S.; Smirnov, S.Y.; Rivera, C.; Kaila, K.; Voipio, J. Carbonic Anhydrase Isoform VII Acts as a Molecular Switch in the Development of Synchronous Gamma-Frequency Firing of Hippocampal CA1 Pyramidal Cells. J. Neurosci. 2004, 24, 2699–2707. [Google Scholar] [CrossRef]
- Ruusuvuori, E.; Kaila, K. Carbonic Anhydrases and Brain pH in the Control of Neuronal Excitability. Subcell. Biochem. 2014, 75, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Kaila, K.; Ruusuvuori, E.; Seja, P.; Voipio, J.; Puskarjov, M. GABA actions and ionic plasticity in epilepsy. Curr. Opin. Neurobiol. 2014, 26, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, I.; Martin, K.F.; Thompson, K.S.; Heal, D.J. GABA-evoked depolarisations in the rat cortical wedge: Involvement of GABAA receptors and HCO3− ions. Brain Res. 1998, 798, 330–332. [Google Scholar] [CrossRef]
- Datta, R.; Waheed, A.; Bonapace, G.; Shah, G.N.; Sly, W.S. Pathogenesis of retinitis pigmentosa associated with apoptosis-inducing mutations in carbonic anhydrase IV. Proc. Natl. Acad. Sci. USA 2009, 106, 3437–3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battke, C.; Kremmer, E.; Mysliwietz, J.; Gondi, G.; Dumitru, C.; Brandau, S.; Lang, S.; Vullo, D.; Supuran, C.; Zeidler, R. Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol. Immunother. 2011, 60, 649–658. [Google Scholar] [CrossRef]
- Hen, N.; Bialer, M.; Yagen, B.; Maresca, A.; Aggarwal, M.; Robbins, A.H.; McKenna, R.; Scozzafava, A.; Supuran, C.T. Anticonvulsant 4-Aminobenzenesulfonamide Derivatives with Branched-Alkylamide Moieties: X-ray Crystallography and Inhibition Studies of Human Carbonic Anhydrase Isoforms I, II, VII, and XIV. J. Med. Chem. 2011, 54, 3977–3981. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin. Emerg. Drugs 2012, 17, 11–15. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Glaucoma and the Applications of Carbonic Anhydrase Inhibitors. Subcell. Biochem. 2013, 75, 349–359. [Google Scholar] [CrossRef]
- Çavuş, I.; Romanyshyn, J.C.; Kennard, J.T.; Farooque, P.; Williamson, A.; Eid, T.; Spencer, S.S.; Duckrow, R.; Dziura, J.; Spencer, D.D. Elevated basal glutamate and unchanged glutamine and GABA in refractory epilepsy: Microdialysis study of 79 patients at the yale epilepsy surgery program. Ann. Neurol. 2016, 80, 35–45. [Google Scholar] [CrossRef]
- Luna-Munguia, H.; Zestos, A.G.; Gliske, S.V.; Kennedy, R.T.; Stacey, W. Chemical biomarkers of epileptogenesis and ictogenesis in experimental epilepsy. Neurobiol. Dis. 2019, 121, 177–186. [Google Scholar] [CrossRef]
- Xiong, Z.-Q.; Stringer, J.L. Regulation of extracellular pH in the developing hippocampus. Dev. Brain Res. 2000, 122, 113–117. [Google Scholar] [CrossRef]
- Koch, A.; Woodbury, D.M. Effects of carbonic anhydrase inhibition of brain excitability. J. Pharmacol. Exp. Ther 1958, 122, 335–342. [Google Scholar] [PubMed]
- Szaflarski, J. University of Alabama at Birmingham and the UAB Epilepsy Center th Avenue South CIRC Birmingham AL USA Cannabis For The Treatment Of Epilepsy. Planta Medica 2016, 82, 1284–1289. [Google Scholar] [CrossRef]
- Rauh, C.E.; Gray, W.D. The anticonvulsant potency of inhibitors of carbonic anhydrase in young and adult rats and mice. J. Pharmacol. Exp. Ther. 1968, 161, 329–334. [Google Scholar]
- Shank, R.P.; Gardocki, J.F.; Vaught, J.L.; Davis, C.B.; Schupsky, J.J.; Raffa, R.B.; Dodgson, S.J.; Nortey, S.O.; Maryanoff, B.E. Topiramate: Preclinical Evaluation of a Structurally Novel Anticonvulsant. Epilepsia 1994, 35, 450–460. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Henriksen, O.; Dam, M.; Mikkelsen, M.; Schmidt, D.; Reid, S.; Reife, R.; Kramer, L.; Pledger, G.; Karim, R. Double-Blind, Placebo-Controlled Trial of Topiramate as Add-on Therapy in Patients with Refractory Partial Seizures. Epilepsia 1996, 37, 539–543. [Google Scholar] [CrossRef]
- Wilensky, A.J.; Friel, P.N.; Ojemann, L.M.; Dodrill, C.B.; McCormick, K.B.; Levy, R.H. Zonisamide in Epilepsy: A Pilot Study. Epilepsia 1985, 26, 212–220. [Google Scholar] [CrossRef]
- Nakamura, J.; Tamura, S.; Kanda, T.; Ishii, A.; Ishihara, K.; Serikawa, T.; Yamada, J.; Sasa, M. Inhibition by topiramate of seizures in spontaneously epileptic rats and DBA/2 mice. Eur. J. Pharmacol. 1994, 254, 83–89. [Google Scholar] [CrossRef]
- Masereel, B.; Rolin, S.; Abbate, F.; Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors: Anticonvulsant Sulfonamides Incorporating Valproyl and Other Lipophilic Moieties. J. Med. Chem. 2002, 45, 312–320. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A.; Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 2003, 23, 146–189. [Google Scholar] [CrossRef]
- Aggarwal, M.; Kondeti, B.; McKenna, R. Anticonvulsant/antiepileptic carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Halmi, P.; Parkkila, S.; Honkaniemi, J. Expression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochem. Int. 2006, 48, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Bootorabi, F.; Jänis, J.; Smith, E.; Waheed, A.; Kukkurainen, S.; Hytönen, V.P.; Valjakka, J.; Supuran, C.T.; Vullo, D.; Sly, W.S.; et al. Analysis of a shortened form of human carbonic anhydrase VII expressed in vitro compared to the full-length enzyme. Biochimie 2010, 92, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Ruusuvuori, E.; Huebner, A.K.; Kirilkin, I.; Yukin, A.Y.; Blaesse, P.; Helmy, M.; Kang, H.J.; El Muayed, M.; Hennings, J.C.; Voipio, J.; et al. Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J. 2013, 32, 2275–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavernet, L.; Funes, J.L.G.; Palestro, P.H.; Blanch, L.E.B.; Estiu, G.L.; Maresca, A.; Barrios, I.; Supuran, C.T. Inhibition pattern of sulfamide-related compounds in binding to carbonic anhydrase isoforms I, II, VII, XII and XIV. Bioorganic Med. Chem. 2013, 21, 1410–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villalba, M.L.; Palestro, P.; Ceruso, M.; Funes, J.L.G.; Talevi, A.; Blanch, L.B.; Supuran, C.T.; Gavernet, L. Sulfamide derivatives with selective carbonic anhydrase VII inhibitory action. Bioorganic Med. Chem. 2016, 24, 894–901. [Google Scholar] [CrossRef]
- De Luca, L.; Ferro, S.; Damiano, F.M.; Supuran, C.T.; Vullo, D.; Chimirri, A.; Gitto, R. Structure-based screening for the discovery of new carbonic anhydrase VII inhibitors. Eur. J. Med. Chem. 2014, 71, 105–111. [Google Scholar] [CrossRef]
- Jelkmann, W. Molecular Biology of Erythropoietin. Intern. Med. 2004, 43, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, A.M. Erythropoiesis stimulating agents: Approaches to modulate activity. Biol. Targets Ther. 2013, 7, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Chikuma, M.; Masuda, S.; Kobayashi, T.; Nagao, M.; Sasaki, R. Tissue-specific regulation of erythropoietin production in the murine kidney, brain, and uterus. Am. J. Physiol. Metab. 2000, 279, E1242–E1248. [Google Scholar] [CrossRef]
- Bunn, H.F. Erythropoietin. Cold Spring Harb. Perspect. Med. 2013, 3, a011619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depping, R.; Kawakami, K.; Ocker, H.; Wagner, J.M.; Heringlake, M.; Noetzold, A.; Sievers, H.-H.; Wagner, K.F. Expression of the erythropoietin receptor in human heart. J. Thorac. Cardiovasc. Surg. 2005, 130, 877–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, Y.; Masuda, S.; Chikuma, M.; Inoue, K.; Nagao, M.; Sasaki, R. Estrogen-dependent Production of Erythropoietin in Uterus and Its Implication in Uterine Angiogenesis. J. Biol. Chem. 1998, 273, 25381–25387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juul, S.E.; Yachnis, A.T.; Rojiani, A.M.; Christensen, R.D. Immunohistochemical Localization of Erythropoietin and Its Receptor in the Developing Human Brain. Pediatr. Dev. Pathol. 1999, 2, 148–158. [Google Scholar] [CrossRef]
- Lappin, T.R. The Cellular Biology of Erythropoietin Receptors. Oncologist 2003, 8, 15–18. [Google Scholar] [CrossRef]
- Morishita, E.; Masuda, S.; Nagao, M.; Yasuda, Y.; Sasaki, R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 1996, 76, 105–116. [Google Scholar] [CrossRef]
- Bernaudin, M.; Marti, H.H.; Roussel, S.; Divoux, D.; Nouvelot, A.; MacKenzie, E.T.; Petit, E. A Potential Role for Erythropoietin in Focal Permanent Cerebral Ischemia in Mice. Br. J. Pharmacol. 1999, 19, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.; Yu, X.; Beleslin-Cokic, B.; Liu, C.; Shen, K.; Mohrenweiser, H.W.; Noguchi, C.T. Production and processing of erythropoietin receptor transcripts in brain. Mol. Brain Res. 2000, 81, 29–42. [Google Scholar] [CrossRef]
- Noguchi, C.T.; Asavaritikrai, P.; Teng, R.; Jia, Y. Role of erythropoietin in the brain. Crit. Rev. Oncol. 2007, 64, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Rabie, T.; Marti, H.H. Brain Protection by Erythropoietin: A Manifold Task. Physiology 2008, 23, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, P.E.; Fares, R.P.; Risso, J.-J.; Bonnet, C.; Bouvard, S.; Le-Cavorsin, M.; Georges, B.; Moulin, C.; Belmeguenai, A.; Bodennec, J.; et al. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc. Natl. Acad. Sci. USA 2009, 106, 9848–9853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, Y.; Chui, D.-H.; Hirose, H.; Kunishita, T.; Tabira, T. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 1993, 609, 29–35. [Google Scholar] [CrossRef]
- Sakanaka, M.; Wen, T.-C.; Matsuda, S.; Morishita, E.; Nagao, M.; Sasaki, R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. USA 1998, 95, 4635–4640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brines, M.; Ghezzi, P.; Keenan, S.; Agnello, D.; De Lanerolle, N.C.; Cerami, C.; Itri, L.M.; Cerami, A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. USA 2000, 97, 10526–10531. [Google Scholar] [CrossRef] [Green Version]
- Sirén, A.-L.; Fratelli, M.; Brines, M.; Goemans, C.; Casagrande, S.; Lewczuk, P.; Keenan, S.; Gleiter, C.; Pasquali, C.; Capobianco, A.; et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. USA 2001, 98, 4044–4049. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.-C.; Sadamoto, Y.; Tanaka, J.; Zhu, P.-X.; Nakata, K.; Ma, Y.-J.; Hata, R.; Sakanaka, M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J. Neurosci. Res. 2002, 67, 795–803. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Kang, J.-Q.; Maiese, K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br. J. Pharmacol. 2003, 138, 1107–1118. [Google Scholar] [CrossRef] [Green Version]
- Mikati, M.A.; El Hokayem, J.A.; El Sabban, M.E. Effects of a Single Dose of Erythropoietin on Subsequent Seizure Susceptibility in Rats Exposed to Acute Hypoxia at P10. Epilepsia 2007, 48, 175–181. [Google Scholar] [CrossRef]
- Chu, K.; Jung, K.-H.; Lee, S.-T.; Kim, J.; Kang, K.-M.; Kim, H.-K.; Park, Y.H.; Park, H.-K.; Kim, M.; Lee, S.K.; et al. Erythropoietin reduces epileptogenic processes following status epilepticus. Epilepsia 2008, 49, 1723–1732. [Google Scholar] [CrossRef]
- Carelli, S.; Giallongo, T.; Viaggi, C.; Latorre, E.; Gombalova, Z.; Raspa, A.; Mazza, M.; Vaglini, F.; Di Giulio, A.M.; Gorio, A. Recovery from experimental parkinsonism by intrastriatal application of erythropoietin or EPO-releasing neural precursors. Neuropharmacol. 2017, 119, 76–90. [Google Scholar] [CrossRef]
