NMR Profiling of Exhaled Breath Condensate Defines Different Metabolic Phenotypes of Non-Cystic Fibrosis Bronchiectasis
Abstract
:1. Introduction
2. Results
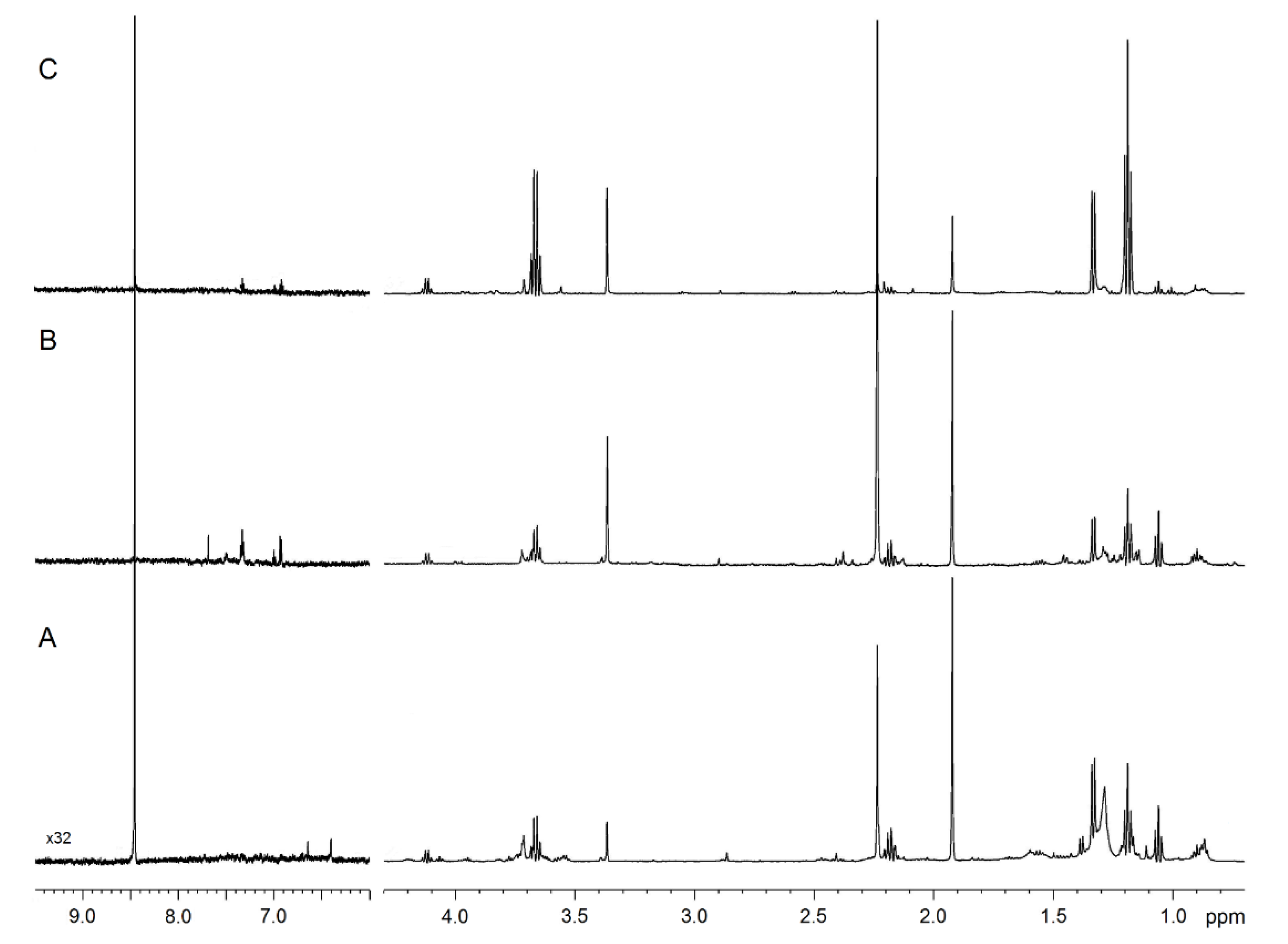
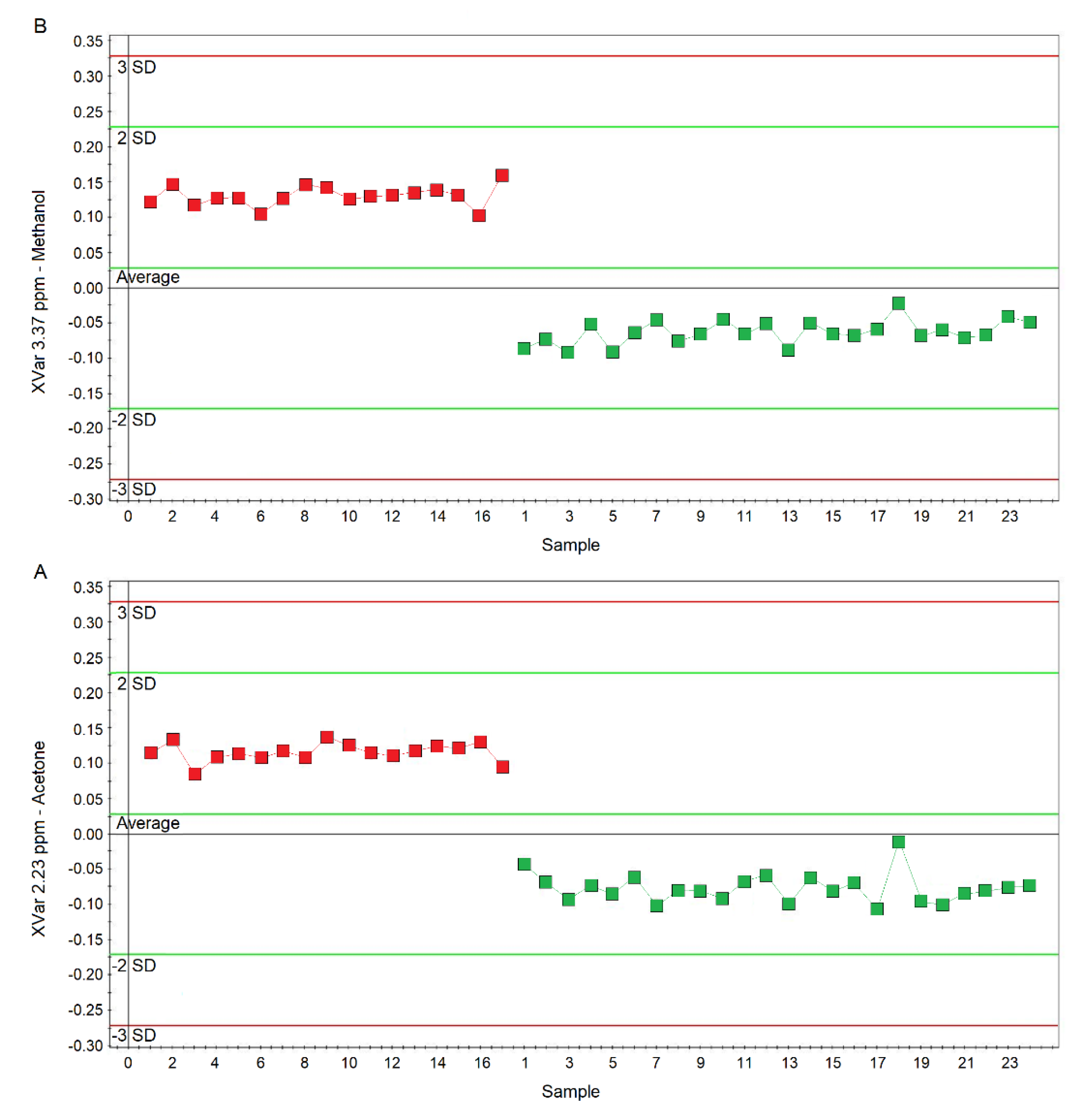
2.1. NMR Profiling of EBC
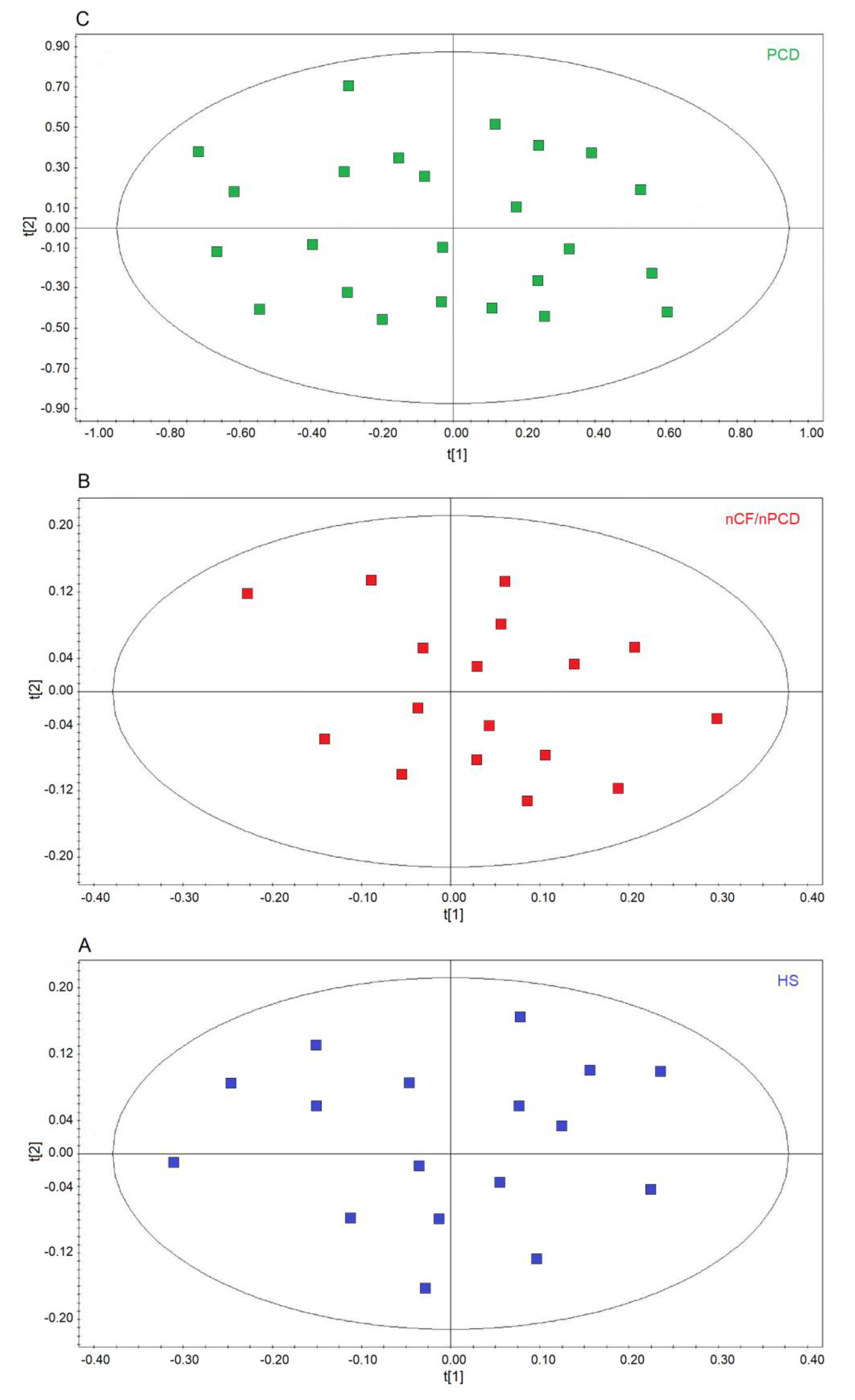
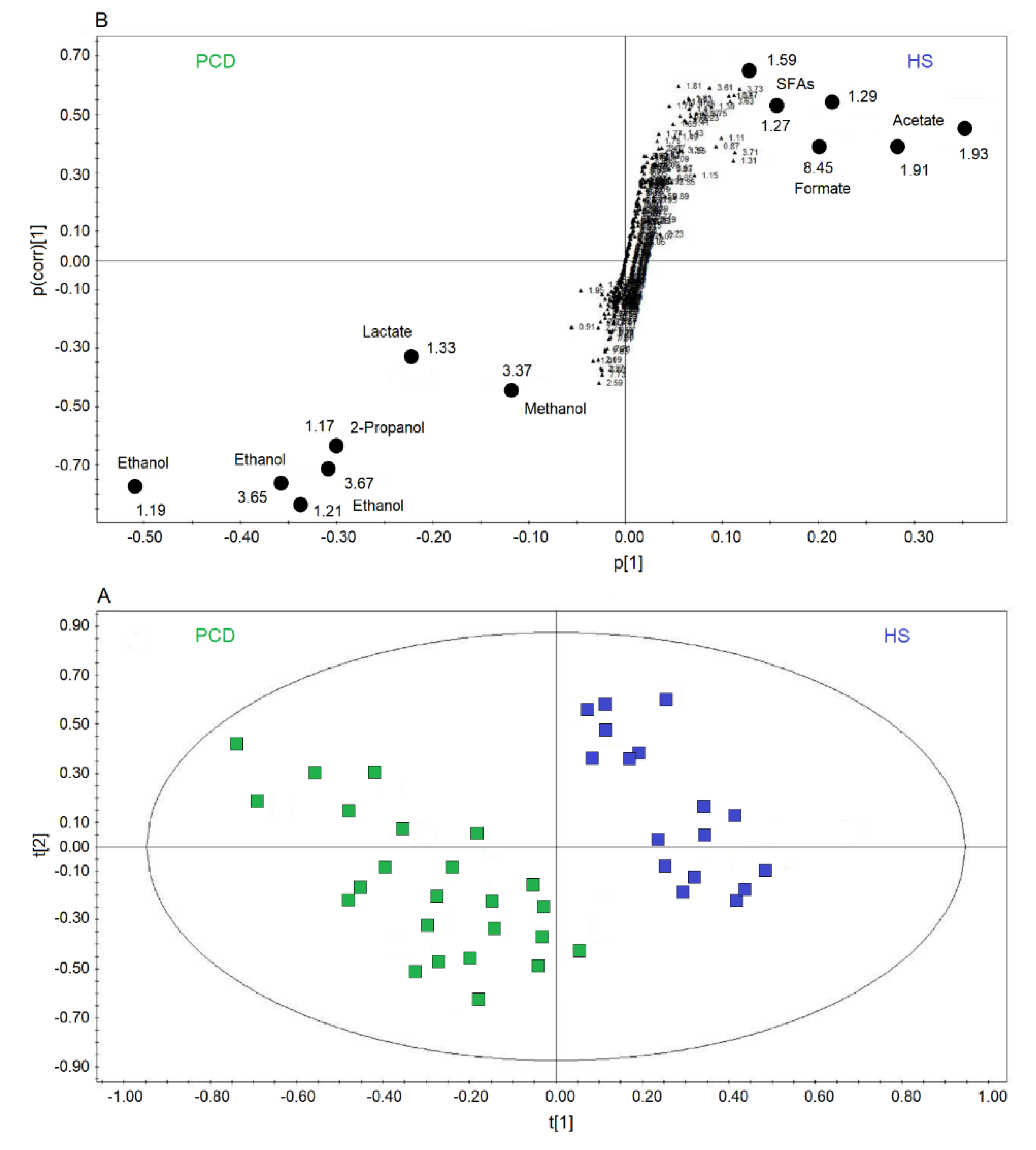
2.2. Comparison of nCF/nPCD and PCD with HS
2.3. All-Class Comparison
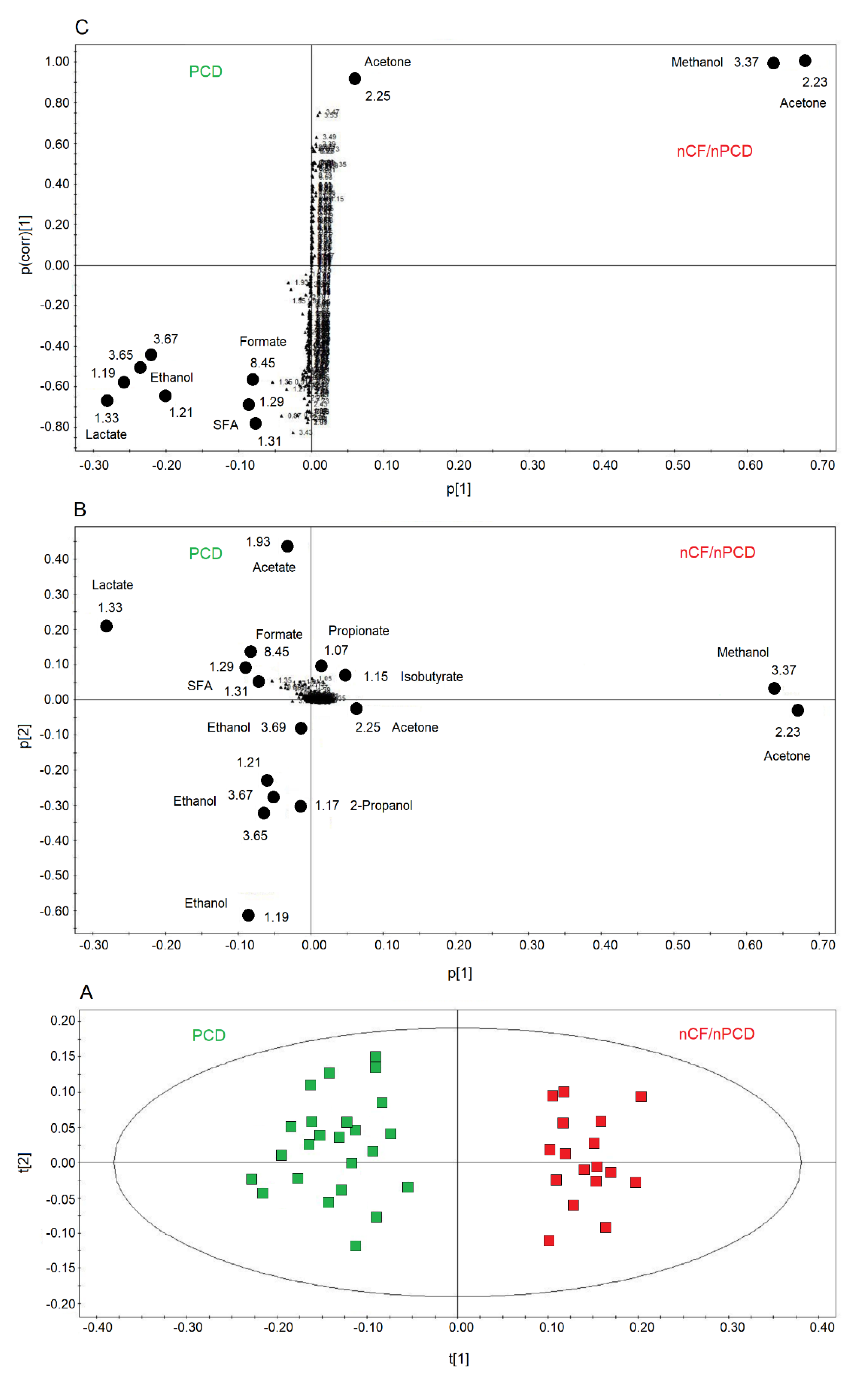
2.4. Comparing nCF/nPCD and PCD Classes
2.5. PCD‒nCF/nPCD Model Validation
2.6. Pathway Topology Analysis for PCD versus nCF/nPCD Comparison
2.7. Correlations
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. EBC Collection
4.3. NMR Spectroscopy Measurements
4.4. Power Analysis
4.5. Demographic Statistical Analysis
4.6. Spectral Statistical Analysis
4.7. Metabolic Pathway Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D | one-dimensional |
| 2D | two-dimensional |
| BAL | bronchoalveolar lavage |
| CF | cystic fibrosis |
| COPD | chronic obstructive pulmonary disease |
| EBC | exhaled breath condensate |
| HRCT | high-resolution computed tomography |
| HS | healthy subjects |
| HSQC | heteronuclear single-quantum coherence; |
| nCF/nPCD | bronchiectasis not associated with CF and PCD |
| NMR | nuclear magnetic resonance |
| OPLS-DA | orthogonal projections to latent structures discriminant analysis |
| OSC | orthogonal signal correction |
| PCA | principal component analysis |
| PCD | bronchiectasis associated with primary ciliary dyskinesia |
| PID | primary immunodeficiency |