- Tamura, T.; Aoyama, M.; Ukai, S.; Kakita, H.; Sobue, K.; Asai, K. Neuroprotective erythropoietin attenuates microglial activation, including morphological changes, phagocytosis, and cytokine production. Brain Res. 2017, 1662, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.-K.; Tsai, T.-H.; Lin, H.-S.; Cheng-Hsien, L.; Sun, C.-K.; Leu, S.; Yuen, C.-M.; Tan, T.-Y.; Lan, M.-Y.; Liou, C.-W.; et al. Effect of erythropoietin on level of circulating endothelial progenitor cells and outcome in patients after acute ischemic stroke. Crit. Care 2011, 15, R40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wustenberg, T.; Begemann, M.; Bartels, C.; Gefeller, O.; Stawicki, S.; Hinze-Selch, D.; Mohr, A.; Falkai, P.; Aldenhoff, J.B.; Knauth, M.; et al. Recombinant human erythropoietin delays loss of gray matter in chronic schizophrenia. Mol. Psychiatry 2010, 16, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, W.; Park, J.; Shin, K.J.; Kim, J.-S.; Kim, J.S.; Youn, J.; Cho, J.W.; Oh, E.; Ahn, J.Y.; Oh, K.-W.; et al. Safety and efficacy of recombinant human erythropoietin treatment of non-motor symptoms in Parkinson’s disease. J. Neurol. Sci. 2014, 337, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Shingo, T.; Yasuhara, T.; Tajiri, N.; Kondo, A.; Morimoto, T.; Yuan, W.J.; Wang, F.; Baba, T.; Tokunaga, K.; et al. Continuous intraventricular infusion of erythropoietin exerts neuroprotective/rescue effects upon Parkinson’s disease model of rats with enhanced neurogenesis. Brain Res. 2009, 1254, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzón, F.; Coimbra, D.; Parcerisas, A.; Rodriguez, Y.; García, J.C.; Soriano, E.; Rama, R. NeuroEPO Preserves Neurons from Glutamate-Induced Excitotoxicity. J. Alzheimer’s Dis. 2018, 65, 1469–1483. [Google Scholar] [CrossRef]
- Im, J.H.; Yeo, I.J.; Hwang, C.J.; Lee, K.S.; Hong, J.T. PEGylated Erythropoietin Protects against Brain Injury in the MCAO-Induced Stroke Model by Blocking NF-κB Activation. Biomol. Ther. 2020, 28, 152–162. [Google Scholar] [CrossRef]
- Renzi, M.J.; Farrell, F.X.; Bittner, A.; Galindo, J.E.; Morton, M.; Trinh, H.; Jolliffe, L.K. Erythropoietin induces changes in gene expression in PC-12 cells. Mol. Brain Res. 2002, 104, 86–95. [Google Scholar] [CrossRef]
- Chu, H.; Ding, H.; Tang, Y.; Dong, Q. Erythropoietin protects against hemorrhagic blood–brain barrier disruption through the effects of aquaporin-4. Lab. Investig. 2014, 94, 1042–1053. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Luo, C.; Yu, S.P.; Gao, J.; Liu, C.; Wei, Z.Z.; Zhang, Z.; Wei, L.; Yi, B. Erythropoietin ameliorates early brain injury after subarachnoid haemorrhage by modulating microglia polarization via the EPOR/JAK2-STAT3 pathway. Exp. Cell Res. 2017, 361, 342–352. [Google Scholar] [CrossRef]
- Koshimura, K.; Murakami, Y.; Sohmiya, M.; Tanaka, J.; Kato, Y. Effects of erythropoietin on neuronal activity. J. Neurochem. 1999, 72, 2565–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, S.; Nagao, M.; Takahata, K.; Konishi, Y.; Gallyas, F.; Tabira, T.; Sasaki, R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 1993, 268, 11208–11216. [Google Scholar] [PubMed]
- Kondo, A.; Shingo, T.; Yasuhara, T.; Kuramoto, S.; Kameda, M.; Kikuchi, Y.; Matsui, T.; Miyoshi, Y.; Agari, T.; Borlongan, C.V.; et al. Erythropoietin exerts anti-epileptic effects with the suppression of aberrant new cell formation in the dentate gyrus and upregulation of neuropeptide Y in seizure model of rats. Brain Res. 2009, 1296, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Üzüm, G.; Diler, A.S.; Bahçekapılı, N.; Ziylan, Y.Z. Erythropoietin prevents the increase in blood–brain barrier permeability during pentylentetrazol induced seizures. Life Sci. 2006, 78, 2571–2576. [Google Scholar] [CrossRef]
- Bahçekapılı, N.; Akgun-Dar, K.; Albeniz, I.; Kapucu, A.; Kandil, A.; Yağız, O.; Üzüm, G.; Bahcekapili, N.; Kandil, A.; Yagiz, O. Erythropoietin pretreatment suppresses seizures and prevents the increase in inflammatory mediators during pentylenetetrazole-induced generalized seizures. Int. J. Neurosci. 2014, 124, 762–770. [Google Scholar] [CrossRef]
- Nadam, J.; Navarro, F.; Sánchez, P.; Moulin, C.; Georges, B.; Laglaine, A.; Pequignot, J.-M.; Morales, A.; Ryvlin, P.; Bezin, L. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol. Dis. 2007, 25, 412–426. [Google Scholar] [CrossRef] [Green Version]
- Sözmen, Ş.Ç.; Kurul, S.H.; Yiş, U.; Tuğyan, K.; Baykara, B.; Yılmaz, O. Neuroprotective effects of recombinant human erythropoietin in the developing brain of rat after lithium-pilocarpine induced status epilepticus. Brain Dev. 2012, 34, 189–195. [Google Scholar] [CrossRef]
- Seeger, N.; Zellinger, C.; Rode, A.; Roloff, F.; Bicker, G.; Russmann, V.; Fischborn, S.; Wendt, H.; Potschka, H. The erythropoietin-derived peptide mimetic pHBSP affects cellular and cognitive consequences in a rat post-status epilepticus model. Epilepsia 2011, 52, 2333–2343. [Google Scholar] [CrossRef]
- Zellinger, C.; Seeger, N.; Hadamitzky, M.; Fischborn, S.; Russmann, V.; Wendt, H.; Pankratova, S.; Bock, E.; Berezin, V.; Potschka, H. Impact of the erythropoietin-derived peptide mimetic Epotris on the histopathological consequences of status epilepticus. Epilepsy Res. 2011, 96, 241–249. [Google Scholar] [CrossRef]
- Ott, C.; Martens, H.; Hassouna, I.; Oliveira, B.; Erck, C.; Zafeiriou, M.-P.; Peteri, U.-K.; Hesse, D.; Gerhart, S.; Altas, B.; et al. Widespread Expression of Erythropoietin Receptor in Brain and Its Induction by Injury. Mol. Med. 2015, 21, 803–815. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Yu, X.; Sun, H.; Li, Y.; Deng, Y. Erythropoietin preconditioning suppresses neuronal death following status epilepticus in rats. Acta Neurobiol. Exp. 2007, 67, 141–148. [Google Scholar]
- Kilic, E.; Kilic, Ü.; Soliz, J.; Bassetti, C.L.; Gassmann, M.; Hermann, D.M. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J. 2005, 19, 2026–2028. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-Z.; Gu, X.-H.; Cheng, S.-F.; Li, L.; Liu, H.; Hu, L.-P.; Gao, F. The oncogenetic role of stanniocalcin 1 in lung adenocarcinoma: A promising serum candidate biomarker for tracking lung adenocarcinoma progression. Tumor Biol. 2015, 37, 5633–5644. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Prolonged Epileptic Seizures in Primates. Arch. Neurol. 1973, 28, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Moseley, B.D.; Nickels, K.; Britton, J.; Wirrell, E. How common is ictal hypoxemia and bradycardia in children with partial complex and generalized convulsive seizures? Epilepsia 2010, 51, 1219–1224. [Google Scholar] [CrossRef]
- Luna-Munguia, H.; Orozco-Suã¡rez, S.; Rocha, L. Effects of high frequency electrical stimulation and R-verapamil on seizure susceptibility and glutamate and GABA release in a model of phenytoin-resistant seizures. Neuropharmacology 2011, 61, 807–814. [Google Scholar] [CrossRef]
- Socodato, R.; Portugal, C.C.; Rodrigues, A.; Henriques, J.; Rodrigues, C.; Figueira, C.; Relvas, J.B. Redox tuning of Ca 2+ signaling in microglia drives glutamate release during hypoxia. Free. Radic. Biol. Med. 2018, 118, 137–149. [Google Scholar] [CrossRef]
- Erecińska, M.; Silver, I.A. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001, 128, 263–276. [Google Scholar] [CrossRef]
- Gorter, J.A.; Pereira, P.M.G.; Van Vliet, E.A.; Aronica, E.; Da Silva, F.H.L.; Lucassen, P.J. Neuronal Cell Death in a Rat Model for Mesial Temporal Lobe Epilepsy Is Induced by the Initial Status Epilepticus and Not by Later Repeated Spontaneous Seizures. Epilepsia 2003, 44, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Weir, E.K.; Olschewski, A. Role of ion channels in acute and chronic responses of the pulmonary vasculature to hypoxia. Cardiovasc. Res. 2006, 71, 630–641. [Google Scholar] [CrossRef] [Green Version]
- Auzmendi, J.; Akyuz, E.; Lazarowski, A. The role of P-glycoprotein (P-gp) and inwardly rectifying potassium (Kir) channels in sudden unexpected death in epilepsy (SUDEP). Epilepsy Behav. 2019, 106590. [Google Scholar] [CrossRef]
- Callaghan, R.; Crowley, E.; Biochem, M.; Potter, S.; Kerr, I.D. P-glycoprotein: So Many Ways to Turn It On. J. Clin. Pharmacol. 2008, 48, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Avemary, J.; Salvamoser, J.D.; Peraud, A.; Rémi, J.; Noachtar, S.; Fricker, G.; Potschka, H. Dynamic Regulation of P-glycoprotein in Human Brain Capillaries. Mol. Pharm. 2013, 10, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Free Radicals, Calcium., and The Synaptic Plasticity-Cell Death Continuum: Emerging Roles of The Transcription Factor Nfκb. Int. Rev. Neurobiol. 1998, 42, 103–168. [Google Scholar] [CrossRef]
- Auzmendi, J.; Orozco-Suã¡rez, S.; Bañuelos-Cabrera, I.; González-Trujano, M.E.; González, E.C.; Rocha, L.; Lazarowski, A. P-Glycoprotein Contributes to Cell Membrane Depolarization of Hippocampus and Neocortex in a Model of Repetitive Seizures Induced by Pentylenetetrazole in Rats. Curr. Pharm. Des. 2013, 19, 6732–6738. [Google Scholar] [CrossRef]
- Marti, H.H. Erythropoietin and the hypoxic brain. J. Exp. Biol. 2004, 207, 3233–3242. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, J.; Shah, S.; McCloskey, D.P.; Goodman, J.; Elkady, A.; Atassi, H.; Hylton, D.; Rudge, J.; Scharfman, H.; Croll, S.D. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience 2008, 151, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Feast, A.; Martinian, L.; Liu, J.; Catarino, C.B.; Thom, M.; Sisodiya, S.M. Investigation of hypoxia-inducible factor-1α in hippocampal sclerosis: A postmortem study. Epilepsia 2012, 53, 1349–1359. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Merelli, A.; Czornyj, L.; Lazarowski, A. Erythropoietin: A Neuroprotective Agent in Cerebral Hypoxia, Neurodegeneration, and Epilepsy. Curr. Pharm. Des. 2013, 19, 6791–6801. [Google Scholar] [CrossRef]
- McPherson, R.J.; Juul, S.E. Recent trends in erythropoietin-mediated neuroprotection. Int. J. Dev. Neurosci. 2007, 26, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomsig, J.L.; Creutz, C.E. Copines: A ubiquitous family of Ca 2+ -dependent phospholipid-binding proteins. Cell. Mol. Life Sci. 2002, 59, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Nalefski, E.A.; Falke, J.J. The C2 domain calcium-binding motif: Structural and functional diversity. Protein Sci. 1996, 5, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creutz, C.E.; Tomsig, J.L.; Snyder, S.L.; Gautier, M.-C.; Skouri, F.; Beisson, J.; Cohen, J. The Copines, a Novel Class of C2 Domain-containing, Calciumdependent, Phospholipid-binding Proteins Conserved fromParameciumto Humans. J. Biol. Chem. 1998, 273, 1393–1402. [Google Scholar] [CrossRef] [Green Version]