| PLS-DA | projection to latent structures discriminant analysis |
| Q2 | goodness-of-prediction parameter |
| R2 | goodness-of-fit parameter |
| ROS | reactive oxygen species |
| SCFAs | short-chain fatty acids |
| SFAs | saturated fatty acids |
| TOCSY | clean total correlation spectroscopy |
| TSP | sodium 3-trimethylsilyl [2,2,3,3-2H4] propionate |
| VIP | variable importance in projection. |
References
- O’Donnell, A.E. Bronchiectasis. Chest 2008, 134, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Aksamit, T.; Santamaria, F. Paediatric and adult bronchiectasis: Specific management with coexisting asthma, COPD, rheumatological disease and inflammatory bowel disease. Respirology 2019, 24, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Montella, S.; Corcione, A.; Santamaria, F. Recurrent Pneumonia in Children: A Reasoned Diagnostic Approach and a Single Centre Experience. Int. J. Mol. Sci. 2017, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Poeta, M.; Maglione, M.; Borrelli, M.; Santamaria, F. Non-cystic fibrosis bronchiectasis in children and adolescents: Neglected and emerging issues. Pediatr. Neonatol. 2020, 61, 255–262. [Google Scholar] [CrossRef]
- Maniscalco, M.; Motta, A. Clinical and Inflammatory Phenotyping: Can Electronic Nose and NMR-based Metabolomics Work at the Bedside? Arch. Med. Res. 2018, 49, 74–76. [Google Scholar] [CrossRef]
- De Laurentiis, G.; Paris, D.; Melck, D.; Montuschi, P.; Maniscalco, M.; Bianco, A.; Sofia, M.; Motta, A. Separating smoking-related diseases using NMR-based metabolomics of exhaled breath condensate. J. Proteome Res. 2013, 12, 1502–1511. [Google Scholar] [CrossRef]