- Tomsig, J.L. Identification of Targets for Calcium Signaling through the Copine Family of Proteins. Characterization of a coiled-coil copine-binding motif. J. Biol. Chem. 2003, 278, 10048–10054. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Yaoi, T.; Yasui, M.; Kuwajima, G. N-copine: A novel two C2-domain-containing protein with neuronal activity-regulated expression. FEBS Lett. 1998, 428, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Savino, M.; D’Apolito, M.; Centra, M.; Van Beerendonk, H.M.; Cleton-Jansen, A.-M.; Whitmore, S.A.; Crawford, J.; Callen, D.F.; Zelante, L.; Savoia, A. Characterization of Copine VII, a New Member of the Copine Family, and Its Exclusion as a Candidate in Sporadic Breast Cancers with Loss of Heterozygosity at 16q24.3. Genomics 1999, 61, 219–226. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Creutz, C.E. Biochemical Characterization of Copine: A Ubiquitous Ca2+-Dependent, Phospholipid-Binding Protein. Biochemistry 2000, 39, 16163–16175. [Google Scholar] [CrossRef]
- Cowland, J.B.; Carter, D.; Bjerregaard, M.D.; Johnsen, A.H.; Borregaard, N.; Lollike, K. Tissue expression of copines and isolation of copines I and III from the cytosol of human neutrophils. J. Leukoc. Biol. 2003, 74, 379–388. [Google Scholar] [CrossRef]
- Maitra, R.; Grigoryev, D.; Bera, T.K.; Pastan, I.; Lee, B. Cloning, molecular characterization, and expression analysis of Copine 8. Biochem. Biophys. Res. Commun. 2003, 303, 842–847. [Google Scholar] [CrossRef] [Green Version]
- Perestenko, P.V.; Pooler, A.M.; Noorbakhshnia, M.; Gray, A.; Bauccio, C.; McIlhinney, R.A.J. Copines-1, -2, -3, -6 and -7 show different calcium-dependent intracellular membrane translocation and targeting. FEBS J. 2010, 277, 5174–5189. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, H.; Zhu, J.; Ding, H.; Zeng, Y.; Du, W.; Ding, Z.; Song, P.; Zhang, Y.; Liu, Z.; et al. High expression of Copine 1 promotes cell growth and metastasis in human lung adenocarcinoma. Int. J. Oncol. 2018, 53, 2369–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbalan-Garcia, S.; Gomez-Fernández, J.C. Signaling through C2 domains: More than one lipid target. Biochim. Biophys. Acta 2014, 1838, 1536–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, M.; Li, T.; Badea, T.C. Differential expression and subcellular localization of Copines in mouse retina. J. Comp. Neurol. 2019, 527, 2245–2262. [Google Scholar] [CrossRef]
- Reinhard, J.R.; Kriz, A.; Galic, M.; Angliker, N.; Rajalu, M.; Vogt, K.E.; Rüegg, M.A. The calcium sensor Copine-6 regulates spine structural plasticity and learning and memory. Nat. Commun. 2016, 7, 11613. [Google Scholar] [CrossRef] [Green Version]
- Burk, K.; Ramachandran, B.; Ahmed, S.; Hurtado-Zavala, J.I.; Awasthi, A.; Benito, E.; Faram, R.; Ahmad, H.; Swaminathan, A.; McIlhinney, J.; et al. Regulation of Dendritic Spine Morphology in Hippocampal Neurons by Copine-6. Cereb. Cortex 2017, 28, 1087–1104. [Google Scholar] [CrossRef]
- Kaufmann, W.E. Dendritic Anomalies in Disorders Associated with Mental Retardation. Cereb. Cortex 2000, 10, 981–991. [Google Scholar] [CrossRef]
- Pfeiffer, B.E.; Huber, K.M. The State of Synapses in Fragile X Syndrome. Neuroscience 2009, 15, 549–567. [Google Scholar] [CrossRef]
- Nishiyama, J. Plasticity of dendritic spines: Molecular function and dysfunction in neurodevelopmental disorders. Psychiatry Clin. Neurosci. 2019, 73, 541–550. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease Is a Synaptic Failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [Green Version]
- Wilke, S.A.; Raam, T.; Antonios, J.K.; Bushong, E.A.; Koo, E.H.; Ellisman, M.H.; Ghosh, A. Specific Disruption of Hippocampal Mossy Fiber Synapses in a Mouse Model of Familial Alzheimer’s Disease. PLoS ONE 2014, 9, e84349. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Parnetti, L.; Di Filippo, M. A convergent model for cognitive dysfunctions in Parkinson’s disease: The critical dopamine–acetylcholine synaptic balance. Lancet Neurol. 2006, 5, 974–983. [Google Scholar] [CrossRef]
- Murphy, B.L.; Hofacer, R.D.; Faulkner, C.N.; Loepke, A.W.; Danzer, S.C. Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis. Epilepsia 2012, 53, 908–921. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; He, X.; McNamara, J.O.; Danzer, S.C. Morphological changes among hippocampal dentate granule cells exposed to early kindling-epileptogenesis. Hippocampus 2013, 23, 1309–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Phillips, J.; Delanty, N.; O’Connor, W. Elevated extracellular levels of glutamate, aspartate and gamma-aminobutyric acid within the intraoperative, spontaneously epileptiform human hippocampus. Epilepsy Res. 2003, 54, 73–79. [Google Scholar] [CrossRef]
- Thomas, P.M.; Phillips, J.P.; O’Connor, W.T. Hippocampal microdialysis during spontaneous intraoperative epileptiform activity. Acta Neurochir. 2004, 146, 143–151. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Cell death and synaptic reorganizations produced by seizures. Epilepsia 2001, 42, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutula, T.; Dudek, F.E. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: An emergent property of a complex system. Prog. Brain Res. 2007, 163, 541–563. [Google Scholar] [CrossRef]
- Nakayama, T.; Yaoi, T.; Kuwajima, G. Localization and Subcellular Distribution ofN-Copine in Mouse Brain. J. Neurochem. 1999, 72, 373–379. [Google Scholar] [CrossRef]
- Zhu, B.; Zha, J.; Long, Y.; Hu, X.; Chen, G.; Wang, X. Increased expression of copine VI in patients with refractory epilepsy and a rat model. J. Neurol. Sci. 2016, 360, 30–36. [Google Scholar] [CrossRef]
- Lai, Y.; Hu, X.; Chen, G.-J.; Wang, X.; Zhu, B.-L. Down-regulation of adenylate kinase 5 in temporal lobe epilepsy patients and rat model. J. Neurol. Sci. 2016, 366, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Barnum, S.R. Complement: A primer for the coming therapeutic revolution. Pharmacol. Ther. 2017, 172, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolev, M.; Le Friec, G.; Kemper, C. Complement — tapping into new sites and effector systems. Nat. Rev. Immunol. 2014, 14, 811–820. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef] [Green Version]
- Nesargikar, P.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I – Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.P. Regulation of the complement membrane attack pathway. Crit. Rev. Immunol. 1999, 19, 26. [Google Scholar] [CrossRef]
- Bubeck, D. The Making of a Macromolecular Machine: Assembly of the Membrane Attack Complex. Biochemistry 2014, 53, 1908–1915. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Younkin, E.M.; Sarma, J.V.; Riedemann, N.; McGuire, S.R.; Lu, K.T.; Kunkel, R.; Younger, J.G.; Zetoune, F.S.; Ward, P.A. Generation of C5a by Phagocytic Cells. Am. J. Pathol. 2002, 161, 1849–1859. [Google Scholar] [CrossRef] [Green Version]
- Klos, A.; Tenner, A.J.; Johswich, K.-O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, T.M.; Ager, R.R.; Tenner, A.J.; Noakes, P.G.; Taylor, S.M. The Role of the Complement System and the Activation Fragment C5a in the Central Nervous System. Neuro Mol. Med. 2009, 12, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Gerard, N.P.; Gerard, C. The chemotactic receptor for human C5a anaphylatoxin. Nat. Cell Biol. 1991, 349, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.J.; Chen, J.; Zien, A.; Sochivko, D.; Normann, S.; Schramm, J.; Elger, C.E.; Wiestler, O.D.; Blümcke, I. Correlated stage- and subfield-associated hippocampal gene expression patterns in experimental and human temporal lobe epilepsy. Eur. J. Neurosci. 2003, 18, 2792–2802. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Bartolomei, F.; Robaglia-Schlupp, A.; Massacrier, A.; Peragut, J.-C.; Régis, J.; Dufour, H.; Ravid, R.; Roll, P.; Pereira, S.; et al. Large-scale expression study of human mesial temporal lobe epilepsy: Evidence for dysregulation of the neurotransmission and complement systems in the entorhinal cortex. Brain 2006, 129, 625–641. [Google Scholar] [CrossRef] [Green Version]
- Jamali, S.; Salzmann, A.; Perroud, N.; Ponsole-Lenfant, M.; Cillario, J.; Roll, P.; Roeckel-Trevisiol, N.; Crespel, A.; Balzar, J.; Schlachter, K.; et al. Functional Variant in Complement C3 Gene Promoter and Genetic Susceptibility to Temporal Lobe Epilepsy and Febrile Seizures. PLoS ONE 2010, 5, e12740. [Google Scholar] [CrossRef] [Green Version]
- Aronica, E.; Boer, K.; Van Vliet, E.A.; Redeker, S.; Baayen, J.C.; Spliet, W.G.; Van Rijen, P.C.; Troost, D.; Da Silva, F.L.; Wadman, W.J.; et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol. Dis. 2007, 26, 497–511. [Google Scholar] [CrossRef]
- Gorter, J.A.; Van Vliet, E.A.; Aronica, E.; Breit, T.; Rauwerda, H.; Da Silva, F.H.L.; Wadman, W.J. Potential New Antiepileptogenic Targets Indicated by Microarray Analysis in a Rat Model for Temporal Lobe Epilepsy. J. Neurosci. 2006, 26, 11083–11110. [Google Scholar] [CrossRef]
- Kharatishvili, I.; Shan, Z.Y.; She, D.; Foong, S.; Kurniawan, N.D.; Reutens, D.C. MRI changes and complement activation correlate with epileptogenicity in a mouse model of temporal lobe epilepsy. Brain Struct. Funct. 2013, 219, 683–706. [Google Scholar] [CrossRef]
- Xiong, Z.-Q.; Qian, W.; Suzuki, K.; McNamara, J.O. Formation of Complement Membrane Attack Complex in Mammalian Cerebral Cortex Evokes Seizures and Neurodegeneration. J. Neurosci. 2003, 23, 955–960. [Google Scholar] [CrossRef]
- Libbey, J.E.; Kirkman, N.J.; Wilcox, K.S.; White, H.S.; Fujinami, R.S. Role for Complement in the Development of Seizures following Acute Viral Infection. J. Virol. 2010, 84, 6452–6460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtman, L.; Van Vliet, E.; Baas, F.; Aronica, E.; Gorter, J.A. Complement protein 6 deficiency in PVG/c rats does not lead to neuroprotection against seizure induced cell death. Neuroscience 2011, 188, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.J.; Manzanero, S.; Borges, K. The effects of C5aR1 on leukocyte infiltration following pilocarpine-induced status epilepticus. Epilepsia 2017, 58, e54–e58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, M.J.; Thomas, N.K.; Talwar, S.; Hodson, M.P.; Lynch, J.W.; Woodruff, T.M.; Borges, K. A novel anticonvulsant mechanism via inhibition of complement receptor C5ar1 in murine epilepsy models. Neurobiol. Dis. 2015, 76, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Nomaru, H.; Sakumi, K.; Katogi, A.; Ohnishi, Y.N.; Kajitani, K.; Tsuchimoto, D.; Nestler, E.J.; Nakabeppu, Y. Fosb gene products contribute to excitotoxic microglial activation by regulating the expression of complement C5a receptors in microglia. Glia 2014, 62, 1284–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyatt, S.K.; Witt, T.; Barbaro, N.M.; Cohen-Gadol, A.A.; Brewster, A.L. Enhanced classical complement pathway activation and altered phagocytosis signaling molecules in human epilepsy. Exp. Neurol. 2017, 295, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Schartz, N.D.; Wyatt-Johnson, S.K.; Price, L.R.; Colin, S.A.; Brewster, A.L. Status epilepticus triggers long-lasting activation of complement C1q-C3 signaling in the hippocampus that correlates with seizure frequency in experimental epilepsy. Neurobiol. Dis. 2018, 109, 163–173. [Google Scholar] [CrossRef]
- Başaran, N.; Hincal, F.; Kansu, E.; Cidotǧer, A.; Cǧer, A. Humoral and cellular immune parameters in untreated and phenytoin- or carbamazepine-treated epileptic patients. Int. J. Immunopharmacol. 1994, 16, 1071–1077. [Google Scholar] [CrossRef]
- Kopczynska, M.; Zelek, W.M.; Vespa, S.; Touchard, S.; Wardle, M.; Loveless, S.; Thomas, R.H.; Hamandi, K.; Morgan, B.P. Complement system biomarkers in epilepsy. Seizure 2018, 60, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hillmen, P.; Young, N.S.; Hubert, S.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The Complement Inhibitor Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef]
- Hillmen, P.; Muus, P.; Röth, A.; Elebute, M.O.; Risitano, A.M.; Schrezenmeier, H.; Szer, J.; Browne, P.; Maciejewski, J.P.; Schubert, J.; et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2013, 162, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.K.; Kavanagh, D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl. Res. 2015, 165, 306–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabrizi, M.A.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2016, 37, 936–983. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.-H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nat. Cell Biol. 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Shin, J.; Cho, H.; Hwang, S.W.; Jung, J.; Shin, C.Y.; Lee, S.-Y.; Kim, S.H.; Lee, M.G.; Choi, Y.H.; Kim, J.; et al. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. USA 2002, 99, 10150–10155. [Google Scholar] [CrossRef] [Green Version]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nat. Cell Biol. 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Mori, F.; Ribolsi, M.; Kusayanagi, H.; Monteleone, F.; Mantovani, V.; Buttari, F.; Marasco, E.; Bernardi, G.; Maccarrone, M.; Centonze, D. TRPV1 Channels Regulate Cortical Excitability in Humans. J. Neurosci. 2012, 32, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Miyake, T.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration. Glia 2015, 63, 1870–1882. [Google Scholar] [CrossRef] [Green Version]
- Mezey, É.; Tóth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Puente, N.; Reguero, L.; Elezgarai, I.; Canduela, M.-J.; Mendizabal-Zubiaga, J.; Ramos, A.; Fernandez-Espejo, E.; Grandes, P. The transient receptor potential vanilloid-1 is localized at excitatory synapses in the mouse dentate gyrus. Brain Struct. Funct. 2014, 220, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Canduela, M.-J.; Mendizabal-Zubiaga, J.; Puente, N.; Reguero, L.; Elezgarai, I.; Ramos, A.; Gerrikagoitia, I.; Grandes, P. Visualization by High Resolution Immunoelectron Microscopy of the Transient Receptor Potential Vanilloid-1 at Inhibitory Synapses of the Mouse Dentate Gyrus. PLoS ONE 2015, 10, e0119401. [Google Scholar] [CrossRef] [PubMed]
- Southall, M.D.; Li, T.; Gharibova, L.S.; Pei, Y.; Nicol, G.D.; Travers, J.B. Activation of Epidermal Vanilloid Receptor-1 Induces Release of Proinflammatory Mediators in Human Keratinocytes. J. Pharmacol. Exp. Ther. 2003, 304, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birder, L.A.; Kanai, A.J.; De Groat, W.C.; Kiss, S.; Nealen, M.L.; Burke, N.E.; Dineley, K.E.; Watkins, S.; Reynolds, I.J.; Caterina, M.J. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc. Natl. Acad. Sci. USA 2001, 98, 13396–13401. [Google Scholar] [CrossRef] [Green Version]
- Akiba, Y.; Kato, S.; Katsube, K.-I.; Nakamura, M.; Takeuchi, K.; Ishii, H.; Hibi, T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet β cells modulates insulin secretion in rats. Biochem. Biophys. Res. Commun. 2004, 321, 219–225. [Google Scholar] [CrossRef]
- Heiner, I.; Eisfeld, J.; Halaszovich, C.R.; Wehage, E.M.; Jüngling, E.; Zitt, C.; Lückhoff, A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: Evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem. J. 2003, 371, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef]
- Saunders, C.I.; Kunde, D.; Crawford, A.; Geraghty, D.P. Expression of transient receptor potential vanilloid 1 (TRPV1) and 2 (TRPV2) in human peripheral blood. Mol. Immunol. 2007, 44, 1429–1435. [Google Scholar] [CrossRef]
- Gibson, H.E.; Edwards, J.G.; Page, R.S.; Van Hook, M.J.; Kauer, J.A. TRPV1 Channels Mediate Long-Term Depression at Synapses on Hippocampal Interneurons. Neuron 2008, 57, 746–759. [Google Scholar] [CrossRef] [Green Version]
- Marinelli, S.; Pascucci, T.; Bernardi, G.; Puglisi-Allegra, S.; Mercuri, N.B. Activation of TRPV1 in the VTA Excites Dopaminergic Neurons and Increases Chemical- and Noxious-Induced Dopamine Release in the Nucleus Accumbens. Neuropsychopharmacology 2004, 30, 864–870. [Google Scholar] [CrossRef]
- Shoudai, K.; Peters, J.H.; McDougall, S.J.; Fawley, J.A.; Andresen, M.C. Thermally Active TRPV1 Tonically Drives Central Spontaneous Glutamate Release. J. Neurosci. 2010, 30, 14470–14475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köles, L.; Garção, P.; Zádori, Z.S.; Ferreira, S.G.; Pinheiro, B.S.; Da Silva-Santos, C.S.; Ledent, C.; Köfalvi, A. Presynaptic TRPV1 vanilloid receptor function is age- but not CB1 cannabinoid receptor-dependent in the rodent forebrain. Brain Res. Bull. 2013, 97, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskaran, M.D.; Smith, B.N. Effects of TRPV1 activation on synaptic excitation in the dentate gyrus of a mouse model of temporal lobe epilepsy. Exp. Neurol. 2010, 222, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.H.; McDougall, S.J.; Fawley, J.A.; Smith, S.M.; Andresen, M.C. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010, 65, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovszki, Z.; Adam, G.; Kekesi, G.; Tuboly, G.; Morvay, Z.; Nagy, E.; Benedek, G.; Horvath, G. The effects of juvenile capsaicin desensitization in rats: Behavioral impairments. Physiol. Behav. 2014, 125, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzi, M.; Felici, R.; Cavone, L.; Gerace, E.; Minassi, A.; Appendino, G.; Moroni, F.; Chiarugi, A. Ischemic Neuroprotection by TRPV1 Receptor-Induced Hypothermia. Br. J. Pharmacol. 2012, 32, 978–982. [Google Scholar] [CrossRef] [Green Version]
- Keeble, J.; Russell, F.; Curtis, B.; Starr, A.; Pintér, E.; Brain, S.D. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005, 52, 3248–3256. [Google Scholar] [CrossRef]
- Abdelhamid, R.E.; Kovács, K.J.; Nunez, M.G.; Larson, A.A. Depressive behavior in the forced swim test can be induced by TRPV1 receptor activity and is dependent on NMDA receptors. Pharmacol. Res. 2014, 79, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Marsch, R.; Foeller, E.; Rammes, G.; Bunck, M.; Kössl, M.; Holsboer, F.; Zieglgänsberger, W.; Landgraf, R.; Lutz, B.; Wotjak, C.T. Reduced Anxiety, Conditioned Fear, and Hippocampal Long-Term Potentiation in Transient Receptor Potential Vanilloid Type 1 Receptor-Deficient Mice. J. Neurosci. 2007, 27, 832–839. [Google Scholar] [CrossRef]
- Razavinasab, M.; Shamsizadeh, A.; Shabani, M.; Nazeri, M.; Allahtavakoli, M.; Asadi-Shekaari, M.; Esmaeili-Mahani, S.; Sheibani, V. Pharmacological blockade of TRPV1 receptors modulates the effects of 6-OHDA on motor and cognitive functions in a rat model of Parkinson’s disease. Fundam. Clin. Pharmacol. 2012, 27, 632–640. [Google Scholar] [CrossRef]
- Nazıroğlu, M. TRPV1 Channel: A Potential Drug Target for Treating Epilepsy. Curr. Neuropharmacol. 2015, 13, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-X.; Yu-Qiang, L.; Sanchez, R.M.; Liu, Y.-Q.; Min, J.-W.; Hu, J.-J.; Bsoul, N.B.; Han, S.; Yin, J.; Liu, W.-H.; et al. TRPV1 promotes repetitive febrile seizures by pro-inflammatory cytokines in immature brain. Brain Behav. Immun. 2015, 48, 68–77. [Google Scholar] [CrossRef]
- Kong, W.-L.; Min, J.-W.; Liu, Y.-L.; Li, J.-X.; He, X.-H.; Peng, B. Role of TRPV1 in susceptibility to PTZ-induced seizure following repeated hyperthermia challenges in neonatal mice. Epilepsy Behav. 2014, 31, 276–280. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, L.E.; Ladas, T.P.; Chiang, C.-C.; Durand, D.M. TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo. Exp. Neurol. 2013, 250, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.J.; Vaca, M.A.; Miranda, C.J.; N’Gouemo, P. Inhibition of transient potential receptor vanilloid type 1 suppresses seizure susceptibility in the genetically epilepsy-prone rat. CNS Neurosci. Ther. 2017, 24, 18–28. [Google Scholar] [CrossRef]
- Manna, S.S.; Umathe, S.N. Involvement of transient receptor potential vanilloid type 1 channels in the pro-convulsant effect of anandamide in pentylenetetrazole-induced seizures. Epilepsy Res. 2012, 100, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Nieoczym, D.; Pieróg, M.; Wlaź, P. α-Spinasterol, a TRPV1 receptor antagonist, elevates the seizure threshold in three acute seizure tests in mice. J. Neural Transm. 2015, 122, 1239–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.-F.; Li, Y.-C.; Tang, Y.-P.; Cao, J.; Wang, L.-P.; Yang, Y.-X.; Xu, L.; Mao, R.-R. Interference of TRPV1 function altered the susceptibility of PTZ-induced seizures. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazıroğlu, M.; Taner, A.N.; Balbay, E.; Çiğ, B. Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model. Mol. Cell. Biochem. 2019, 453, 143–155. [Google Scholar] [CrossRef]
- Saffarzadeh, F.; Eslamizade, M.J.; Ghadiri, T.; Mousavi, S.M.M.; Hadjighassem, M.; Gorji, A. Effects of TRPV1 on the hippocampal synaptic plasticity in the epileptic rat brain. Synapse 2015, 69, 375–383. [Google Scholar] [CrossRef]
- Saffarzadeh, F.; Eslamizade, M.; Mousavi, S.; Abraki, S.; Hadjighassem, M.; Gorji, A. TRPV1 receptors augment basal synaptic transmission in CA1 and CA3 pyramidal neurons in epilepsy. Neuroscience 2016, 314, 170–178. [Google Scholar] [CrossRef]
- Shirazi, M.; Izadi, M.; Amin, M.; Rezvani, M.E.; Roohbakhsh, A.; Shamsizadeh, A. Involvement of central TRPV1 receptors in pentylenetetrazole and amygdala-induced kindling in male rats. Neurol. Sci. 2014, 35, 1235–1241. [Google Scholar] [CrossRef]
- Sun, F.-J.; Guo, W.; Zheng, D.-H.; Zhang, C.-Q.; Li, S.; Liu, S.-Y.; Yin, Q.; Yang, H.; Shu, H.-F. Increased Expression of TRPV1 in the Cortex and Hippocampus from Patients with Mesial Temporal Lobe Epilepsy. J. Mol. Neurosci. 2012, 49, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Vrontakis, M.E. Galanin: A Biologically Active Peptide. Curr. Drug Target -CNS Neurol. Disord. 2002, 1, 531–541. [Google Scholar] [CrossRef]
- Langel, Ü.; Bartfai, T. Chemistry and molecular biology of galanin receptor ligands. Ann. N. Y. Acad. Sci. 1998, 863, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Vrontakis, M.E.; Yamamoto, T.; Schroedter, I.C.; Nagy, J.I.; Friesen, H.G. Estrogen induction of galanin synthesis in the rat anterior pituitary gland demonstrated by in situ hybridization and immunohistochemistry. Neurosci. Lett. 1989, 100, 59–64. [Google Scholar] [CrossRef]
- Mohney, R.P.; Zigmond, R.E. Galanin expression is decreased by cAMP-elevating agents in cultured sympathetic ganglia. Neuro Rep. 1999, 10, 1221–1224. [Google Scholar] [CrossRef]
- Hooi, S.C.; Koenig, J.I.; Abraczinskas, D.R.; Kaplan, L.M. Regulation of anterior pituitary galanin gene expression by thyroid hormone. Mol. Brain Res. 1997, 51, 15–22. [Google Scholar] [CrossRef]