- Motta, A.; Paris, D.; D’Amato, M.; Melck, D.; Calabrese, C.; Vitale, C.; Stanziola, A.A.; Corso, G.; Sofia, M.; Maniscalco, M. NMR metabolomic analysis of exhaled breath condensate of asthmatic patients at two different temperatures. J. Proteome Res. 2014, 13, 6107–6120. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Melck, D.J.; D’Amato, M.; Zedda, A.; Sofia, M.; Stellato, C.; Motta, A. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J. Allergy Clin. Immunol. 2017, 139, 1536–1547. [Google Scholar] [CrossRef] [Green Version]
- Maniscalco, M.; Motta, A. Metabolomics of exhaled breath condensate: A means for phenotyping respiratory diseases? Biomark. Med. 2017, 11, 405–407. [Google Scholar] [CrossRef] [Green Version]
- Paris, D.; Maniscalco, M.; Motta, A. Nuclear magnetic resonance-based metabolomics in respiratory medicine. Eur. Respir. J. 2018, 52, 1801107. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Melck, D.J.; Molino, A.; Carone, M.; Ruggeri, P.; Caramori, G.; Motta, A. Differential diagnosis between newly diagnosed asthma and COPD using exhaled breath condensate metabolomics: A pilot study. Eur. Respir. J. 2018, 51, 1701825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertini, I.; Luchinat, C.; Miniati, M.; Monti, S.; Tenori, L. Phenotyping COPD by 1H NMR metabolomics of exhaled breath condensate. Metabolomics 2014, 10, 302–311. [Google Scholar] [CrossRef]