- Brann, D.W.; Chorich, L.P.; Mahesh, V.B. Effect of Progesterone on Galanin mRNA Levels in the Hypothalamus and the Pituitary: Correlation with the Gonadotropin Surge. Neuroendocrinology 1993, 58, 531–538. [Google Scholar] [CrossRef]
- Corness, J.; Stevens, B.; Fields, R.D.; Hökfelt, T. NGF and LIF both regulate galanin gene expression in primary DRG cultures. Neuroreport 1998, 9, 1533–1536. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.L.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hökfelt, T.; Kofler, B. Physiology, Signaling, and Pharmacology of Galanin Peptides and Receptors: Three Decades of Emerging Diversity. Pharmacol. Rev. 2014, 67, 118–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melander, T.; Hökfelt, T.; Rökaeus, A.; Cuello, A.C.; Oertel, W.H.; Verhofstad, A.; Goldstein, M. Coexistence of galanin-like immunoreactivity with catecholamines, 5- hydroxytryptamine, GABA and neuropeptides in the rat CNS. J. Neurosci. 1986, 6, 3640–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Nicholas, A.; Ho¨kfelt, T. Ultrastructural studies on peptides in the dorsal horn of the spinal cord—I. Co-existence of galanin with other peptides in primary afferents in normal rats. Neuroscience 1993, 57, 365–384. [Google Scholar] [CrossRef]
- Crawley, J. The role of galanin in feeding behavior. Neuropeptides 1999, 33, 369–375. [Google Scholar] [CrossRef]
- Liu, H.-X.; Hökfelt, T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol. Sci. 2002, 23, 468–474. [Google Scholar] [CrossRef]
- Xu, X.-F.; Zhang, D.-D.; Liao, J.-C.; Xiao, L.; Wang, Q.; Qiu, W. Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats. Neural Regen. Res. 2016, 11, 1517–1526. [Google Scholar] [CrossRef]
- Steininger, T.L.; Gong, H.; McGinty, D.; Szymusiak, R. Subregional organization of preoptic area /anterior hypothalamic projections to arousal-related monoaminergic cell groups. J. Comp. Neurol. 2001, 429, 638–653. [Google Scholar] [CrossRef]
- Fraley, G.; Thomas-Smith, S.; Acohido, B.; Steiner, R.; Clifton, D. Stimulation of sexual behavior in the male rat by galanin-like peptide. Horm. Behav. 2004, 46, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Ögren, S.O.; Schött, P.A.; Kehr, J.; Misane, I.; Razani, H. Galanin and learning. Brain Res. 1999, 848, 174–182. [Google Scholar] [CrossRef]
- Ögren, S.O.; Kuteeva, E.; Elvander-Tottie, E.; Hökfelt, T. Neuropeptides in learning and memory processes with focus on galanin. Eur. J. Pharmacol. 2010, 626, 9–17. [Google Scholar] [CrossRef]
- Bartfai, T.; Hökfelt, T.; Langel, U. Galanin--a neuroendocrine peptide. Crit. Rev. Neurobiol. 1993, 7, 229–274. [Google Scholar]
- Bauer, F.; Zintel, A.; Kenny, M.; Calder, D.; Ghatei, M.; Bloom, S. Inhibitory effect of galanin on postprandial gastrointestinal motility and gut hormone release in humans. Gastroenterol. 1989, 97, 260–264. [Google Scholar] [CrossRef]
- Holm, L.; Hilke, S.; Ádori, C.; Theodorsson, E.; Hökfelt, T.; Theodorsson, A. Changes in galanin and GalR1 gene expression in discrete brain regions after transient occlusion of the middle cerebral artery in female rats. Neuropeptides 2012, 46, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.V.; Blanchard, D.; Blanchard, R.J.; Brady, L.S.; Crawley, J.N. Chronic social stress increases levels of preprogalanin mRNA in the rat locus coeruleus. Pharmacol. Biochem. Behav. 1995, 50, 655–660. [Google Scholar] [CrossRef]
- Flatters, S.J.; Fox, A.J.; Dickenson, A.H. Nerve injury induces plasticity that results in spinal inhibitory effects of galanin. Pain 2002, 98, 249–258. [Google Scholar] [CrossRef]
- Wraith, D.C.; Pope, R.; Butzkueven, H.; Holder, H.; Vanderplank, P.; Lowrey, P.; Day, M.J.; Gundlach, A.L.; Kilpatrick, T.J.; Scolding, N.J.; et al. A role for galanin in human and experimental inflammatory demyelination. Proc. Natl. Acad. Sci. USA 2009, 106, 15466–15471. [Google Scholar] [CrossRef] [Green Version]
- Baraka, A.; ElGhotny, S. Study of the effect of inhibiting galanin in Alzheimer’s disease induced in rats. Eur. J. Pharmacol. 2010, 641, 123–127. [Google Scholar] [CrossRef]
- Branchek, T.A.; Smith, K.E.; Gerald, C.; Walker, M.W. Galanin receptor subtypes. Trends Pharmacol. Sci. 2000, 21, 109–117. [Google Scholar] [CrossRef]
- Skofitsch, G.; Sills, M.A.; Jacobowitz, D.M. Autoradiographic distribution of 125I-galanin binding sites in the rat central nervous system. Peptides 1986, 7, 1029–1042. [Google Scholar] [CrossRef]
- Melander, T.; Hökfelt, T.; Rökaeus, A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J. Comp. Neurol. 1986, 248, 475–517. [Google Scholar] [CrossRef]
- Nicholl, J.; Kofler, B.; Sutherland, G.; Shine, J.; Iismaa, T. Assignment of the Gene Encoding Human Galanin Receptor (GALNR) to 18q23 by in Situ Hybridization. Genomics 1995, 30, 629–630. [Google Scholar] [CrossRef]
- Zini, S.; Roisin, M.-P.; Armengaud, C.; Ben-Ari, Y. Effect of potassium channel modulators on the release of glutamate induced by ischaemic-like conditions in rat hippocampal slices. Neurosci. Lett. 1993, 153, 202–205. [Google Scholar] [CrossRef]
- Zini, S.; Roisin, M.-P.; Langel, Ü.; Bartfai, T.; Ben-Ari, Y. Galanin reduces release of endogeneous excitatory amino acids in the rat hippocampus. Eur. J. Pharmacol. Mol. Pharmacol. 1993, 245, 1–7. [Google Scholar] [CrossRef]
- Endoh, T.; Sato, D.; Wada, Y.; Shibukawa, Y.; Ishihara, K.; Hashimoto, S.; Yoshinari, M.; Matsuzaka, K.; Tazaki, M.; Inoue, T. Galanin inhibits calcium channels via Gαi-protein mediated by GalR1 in rat nucleus tractus solitarius. Brain Res. 2008, 1229, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badie-Mahdavi, H.; Lu, X.; Behrens, M.; Bartfai, T. Role of galanin receptor 1 and galanin receptor 2 activation in synaptic plasticity associated with 3′,5′-cyclic AMP response element-binding protein phosphorylation in the dentate gyrus: Studies with a galanin receptor 2 agonist and galanin receptor 1 knockout mice. Neuroscience 2005, 133, 591–604. [Google Scholar] [CrossRef]
- Blackshear, A.; Yamamoto, M.; Anderson, B.J.; Holmes, P.V.; Lundström, L.; Langel, Ü.; Robinson, J.K. Intracerebroventricular administration of galanin or galanin receptor subtype 1 agonist M617 induces c-Fos activation in central amygdala and dorsomedial hypothalamus. Peptides 2007, 28, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Laburthe, M.; Amiranoff, B. Galanin inhibits adenylate cyclase of rat brain membranes. Peptides 1992, 13, 339–341. [Google Scholar] [CrossRef]
- Wang, S.; Hashemi, T.; Fried, S.; Clemmons, A.L.; Hawes, B.E. Differential Intracellular Signaling of the GalR1 and GalR2 Galanin Receptor Subtypes. Biochemistry 1998, 37, 6711–6717. [Google Scholar] [CrossRef]
- Takigawa, T.; Alzheimer, C. G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J. Physiol. 1999, 517, 385–390. [Google Scholar] [CrossRef]
- Fathi, Z.; Battaglino, P.M.; Iben, L.G.; Li, H.; Baker, E.; Zhang, D.; McGovern, R.; Mahle, C.D.; Sutherland, G.R.; Iismaa, T.P.; et al. Molecular characterization, pharmacological properties and chromosomal localization of the human GALR2 galanin receptor. Mol. Brain Res. 1998, 58, 156–169. [Google Scholar] [CrossRef]
- Seufferlein, T.; Rozengurt, E. Galanin, neurotensin, and phorbol esters rapidly stimulate activation of mitogen-activated protein kinase in small cell lung cancer cells. Cancer Res. 1996, 56, 5758–5764. [Google Scholar] [PubMed]
- Hawes, J.J.; Narasimhaiah, R.; Picciotto, M.R. Galanin and galanin-like peptide modulate neurite outgrowth via protein kinase C-mediated activation of extracellular signal-related kinase. Eur. J. Neurosci. 2006, 23, 2937–2946. [Google Scholar] [CrossRef]
- Ifuku, M.; Okuno, Y.; Yamakawa, Y.; Izumi, K.; Seifert, S.; Kettenmann, H.; Noda, M. Functional importance of inositol-1,4,5-triphosphate-induced intracellular Ca2+ mobilization in galanin-induced microglial migration. J. Neurochem. 2011, 117, 61–70. [Google Scholar] [CrossRef]
- Kolakowski, L.F., Jr.; O’Neill, G.P.; Howard, A.D.; Broussard, S.R.; Sullivan, K.A.; Feighner, S.D.; Sawzdargo, M.; Nguyen, T.; Kargman, S.; Shiao, L.-L.; et al. Molecular Characterization and Expression of Cloned Human Galanin Receptors GALR2 and GALR3. J. Neurochem. 2002, 71, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Barde, S.; Ortsäter, H.; Eweida, M.; Darsalia, V.; Langel, Ü.; Sjöholm, Å.; Hökfelt, T.; Patrone, C. GalR3 activation promotes adult neural stem cell survival in response to a diabeticmilieu. J. Neurochem. 2013, 127, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.E.; Walker, M.W.; Artymyshyn, R.; A Bard, J.; Borowsky, B.; Tamm, J.A.; Yao, W.-J.; Vaysse, P.J.-J.; Branchek, T.A.; Gerald, C.; et al. Cloned Human and Rat Galanin GALR3 Receptors. J. Biol. Chem. 1998, 273, 23321–23326. [Google Scholar] [CrossRef] [Green Version]
- Mennicken, F.; Hoffert, C.; Pelletier, M.; Ahmad, S.; O’Donnell, D. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J. Chem. Neuroanat. 2002, 24, 257–268. [Google Scholar] [CrossRef]
- Mazarati, A.; Langel, Ü.; Bartfai, T. Book Review: Galanin: An Endogenous Anticonvulsant? Neuroscience 2001, 7, 506–517. [Google Scholar] [CrossRef]
- Mazarati, A.; Liu, H.; Soomets, U.; Sankar, R.; Shin, D.; Katsumori, H.; Langel, Ü.; Wasterlain, C.G. Galanin Modulation of Seizures and Seizure Modulation of Hippocampal Galanin in Animal Models of Status Epilepticus. J. Neurosci. 1998, 18, 10070–10077. [Google Scholar] [CrossRef]
- Wilson, D.N.; Chung, H.; Elliott, R.C.; Bremer, E.; George, D.; Koh, S. Microarray Analysis of Postictal Transcriptional Regulation of Neuropeptides. J. Mol. Neurosci. 2005, 25, 285–298. [Google Scholar] [CrossRef]
- Mazarati, A.M.; Halászi, E.; Telegdy, G. Anticonvulsive effects of galanin administered into the central nervous system upon the picrotoxin-kindled seizure syndrome in rats. Brain Res. 1992, 589, 164–166. [Google Scholar] [CrossRef]
- Ledri, M.; Sørensen, A.T.; Madsen, M.G.; Christiansen, S.H.; Ledri, L.N.; Cifra, A.; Bengzon, J.; Hellström-Lindberg, E.; Pinborg, L.H.; Jespersen, B.; et al. Differential Effect of Neuropeptides on Excitatory Synaptic Transmission in Human Epileptic Hippocampus. J. Neurosci. 2015, 35, 9622–9631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guipponi, M.; Chentouf, A.; Webling, K.E.; Freimann, K.; Crespel, A.; Nobile, C.; Lemke, J.R.; Hansen, J.; Dorn, T.; Lesca, G.; et al. Galanin pathogenic mutations in temporal lobe epilepsy. Hum. Mol. Genet. 2015, 24, 3082–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazarati, A.; Lu, X.; Shinmei, S.; Badie-Mahdavi, H.; Bartfai, T. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience 2004, 128, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, A.S.; Hort, Y.J.; Constantinescu, G.; Shine, J.; Iismaa, T.P. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Mol. Brain Res. 2002, 107, 195–200. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Jacoby, A.S.; Brumovsky, P.R.; Shine, J.; Iismaa, T.P.; Hökfelt, T. Altered Hippocampal Expression of Neuropeptides in Seizure-prone GALR1 Knockout Mice. Epilepsia 2003, 44, 1022–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottsch, M.L.; Zeng, H.; Hohmann, J.G.; Weinshenker, D.; Clifton, D.K.; Steiner, R.A. Phenotypic Analysis of Mice Deficient in the Type 2 Galanin Receptor (GALR2). Mol. Cell. Biol. 2005, 25, 4804–4811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazarati, A.; Lu, X.; Kilk, K.; Langel, Ü.; Wasterlain, C.; Bartfai, T. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur. J. Neurosci. 2004, 19, 3235–3244. [Google Scholar] [CrossRef]
- McColl, C.D.; Jacoby, A.S.; Shine, J.; Iismaa, T.P.; Bekkers, J.M. Galanin receptor-1 knockout mice exhibit spontaneous epilepsy, abnormal EEGs and altered inhibition in the hippocampus. Neuropharmacology 2006, 50, 209–218. [Google Scholar] [CrossRef]
- Mazarati, A.; Lundström, L.; Sollenberg, U.; Shin, D.; Langel, Ü.; Sankar, R. Regulation of Kindling Epileptogenesis by Hippocampal Galanin Type 1 and Type 2 Receptors: The Effects of Subtype-Selective Agonists and the Role of G-Protein-Mediated Signaling. J. Pharmacol. Exp. Ther. 2006, 318, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Bartfai, T.; Lu, X.; Badie-Mahdavi, H.; Barr, A.M.; Mazarati, A.; Hua, X.-Y.; Yaksh, T.; Haberhauer, G.; Ceide, S.C.; Trembleau, L.; et al. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc. Natl. Acad. Sci. USA 2004, 101, 10470–10475. [Google Scholar] [CrossRef] [Green Version]
- Bulaj, G.; Green, B.R.; Lee, H.-K.; Robertson, C.R.; White, K.; Zhang, L.; Sochanska, M.; Flynn, S.P.; Scholl, E.A.; Pruess, T.H.; et al. Design, Synthesis, and Characterization of High-Affinity, Systemically-Active Galanin Analogues with Potent Anticonvulsant Activities. J. Med. Chem. 2008, 51, 8038–8047. [Google Scholar] [CrossRef]
- White, H.S.; Scholl, E.A.; Klein, B.D.; Flynn, S.P.; Pruess, T.H.; Green, B.R.; Zhang, L.; Bulaj, G. Developing novel antiepileptic drugs: Characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics 2009, 6, 372–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walls, A.B.; Flynn, S.P.; West, P.J.; Müller, M.S.; Bak, L.K.; Bulaj, G.; Schousboe, A.; White, H.S. The anticonvulsant action of the galanin receptor agonist NAX-5055 involves modulation of both excitatory- and inhibitory neurotransmission. Epilepsy Res. 2016, 121, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Saar, K.; Mazarati, A.M.; Mahlapuu, R.; Hallnemo, G.; Soomets, U.; Kilk, K.; Hellberg, S.; Pooga, M.; Tolf, B.-R.; Shi, T.S.; et al. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc. Natl. Acad. Sci. USA 2002, 99, 7136–7141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, C.S.; Klein, B.D.; McDougle, D.R.; Zhang, L.; Kaufmann, D.; Bulaj, G.; White, H.S. Preclinical evaluation of intravenous NAX 810-2, a novel GalR2-preferring analog, for anticonvulsant efficacy and pharmacokinetics. Epilepsia 2017, 58, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Roberts, E.; Xia, F.; Sanchez-Alavez, M.; Liu, T.; Baldwin, R.; Wu, S.; Chang, J.; Wasterlain, C.G.; Bartfai, T. GalR2-positive allosteric modulator exhibits anticonvulsant effects in animal models. Proc. Natl. Acad. Sci. USA 2010, 107, 15229–15234. [Google Scholar] [CrossRef] [Green Version]
- Kokaia, M.; Holmberg, K.; Nanobashvili, A.; Xu, Z.-Q.D.; Kokaia, Z.; Lendahl, U.; Hilke, S.; Theodorsson, E.; Kahl, U.; Bartfai, T.; et al. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proc. Natl. Acad. Sci. USA 2001, 98, 14006–14011. [Google Scholar] [CrossRef] [Green Version]
- Schlifke, I.; Kuteeva, E.; Hökfelt, T.; Kokaia, M. Galanin expressed in the excitatory fibers attenuates synaptic strength and generalized seizures in the piriform cortex of mice. Exp. Neurol. 2006, 200, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Kanter-Schlifke, I.; Sørensen, A.T.; Ledri, M.; Kuteeva, E.; Hokfelt, T.; Kokaia, M. Galanin gene transfer curtails generalized seizures in kindled rats without altering hippocampal synaptic plasticity. Neuroscience 2007, 150, 984–992. [Google Scholar] [CrossRef]
- McCown, T.J. Adeno-associated Virus-Mediated Expression and Constitutive Secretion of Galanin Suppresses Limbic Seizure Activity in Vivo. Mol. Ther. 2006, 14, 63–68. [Google Scholar] [CrossRef] [PubMed]
- McCown, T.J. Adeno-associated virus vector-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity. Neurotherapeutics 2009, 6, 307–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, S.J.; Blake, B.L.; Criswell, H.E.; Nicolson, S.C.; Samulski, R.J.; McCown, T.J. Directed Evolution of a Novel Adeno-associated Virus (AAV) Vector That Crosses the Seizure-compromised Blood–Brain Barrier (BBB). Mol. Ther. 2010, 18, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, A. Melatonin in Humans. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef]
- Axelrod, J.; Weissbach, H. Enzymatic O-Methylation of N-Acetylserotonin to Melatonin. Science 1960, 131, 1312. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.; Hermes, M.H.; Kalsbeek, A. The suprachiasmatic nucleus—paraventricular nucleus interactions: A bridge to the neuroendocrine and autonomic nervous system. Prog. Brain Res. 1999, 119, 365–382. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Rosner, J.M. Retinal Localization of the Hydroxyindole-O-methyl Transferase (HIOMT) in the Rat. Endocrinology 1971, 89, 301–303. [Google Scholar] [CrossRef]
- Slominski, A.T.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002, 16, 896–898. [Google Scholar] [CrossRef] [Green Version]
- Bubenik, G.A. REVIEW: Gastrointestinal Melatonin: Localization, Function, and Clinical Relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, G.J. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; García-Mauriño, S.; Reiter, R.J.; Guerrero, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.-J.; Cajochen, C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J. Biol. Rhythm. 1997, 12, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Talpur, H.; Chandio, I.; Brohi, R.; Worku, T.; Rehman, Z.; Bhattarai, D.; Ullah, F.; Jiajia, L.; Yang, L. Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod. Domest. Anim. 2018, 53, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-C.; Lin, S.-H.; Chang, J.-S.; Chien, Y.-W. Effects of Melatonin on Glucose Homeostasis, Antioxidant Ability, and Adipokine Secretion in ICR Mice with NA/STZ-Induced Hyperglycemia. Nutrients 2017, 9, 1187. [Google Scholar] [CrossRef] [Green Version]
- Gorfine, T.; Zisapel, N. Melatonin and the human hippocampus, a time dependant interplay. J. Pineal Res. 2007, 43, 80–86. [Google Scholar] [CrossRef]
- Baker, J.; Kimpinski, K. Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 2018, 45, 755–766. [Google Scholar] [CrossRef]
- Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Esquifino, A.I.; Pandi-Perumal, S.R.; Miller, S.C. Melatonin, immune function and aging. Immun. Ageing 2005, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molcules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.P.H. The analgesic effects of exogenous melatonin in humans. Acta Anaesthesiol. Scand. 2016, 60, 1024–1025. [Google Scholar] [CrossRef]
- Hansen, M.; Danielsen, A.K.; Hageman, I.; Rosenberg, J.; Gögenur, I. The therapeutic or prophylactic effect of exogenous melatonin against depression and depressive symptoms: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2014, 24, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Antioxidative Protection by Melatonin: Multiplicity of Mechanisms from Radical Detoxification to Radical Avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Huang, F.; Yang, Z.; Li, C.-Q. The Melatonergic System in Anxiety Disorders and the Role of Melatonin in Conditional Fear. Vitam. Horm. 2017, 103, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 Melatonin Receptors in Mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Slaugenhaupt, S.A.; Roca, A.L.; Liebert, C.B.; Altherr, M.R.; Gusella, J.F.; Reppert, S.M. Mapping of the Gene for the Mel1a-Melatonin Receptor to Human Chromosome 4 (MTNR1A) and Mouse Chromosome 8 (Mtnr1a). Genomics 1995, 27, 355–357. [Google Scholar] [CrossRef]
- Brydon, L.; Roka, F.; Petit, L.; De Coppet, P.; Tissot, M.; Barrett, P.; Morgan, P.J.; Nanoff, C.; Strosberg, A.D.; Jockers, R. Dual Signaling of Human Mel1a Melatonin Receptors via Gi2, Gi3, and Gq/11 Proteins. Mol. Endocrinol. 1999, 13, 2025–2038. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1and MT2Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [Green Version]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.A.; Gusella, J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 8734–8738. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, R.S.; Melan, M.A.; Passey, D.K.; Witt-Enderby, P.A. Dual coupling of MT1 and MT2 melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem. Pharmacol. 2002, 63, 587–595. [Google Scholar] [CrossRef]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.-M.; Lefoulon, F.; Fauchère, J.-L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the Melatonin-binding SiteMT3as the Quinone Reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef] [Green Version]
- Acufla-Castroviejo, D.; Escames, G.; Macks, M.; Hoyos, A.M.; Carballo, A.M.; Arauzo, M.; Montes, R.; Vives, F. Minireview: Cell protective role of melatonin in the brain. J. Pineal Res. 1995, 19, 57–63. [Google Scholar] [CrossRef]
- Nilcs, L.P.; Pickering, D.S.; Arciszewski, M.A. Effects of chronic melatonin administration on GABA and diazepam binding in rat brain. J. Neural Transm. 1987, 70, 117–124. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Macías, M.; Escames, G.; Camacho, E.; Khaldy, H.; Martín, M.; Espinosa, A.; Gallo, M.A.; Acuña-Castroviejo, D. Structure-Related Inhibition of Calmodulin-Dependent Neuronal Nitric-Oxide Synthase Activity by Melatonin and Synthetic Kynurenines. Mol. Pharmacol. 2000, 58, 967–975. [Google Scholar] [CrossRef]
- Hamdi, A. Melatonin administration increases the affinity of D2 dopamine receptors in the rat striatum. Life Sci. 1998, 63, 2115–2120. [Google Scholar] [CrossRef]
- Esposito, S.; Laino, D.; D’Alonzo, R.; Mencarelli, A.; Di Genova, L.; Fattorusso, A.; Argentiero, A.; Mencaroni, E. Pediatric sleep disturbances and treatment with melatonin. J. Transl. Med. 2019, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Barchas, J.; Dacosta, F.; Spector, S. Acute Pharmacology of Melatonin. Nat. Cell Biol. 1967, 214, 919–920. [Google Scholar] [CrossRef]
- Fauteck, J.-D.; Böckmann, J.; Wittkowski, W.; Straub, H.; Speckmann, E.-J.; Tuxhorn, I.; Wolf, P.; Pannek, H.; Oppel, F. Melatonin reduces low-Mg2+ epileptiform activity in human temporal slices. Exp. Brain Res. 1995, 107, 321–325. [Google Scholar] [CrossRef]
- Borowicz, K.K.; Kamiński, R.; Gasior, M.; Kleinrok, Z.; Czuczwar, S.J. Influence of melatonin upon the protective action of conventional anti-epileptic drugs against maximal electroshock in mice. Eur. Neuropsychopharmacol. 1999, 9, 185–190. [Google Scholar] [CrossRef]
- Mevissen, M.; Ebert, U. Anticonvulsant effects of melatonin in amygdala-kindled rats. Neurosci. Lett. 1998, 257, 13–16. [Google Scholar] [CrossRef]
- Yildirim, M.; Marangoz, C. Anticonvulsant effects of melatonin on penicillin-induced epileptiform activity in rats. Brain Res. 2006, 1099, 183–188. [Google Scholar] [CrossRef]