- Montuschi, P.; Santini, G.; Mores, N.; Vignoli, A.; Macagno, F.; Shoreh, R.; Tenori, L.; Zini, G.; Fuso, L.; Mondino, C.; et al. Breathomics for assessing the effects of treatment and withdrawal with inhaled beclomethasone/formoterol in patients with COPD. Front. Pharmacol. 2018, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignoli, A.; Santini, G.; Tenori, L.; Macis, G.; Mores, N.; Macagno, F.; Pagano, F.; Higenbottam, T.; Luchinat, C.; Montuschi, P. NMR-based metabolomics for the assessment of inhaled pharmacotherapy in chronic obstructive pulmonary disease patients. J. Proteome Res. 2020, 19, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Rezzi, S.; Reniero, F.; Heberger, K.; Giordano, G.; Zanconato, S.; Guillou, C.; Baraldi, E. Metabolomics applied to exhaled breath condensate in childhood asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 986–990. [Google Scholar] [CrossRef]
- De Laurentiis, G.; Paris, D.; Melck, D.; Maniscalco, M.; Marsico, S.; Corso, G.; Motta, A.; Sofia, M. Metabonomic analysis of exhaled breath condensate in adults by nuclear magnetic resonance spectroscopy. Eur. Respir. J. 2008, 32, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Montuschi, P.; Paris, D.; Melck, D.; Lucidi, V.; Ciabattoni, G.; Raia, V.; Calabrese, C.; Bush, A.; Barnes, P.J.; Motta, A. NMR spectroscopy metabolomic profiling of exhaled breath condensate in patients with stable and unstable cystic fibrosis. Thorax 2012, 67, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Montuschi, P.; Paris, D.; Montella, S.; Melck, D.; Mirra, V.; Santini, G.; Mores, N.; Montemitro, E.; Majo, F.; Lucidi, V.; et al. Nuclear magnetic resonance-based metabolomics discriminates primary ciliary dyskinesia from cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 229–233. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, M.; Paris, D.; Molino, A.; Cuomo, P.; Fulgione, A.; Sorrentino, N.; Palomba, L.; Maniscalco, M.; Motta, A. The Immune-Modulator Pidotimod Affects the Metabolic Profile of Exhaled Breath Condensate in Bronchiectatic Patients: A Metabolomics Pilot Study. Front. Pharmacol. 2019, 10, 1115. [Google Scholar] [CrossRef] [Green Version]
- Ulloa, L.; Ochani, M.; Yang, H.; Tanovic, M.; Halperin, D.; Yang, R.; Czura, C.J.; Fink, M.P.; Tracey, K.J. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl. Acad. Sci. USA 2002, 99, 12351–12356. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.H.; Clark, N.; Brennan, A.L.; Fisher, D.A.; Gyi, K.M.; Hodson, M.E.; Philips, B.J.; Baines, D.L.; Wood, D.M. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J. Appl. Physiol. (1985) 2007, 102, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Philips, B.J.; Redman, J.; Brennan, A.; Wood, D.; Holliman, R.; Baines, D.; Baker, E.H. Glucose in bronchial aspirates increases the risk of respiratory MRSA in intubated patients. Thorax 2005, 60, 761–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; W.H. Freeman & Company: New York, NY, USA, 2017. [Google Scholar]
- Miller, T.L.; Wolin, M.J. Methanosphaera stadtmaniae gen. nov., sp. nov.: A species that forms methane by reducing methanol with hydrogen. Arch. Microbiol. 1985, 141, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Persoz, C.; Achard, S.; Momas, I.; Seta, N. Inflammatory response modulation of airway epithelial cells exposed to formaldehyde. Toxicol. Lett. 2012, 211, 159–163. [Google Scholar] [CrossRef]