- Aydin, L.; Gundogan, N.U.; Yazici, C. Anticonvulsant efficacy of melatonin in an experimental model of hyperthermic febrile seizures. Epilepsy Res. 2015, 118, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Costa-Lotufo, L.V.; Fonteles, M.M.D.F.; Lima, I.S.P.; De Oliveira, A.A.; Nascimento, V.S.; De Bruin, V.M.; Viana, G.S. Attenuating effects of melatonin on pilocarpine-induced seizures in rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 131, 521–529. [Google Scholar] [CrossRef]
- Solmaz, I.; Gurkanlar, D.; Gökçil, Z.; Goksoy, C.; Özkan, M.; Erdogan, E. Antiepileptic activity of melatonin in guinea pigs with pentylenetetrazol-induced seizures. Neurol. Res. 2009, 31, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Janjoppi, L.; De Lacerda, A.F.S.; Scorza, F.A.; Amado, D.; Cavalheiro, E.; Arida, R.M. Influence of pinealectomy on the amygdala kindling development in rats. Neurosci. Lett. 2006, 392, 150–153. [Google Scholar] [CrossRef] [PubMed]
- De Lima, E.; Soares, J.M.; Garrido, Y.D.C.S.; Valente, S.; Priel, M.R.; Baracat, E.C.; Cavalheiro, E.; Mazzacoratti, M.D.G.N.; Amado, D. Effects of pinealectomy and the treatment with melatonin on the temporal lobe epilepsy in rats. Brain Res. 2005, 1043, 24–31. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chun, W.; Kong, P.-J.; Han, J.A.; Cho, B.P.; Kwon, O.-Y.; Lee, H.J.; Kim, S.-S. Sustained activation of Akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J. Pineal Res. 2006, 40, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-Y.; Han, S.-H. Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J. Pineal Res. 2003, 34, 95–102. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Petkova, Z.; Pechlivanova, D.; Moyanova, S.G.; Kortenska, L.; Mitreva, R.; Lozanov, V.; Atanasova, D.; Lazarov, N.; Stoynev, A.; et al. Prophylactic treatment with melatonin after status epilepticus: Effects on epileptogenesis, neuronal damage, and behavioral changes in a kainate model of temporal lobe epilepsy. Epilepsy Behav. 2013, 27, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Cabral, F.R.; Cavalheiro, E.; Mazzacoratti, M.D.G.N.; Amado, D. Melatonin administration after pilocarpine-induced status epilepticus: A new way to prevent or attenuate postlesion epilepsy? Epilepsy Behav. 2011, 20, 607–612. [Google Scholar] [CrossRef]
- Kazemi, M.; Shokri, S.; Ganjkhani, M.; Ali, R.; Iraj, J.A. Modulation of axonal sprouting along rostro-caudal axis of dorsal hippocampus and no neuronal survival in parahippocampal cortices by long-term post-lesion melatonin administration in lithium-pilocarpine model of temporal lobe epilepsy. Anat. Cell Biol. 2016, 49, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.K.A.D.A.; De Lima, E.; Amaral, F.G.; Peres, R.; Cipolla-Neto, J.; Amado, D. Altered MT1 and MT2 melatonin receptors expression in the hippocampus of pilocarpine-induced epileptic rats. Epilepsy Behav. 2017, 71, 23–34. [Google Scholar] [CrossRef]
- Fenoglio-Simeone, K.; Mazarati, A.; Sefidvash-Hockley, S.; Shin, D.; Wilke, J.; Milligan, H.; Sankar, R.; Rho, J.M.; Maganti, R. Anticonvulsant effects of the selective melatonin receptor agonist ramelteon. Epilepsy Behav. 2009, 16, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bialer, M.; Johannessen, S.I.; Levy, R.H.; Perucca, E.; Tomson, T.; White, H.S. Progress report on new antiepileptic drugs: A summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res. 2015, 111, 85–141. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Atanasova, D.; Nenchovska, Z.; Atanasova, M.; Kortenska, L.; Gesheva, R.; Lazarov, N. Agomelatine protects against neuronal damage without preventing epileptogenesis in the kainate model of temporal lobe epilepsy. Neurobiol. Dis. 2017, 104, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ardura, J.; Andres, J.; Garmendia, J.R.; Ardura, F. Melatonin in Epilepsy and Febrile Seizures. J. Child Neurol. 2010, 25, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Dabak, O.; Altun, D.; Arslan, M.; Yaman, H.; Vurucu, S.; Yesilkaya, E.; Unay, B. Evaluation of Plasma Melatonin Levels in Children With Afebrile and Febrile Seizures. Pediatr. Neurol. 2016, 57, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Bazil, C.W.; Short, D.; Crispin, D.; Zheng, W. Patients with intractable epilepsy have low melatonin, which increases following seizures. Neurology 2000, 55, 1746–1748. [Google Scholar] [CrossRef] [Green Version]
- Elkhayat, H.A.; Hassanein, S.M.; Tomoum, H.Y.; Abd-Elhamid, I.A.; Asaad, T.; Elwakkad, A.S. Melatonin and Sleep-Related Problems in Children With Intractable Epilepsy. Pediatr. Neurol. 2010, 42, 249–254. [Google Scholar] [CrossRef]
- Peled, N.; Shorer, Z.; Peled, E.; Pillar, G. Melatonin Effect on Seizures in Children with Severe Neurologic Deficit Disorders. Epilepsia 2002, 42, 1208–1210. [Google Scholar] [CrossRef]
- Molina-Carballo, A.; Hoyos, A.M.; Reiter, R.J.; Sánchez-Forte, M.; Moreno-Madrid, F.; Rufo-Campos, M.; Molina-Font, J.A.; Acuña-Castroviejo, D. Utility of high doses of melatonin as adjunctive anticonvulsant therapy in a child with severe myoclonic epilepsy: Two years’ experience. J. Pineal Res. 1997, 23, 97–105. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, Y.K.; Agarwal, S.; Aneja, S.; Kalaivani, M.; Kohli, K. Effects of Add-on Melatonin Administration on Antioxidant Enzymes in Children with Epilepsy Taking Carbamazepine Monotherapy: A Randomized, Double-blind, Placebo-controlled Trial. Epilepsia 2004, 45, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, Y.K.; Agarwal, S.; Aneja, S.; Kohli, K. A randomized, double-blind, placebo controlled trial of melatonin add-on therapy in epileptic children on valproate monotherapy: Effect on glutathione peroxidase and glutathione reductase enzymes. Br. J. Clin. Pharmacol. 2004, 58, 542–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, S.H. Pro-convulsant effects of oral melatonin in neurologically disabled children. Lancet 1998, 351, 1254. [Google Scholar] [CrossRef]
- Sandyk, R.; Tsagas, N.; Anninos, P. Melatonin as a Proconvulsive Hormone in Humans. Int. J. Neurosci. 1992, 63, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; Hamilton, D.; Seward, N.; Fast, D.K.; Freeman, R.D.; Laudon, M. Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders. J. Pineal Res. 2000, 29, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.; Davies, P.; Whitehouse, W. Melatonin treatment for sleep disorders in children with neurodevelopmental disorders: An observational study. Dev. Med. Child Neurol. 2002, 44, 339–344. [Google Scholar] [CrossRef]
- Jones, C.; Huyton, M.; Hindley, D. Melatonin and epilepsy. Arch. Dis. Child. 2005, 90, 1203. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Nguyen, D.T.; Jiang, J. G protein-coupled receptors in acquired epilepsy: Druggability and translatability. Prog. Neurobiol. 2019, 183, 101682. [Google Scholar] [CrossRef]
- Oliveira, M.; Furian, A.F.; Rambo, L.M.; Ribeiro, L.R.; Royes, L.; Ferreira, J.A.; Calixto, J.B.; Mello, C.F. Modulation of pentylenetetrazol-induced seizures by prostaglandin E2 receptors. Neuroscience 2008, 152, 1110–1118. [Google Scholar] [CrossRef]
- Pekcec, A.; Unkrüer, B.; Schlichtiger, J.; Soerensen, J.; Hartz, A.M.S.; Bauer, B.; Van Vliet, E.A.; Gorter, J.A.; Potschka, H. Targeting Prostaglandin E2 EP1 Receptors Prevents Seizure-Associated P-glycoprotein Up-Regulation. J. Pharmacol. Exp. Ther. 2009, 330, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Fischborn, S.V.; Soerensen, J.; Potschka, H. Targeting the prostaglandin E2 EP1 receptor and cyclooxygenase-2 in the amygdala kindling model in mice. Epilepsy Res. 2010, 91, 57–65. [Google Scholar] [CrossRef]
- Reschke, C.R.; Poersch, A.B.; Masson, C.J.; Jesse, A.C.; Marafiga, J.R.; Lenz, Q.F.; Oliveira, M.S.; Henshall, D.C.; Mello, C.F. Systemic delivery of selective EP1 and EP3 receptor antagonists attenuates pentylenetetrazole-induced seizures in mice. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 47–59. [Google Scholar]
- Jiang, J.; Ganesh, T.; Du, Y.; Quan, Y.; Serrano, G.; Qui, M.; Speigel, I.; Rojas, A.; Lelutiu, N.; Dingledine, R. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc. Natl. Acad. Sci. USA 2012, 109, 3149–3154. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Quan, Y.; Ganesh, T.; Pouliot, W.A.; Dudek, F.E.; Dingledine, R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc. Natl. Acad. Sci. USA 2013, 110, 3591–3596. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Yang, M.-S.; Quan, Y.; Gueorguieva, P.; Ganesh, T.; Dingledine, R. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol. Dis. 2015, 76, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Yu, Y.; Kinjo, E.R.; Du, Y.; Nguyen, H.P.; Dingledine, R. Suppressing pro-inflammatory prostaglandin signaling attenuates excitotoxicity-associated neuronal inflammation and injury. Neuropharmacology 2019, 149, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Gueorguieva, P.; Lelutiu, N.; Quan, Y.; Shaw, R.; Dingledine, R. The prostaglandin EP1 receptor potentiates kainate receptor activation via a protein kinase C pathway and exacerbates status epilepticus. Neurobiol. Dis. 2014, 70, 74–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, A.; Ganesh, T.; Lelutiu, N.; Gueorguieva, P.; Dingledine, R. Inhibition of the prostaglandin EP2 receptor is neuroprotective and accelerates functional recovery in a rat model of organophosphorus induced status epilepticus. Neuropharmacology 2015, 93, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Rojas, A.; Ganesh, T.; Manji, Z.; O’Neill, T.; Dingledine, R. Inhibition of the prostaglandin E2 receptor EP2 prevents status epilepticus-induced deficits in the novel object recognition task in rats. Neuropharmacology 2016, 110, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Chung, J.-I.; Lee, S.H.; Jung, Y.-S.; Moon, C.-H.; Baik, E.J. Involvement of endogenous prostaglandin F2α on kainic acid-induced seizure activity through FP receptor: The mechanism of proconvulsant effects of COX-2 inhibitors. Brain Res. 2008, 1193, 153–161. [Google Scholar] [CrossRef]
- Chung, J.-I.; Kim, A.Y.; Lee, S.H.; Baik, E.J. Seizure susceptibility in immature brain due to lack of COX-2-induced PGF2α. Exp. Neurol. 2013, 249, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nagib, M.M.; Yu, Y.; Jiang, J. Targeting prostaglandin receptor EP2 for adjunctive treatment of status epilepticus. Pharmacol. Ther. 2020, 209, 107504. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [CrossRef]
- DeMuth, D.G.; Molleman, A. Cannabinoid signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef]
- Zavala-Tecuapetla, C.; Rocha, L. Chapter 5: Do cannabinoids represent a good therapeutic strategy for epilepsy? In Antiepileptic Drug Discovery: Novel Approaches; Talevi, A., Rocha, L., Eds.; Humana Press: New York, NY, USA, 2016; pp. 83–96. ISBN 978-1-4939-6353-9. [Google Scholar]