- Lino-dos-Santos-Franco, A.; Correa-Costa, M.; Cardoso dos Santos Durão, A.C.; Ligeiro de Oliveira, A.P.; Breithaupt-Faloppa, A.C.; de Almeida Bertoni, J.; Oliveira-Filho, R.M.; Saraiva Câmara, N.O.; Marcourakis, T.; Tavares-de-Lima, W. Formaldehyde induces lung inflammation by an oxidant and antioxidant enzymes mediated mechanism in the lung tissue. Toxicol. Lett. 2011, 207, 278–285. [Google Scholar] [CrossRef]
- Okubo, T.; Suzuki, T.; Hosaka, M.; Nakae, D. Effects Induced by Organic Acids in a Human Lung Alveolar Carcinoma Cell Line A549. Yakugaku Zasshi 2016, 136, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Bezabeh, T.; Ijare, O.B.; Myers, R.; Alomran, R.; Aliani, M.; Nugent, Z.; Banerji, S.; Kim, J.; Qing, G.; et al. Metabolic Signatures of Lung Cancer in Sputum and Exhaled Breath Condensate Detected by (1)H Magnetic Resonance Spectroscopy: A Feasibility Study. Magn. Reson. Insights 2016, 9, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, M.; Mine, T.; Ohuchi, K.; Ohmori, S. A detoxication route for acetaldehyde: Metabolism of diacetyl, acetoin, and 2,3-butanediol in liver homogenate and perfused liver of rats. J. Biochem. 1996, 119, 246–251. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Pulmonary toxicity and environmental contamination: Radicals, electron transfer, and protection by antioxidants. Rev. Environ. Contam. Toxicol. 2009, 201, 41–69. [Google Scholar] [CrossRef]