- Cheung, K.A.K.; Peiris, H.; Wallace, G.; Holland, O.J.; Mitchell, M.D. The Interplay between the Endocannabinoid System, Epilepsy and Cannabinoids. Int. J. Mol. Sci. 2019, 20, 6079. [Google Scholar] [CrossRef] [Green Version]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutiérrez, S.O.; Van Der Stelt, M.; et al. CB1 Cannabinoid Receptors and On-Demand Defense Against Excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Blair, R.E.; Deshpande, L.S.; Sombati, S.; Falenski, K.W.; Martin, B.R.; DeLorenzo, R.J.; Kinney, G.G.; Burno, M.; Campbell, U.C.; Hernández, L.M.; et al. Activation of the Cannabinoid Type-1 Receptor Mediates the Anticonvulsant Properties of Cannabinoids in the Hippocampal Neuronal Culture Models of Acquired Epilepsy and Status Epilepticus. J. Pharmacol. Exp. Ther. 2006, 317, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Kow, R.L.; Jiang, K.; Naydenov, A.V.; Le, J.H.; Stella, N.; Nathanson, N.M. Modulation of Pilocarpine-Induced Seizures by Cannabinoid Receptor 1. PLoS ONE 2014, 9, e95922. [Google Scholar] [CrossRef]
- Sugaya, Y.; Yamazaki, M.; Uchigashima, M.; Kobayashi, K.; Watanabe, M.; Sakimura, K.; Kano, M. Crucial Roles of the Endocannabinoid 2-Arachidonoylglycerol in the Suppression of Epileptic Seizures. Cell Rep. 2016, 16, 1405–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, S.; Sun, X.; Lima, I.V.; Tavenier, A.; De Oliveira, A.C.P.; Dey, S.K.; Danzer, S.C. Cannabinoid receptor 1/2 double-knockout mice develop epilepsy. Epilepsia 2017, 58, e162–e166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Li, L.; Nguyen, D.T.; Mustafa, S.M.; Moore, B.M.; Jiang, J. Inverse Agonism of Cannabinoid Receptor Type 2 Confers Anti-inflammatory and Neuroprotective Effects Following Status Epileptics. Mol. Neurobiol. 2020, 57, 2830–2845. [Google Scholar] [CrossRef] [PubMed]
- Ludányi, A.; Erőss, L.; Czirják, S.; Vajda, J.; Halász, P.; Watanabe, M.; Palkovits, M.; Maglóczky, Z.; Freund, T.F.; Katona, I. Downregulation of the CB1 Cannabinoid Receptor and Related Molecular Elements of the Endocannabinoid System in Epileptic Human Hippocampus. J. Neurosci. 2008, 28, 2976–2990. [Google Scholar] [CrossRef] [Green Version]
- Rocha, L.; Cinar, R.; Guevara-Guzmán, R.; Alonso-Vanegas, M.; San-Juan, D.; Martínez-Juárez, I.; Castañeda-Cabral, J.L.; Carmona-Cruz, F. Endocannabinoid System and Cannabinoid 1 Receptors in Patients With Pharmacoresistant Temporal Lobe Epilepsy and Comorbid Mood Disorders. Front. Behav. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Nanduri, V.; Jing, S.; Lamballe, F.; Tapley, P.; Bryant, S.; Cordon-Cardo, C.; Jones, K.R.; Reichardt, L.F.; Barbacid, M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 1991, 66, 395–403. [Google Scholar] [CrossRef]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef]
- McNamara, J.O.; Scharfman, H.E. Temporal Lobe Epilepsy and the BDNF Receptor, TrkB. In Jasper’s Basic Mechanisms of the Epilepsies [Internet], 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Lin, T.W.; Harward, S.C.; Huang, Y.Z.; McNamara, J.O. Targeting BDNF/TrkB pathways for preventing or suppressing epilepsy. Neuropharmacology 2020, 167, 107734. [Google Scholar] [CrossRef]
- Isackson, P.J.; Huntsman, M.M.; Murray, K.D.; Gall, C.M. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: Temporal patterns of induction distinct from NGF. Neuron 1991, 6, 937–948. [Google Scholar] [CrossRef]
- Takahashi, M.; Hayashi, S.; Kakita, A.; Wakabayashi, K.; Fukuda, M.; Kameyama, S.; Tanaka, R.; Takahashi, H.; Nawa, H. Patients with temporal lobe epilepsy show an increase in brain-derived neurotrophic factor protein and its correlation with neuropeptide Y. Brain Res. 1999, 818, 579–582. [Google Scholar] [CrossRef]
- Xu, B.; Michalski, B.; Racine, R.; Fahnestock, M. The effects of brain-derived neurotrophic factor (BDNF) administration on kindling induction, Trk expression and seizure-related morphological changes. Neuroscience 2004, 126, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.X.; Del Angel, Y.C.; González, M.I.; Carrel, A.J.; Carlsen, J.; Lam, P.M.; Hempstead, B.L.; Russek, S.J.; Brooks-Kayal, A. Rapid Increases in proBDNF after Pilocarpine-Induced Status Epilepticus in Mice Are Associated with Reduced proBDNF Cleavage Machinery. eNeuro 2016, 3, 0020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, D.K.; Routbort, M.J.; Ryan, T.E.; Yancopoulos, G.D.; McNamara, J.O. Selective Inhibition of Kindling Development by Intraventricular Administration of TrkB Receptor Body. J. Neurosci. 1999, 19, 1424–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.-P.; Minichiello, L.; Klein, R.; McNamara, J.O. Immunohistochemical Evidence of Seizure-Induced Activation of trkB Receptors in the Mossy Fiber Pathway of Adult Mouse Hippocampus. J. Neurosci. 2002, 22, 7502–7508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.-P.; Kotloski, R.; Nef, S.; Luikart, B.W.; Parada, L.F.; McNamara, J.O. Conditional Deletion of TrkB but Not BDNF Prevents Epileptogenesis in the Kindling Model. Neuron 2004, 43, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.P.; Pan, E.; Sciarretta, C.; Minichiello, L.; McNamara, J.O. Disruption of TrkB-Mediated Phospholipase C Signaling Inhibits Limbic Epileptogenesis. J. Neurosci. 2010, 30, 6188–6196. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Gu, B.; He, X.-P.; Joshi, R.B.; Wackerle, H.D.; Rodriguiz, R.M.; Wetsel, W.C.; McNamara, J.O. Transient Inhibition of TrkB Kinase after Status Epilepticus Prevents Development of Temporal Lobe Epilepsy. Neuron 2013, 79, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.; Huang, Y.Z.; He, X.-P.; Joshi, R.B.; Jang, W.; McNamara, J.O. A Peptide Uncoupling BDNF Receptor TrkB from Phospholipase Cγ1 Prevents Epilepsy Induced by Status Epilepticus. Neuron 2015, 88, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Jiang, J. COX-2/PGE 2 axis regulates hippocampal BDNF/TrkB signaling via EP2 receptor after prolonged seizures. Epilepsia Open 2020, 5, 418–431. [Google Scholar] [CrossRef]
- Falcicchia, C.; Paolone, G.; Emerich, D.F.; Lovisari, F.; Bell, W.J.; Fradet, T.; Wahlberg, L.U.; Simonato, M. Seizure-Suppressant and Neuroprotective Effects of Encapsulated BDNF-Producing Cells in a Rat Model of Temporal Lobe Epilepsy. Mol. Ther. Methods Clin. Dev. 2018, 9, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Shestopalov, V.I.; Slepak, V.Z. Molecular pathways of pannexin1-mediated neurotoxicity. Front. Physiol. 2014, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scemes, E.; Velíšková, J. Exciting and not so exciting roles of pannexins. Neurosci. Lett. 2019, 695, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, M.S.; Whyte-Fagundes, P.; Zoidl, G.; Carlen, P.L. Pannexin-1 channels in epilepsy. Neurosci. Lett. 2019, 695, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, R.; Hormuzdi, S.G.; Barbe, M.T.; Herb, A.; Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13644–13649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, A.; Hormuzdi, S.G.; Monyer, H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Mol. Brain Res. 2005, 141, 113–120. [Google Scholar] [CrossRef]
- Zoidl, G.; Petrasch-Parwez, E.; Ray, A.; Meier, C.; Bunse, S.; Habbes, H.-W.; Dahl, G.; Dermietzel, R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 2007, 146, 9–16. [Google Scholar] [CrossRef]
- Cone, A.C.; Ambrosi, C.; Scemes, E.; Martone, M.E.; Sosinsky, G.E. A Comparative Antibody Analysis of Pannexin1 Expression in Four Rat Brain Regions Reveals Varying Subcellular Localizations. Front. Pharmacol. 2013, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, A.; Andó, R.; Turi, G.; Rozsa, B.; Sperlágh, B. K+depolarization evokes ATP, adenosine and glutamate release from glia in rat hippocampus: A microelectrode biosensor study. Br. J. Pharmacol. 2012, 167, 1003–1020. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.J.; Jackson, M.F.; Olah, M.E.; Rungta, R.L.; Hines, D.J.; Beazely, M.A.; Macdonald, J.F.; MacVicar, B.A. Activation of Pannexin-1 Hemichannels Augments Aberrant Bursting in the Hippocampus. Science 2008, 322, 1555–1559. [Google Scholar] [CrossRef] [Green Version]
- Santiago, M.F.; Velísková, J.; Patel, N.K.; Lutz, S.E.; Caille, D.; Charollais, A.; Meda, P.; Scemes, E. Targeting Pannexin1 Improves Seizure Outcome. PLoS ONE 2011, 6, e25178. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-E.; Kang, T.-C. The P2X7 receptor–pannexin-1 complex decreases muscarinic acetylcholine receptor–mediated seizure susceptibility in mice. J. Clin. Investig. 2011, 121, 2037–2047. [Google Scholar] [CrossRef] [Green Version]
- Aquilino, M.S.; Whyte-Fagundes, P.; Lukewich, M.K.; Zhang, L.; Bardakjian, B.L.; Zoidl, G.; Carlen, P.L. Pannexin-1 Deficiency Decreases Epileptic Activity in Mice. Int. J. Mol. Sci. 2020, 21, 7510. [Google Scholar] [CrossRef] [PubMed]
- Mylvaganam, S.; Zhang, L.; Wu, C.; Zhang, Z.J.; Samoilova, M.; Eubanks, J.; Carlen, P.L.; Poulter, M.O. Hippocampal seizures alter the expression of the pannexin and connexin transcriptome. J. Neurochem. 2010, 112, 92–102. [Google Scholar] [CrossRef]
- Jiang, T.; Long, H.; Ma, Y.; Long, L.-L.; Li, Y.; Li, F.; Zhou, P.; Yuan, C.; Xiao, B. Altered expression of pannexin proteins in patients with temporal lobe epilepsy. Mol. Med. Rep. 2013, 8, 1801–1806. [Google Scholar] [CrossRef] [Green Version]
- Cepeda, C.; Chang, J.W.; Owens, G.C.; Huynh, M.N.; Chen, J.Y.; Tran, C.; Vinters, H.V.; Levine, M.S.; Mathern, G.W. In Rasmussen Encephalitis, Hemichannels Associated with Microglial Activation are linked to Cortical Pyramidal Neuron Coupling: A Possible Mechanism for Cellular Hyperexcitability. CNS Neurosci. Ther. 2014, 21, 152–163. [Google Scholar] [CrossRef]
- Li, S.; Zang, Z.; He, J.; Chen, X.; Yu, S.; Pei, Y.; Hou, Z.; An, N.; Yang, H.; Zhang, C.; et al. Expression of pannexin 1 and 2 in cortical lesions from intractable epilepsy patients with focal cortical dysplasia. Oncotarget 2016, 8, 6883–6895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossi, E.; Blauwblomme, T.; Moulard, J.; Chever, O.; Vasile, F.; Guinard, E.; Le Bert, M.; Couillin, I.; Pallud, J.; Capelle, L.; et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci. Transl. Med. 2018, 10, eaar3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
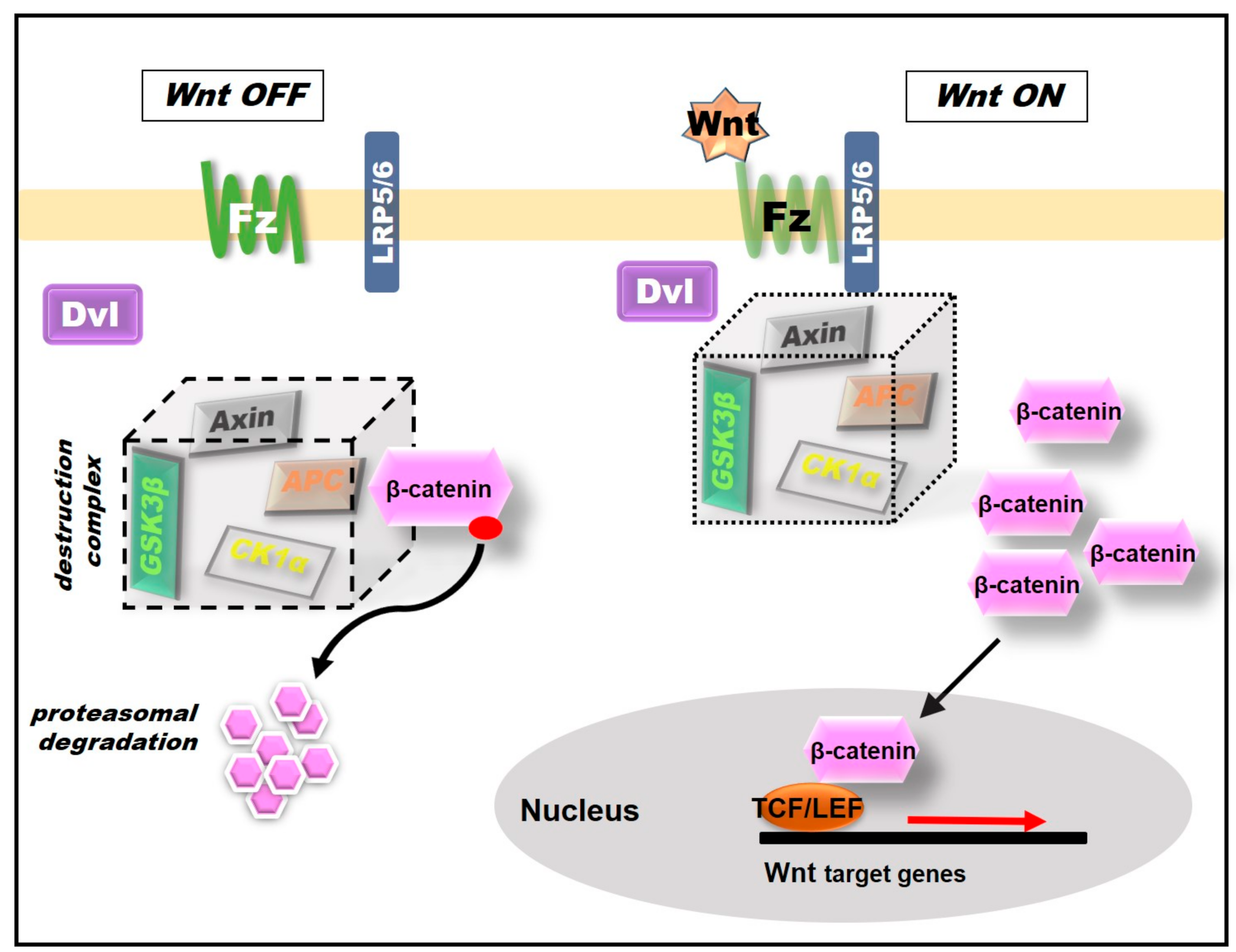
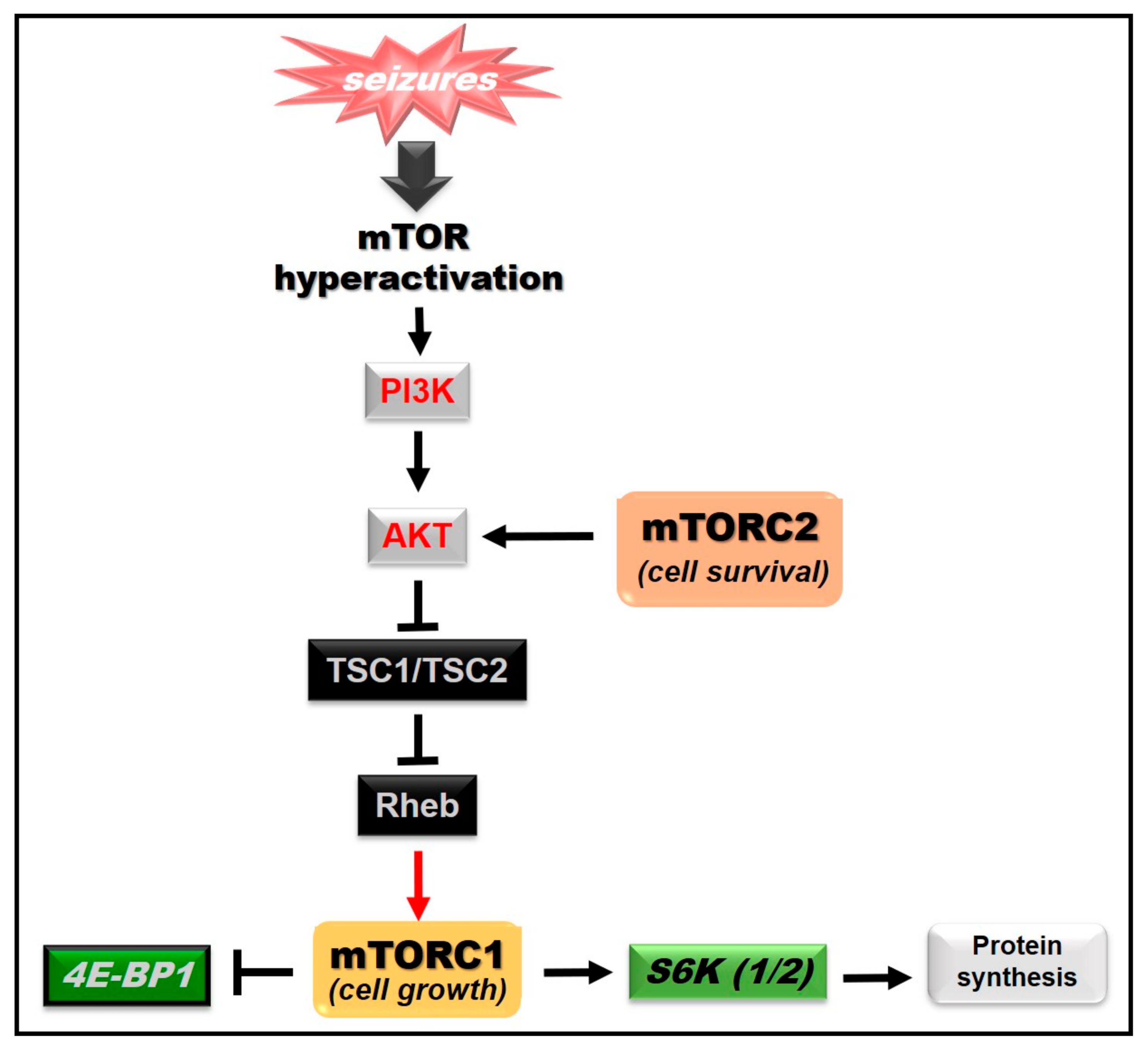
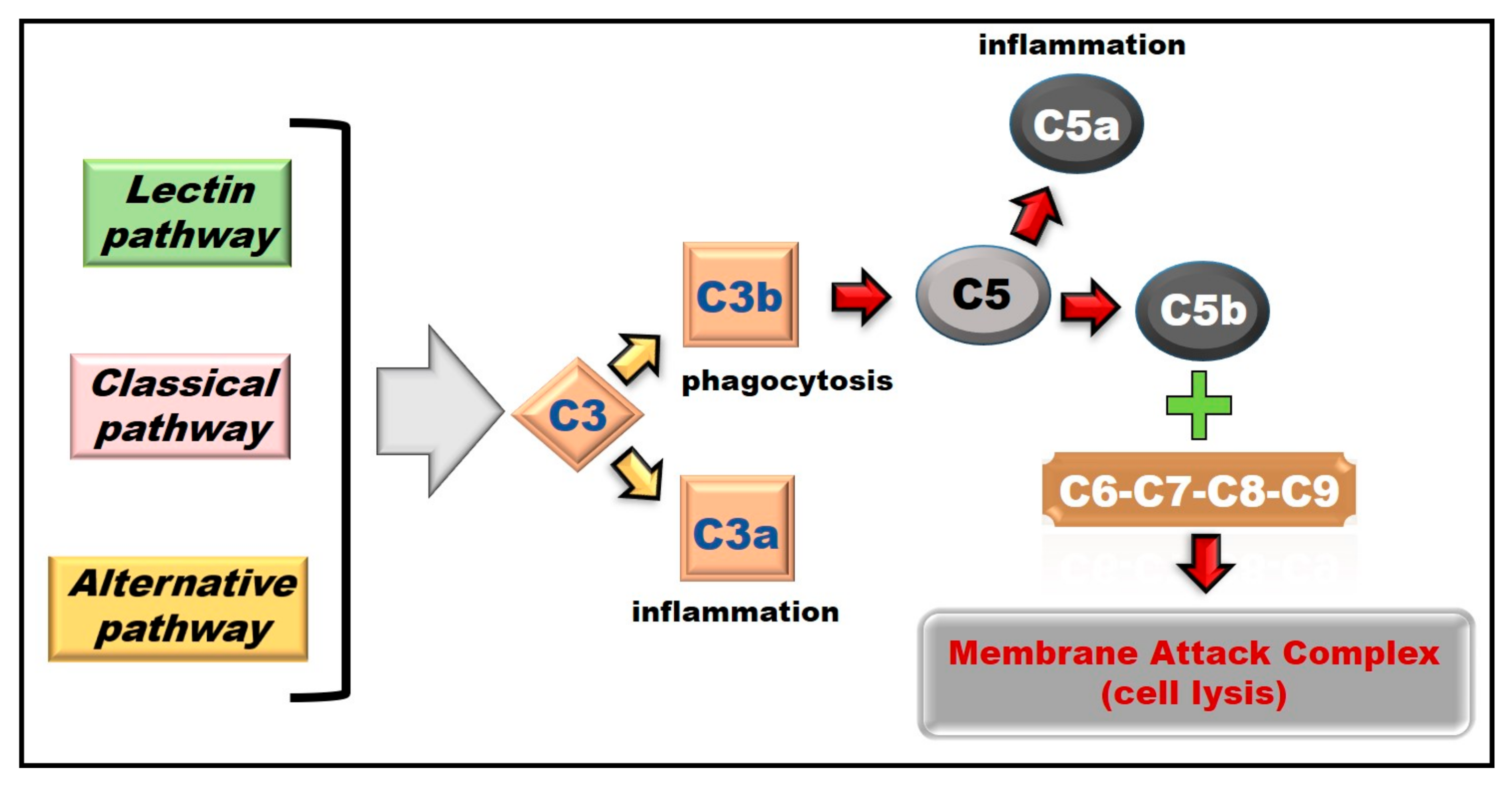
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavala-Tecuapetla, C.; Cuellar-Herrera, M.; Luna-Munguia, H. Insights into Potential Targets for Therapeutic Intervention in Epilepsy. Int. J. Mol. Sci. 2020, 21, 8573. https://doi.org/10.3390/ijms21228573
Zavala-Tecuapetla C, Cuellar-Herrera M, Luna-Munguia H. Insights into Potential Targets for Therapeutic Intervention in Epilepsy. International Journal of Molecular Sciences. 2020; 21(22):8573. https://doi.org/10.3390/ijms21228573
Chicago/Turabian StyleZavala-Tecuapetla, Cecilia, Manola Cuellar-Herrera, and Hiram Luna-Munguia. 2020. "Insights into Potential Targets for Therapeutic Intervention in Epilepsy" International Journal of Molecular Sciences 21, no. 22: 8573. https://doi.org/10.3390/ijms21228573
APA StyleZavala-Tecuapetla, C., Cuellar-Herrera, M., & Luna-Munguia, H. (2020). Insights into Potential Targets for Therapeutic Intervention in Epilepsy. International Journal of Molecular Sciences, 21(22), 8573. https://doi.org/10.3390/ijms21228573