- Airoldi, C.; Ciaramelli, C.; Fumagalli, M.; Bussei, R.; Mazzoni, V.; Viglio, S.; Iadarola, P.; Stolk, J. (1)H NMR To Explore the Metabolome of Exhaled Breath Condensate in alpha1-Antitrypsin Deficient Patients: A Pilot Study. J. Proteome Res. 2016, 15, 4569–4578. [Google Scholar] [CrossRef]
- Whiteson, K.L.; Meinardi, S.; Lim, Y.W.; Schmieder, R.; Maughan, H.; Quinn, R.; Blake, D.R.; Conrad, D.; Rohwer, F. Breath gas metabolites and bacterial metagenomes from cystic fibrosis airways indicate active pH neutral 2,3-butanedione fermentation. ISME J. 2014, 8, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.; Kisiela, M.; Maser, E. Human DCXR-another ‘moonlighting protein’ involved in sugar metabolism, carbonyl detoxification, cell adhesion and male fertility? Biol. Rev. Camb. Philos. Soc. 2015, 90, 254–278. [Google Scholar] [CrossRef] [PubMed]
- Traphagen, N.; Tian, Z.; Allen-Gipson, D. Chronic Ethanol Exposure: Pathogenesis of Pulmonary Disease and Dysfunction. Biomolecules 2015, 5, 2840–2853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisson, J.H.; Pavlik, J.A.; Wyatt, T.A. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol. Clin. Exp. Res. 2009, 33, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolak, J.E.; Esther, C.R., Jr.; O’Connell, T.M. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers 2009, 14, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Sanchez, L.M.; Jurado-Gamez, B.; Feu-Collado, N.; Valverde, A.; Canas, A.; Fernandez-Rueda, J.L.; Aranda, E.; Rodriguez-Ariza, A. Exhaled breath condensate biomarkers for the early diagnosis of lung cancer using proteomics. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L664–L676. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.L.; Dal Cortivo, L.A.; Maturo, J. Endogenous isopropanol: Forensic and biochemical implications. J. Anal. Toxicol. 1984, 8, 209–212. [Google Scholar] [CrossRef]
- Lewis, G.D.; Laufman, A.K.; McAnalley, B.H.; Garriott, J.C. Metabolism of acetone to isopropyl alcohol in rats and humans. J. Forensic. Sci. 1984, 29, 541–549. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Chang, Y.J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Korithoski, B.; Levesque, C.M.; Cvitkovitch, D.G. The involvement of the pyruvate dehydrogenase E1alpha subunit, in Streptococcus mutans acid tolerance. FEMS Microbiol. Lett. 2008, 289, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Costanzo, A.P.D.; Melck, D.; Angiolillo, A.; Corso, G.; Maniscalco, M.; Motta, A. Blood biomarkers indicate that the preclinical stages of Alzheimer’s disease present overlapping molecular features. Sci. Rep. 2020, 10, 15612. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Lan, H.; Yu, Z.; Wang, M.; Wang, S.; Chen, Y.; Rao, H.; Li, J.; Sheng, Z.; Shao, J. Blockage of glycolysis by targeting PFKFB3 alleviates sepsis-related acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2017, 491, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2017, 49, 1601090. [Google Scholar] [CrossRef]
- Hwang, T.L.; Shaka, A.J. Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. Ser. A 1995, 112, 275–279. [Google Scholar] [CrossRef]
- Griesinger, C.O.G.; Wuethrich, K.; Ernst, R. Clean TOCSY for 1H spin system identification in macromolecules. J. Am. Chem. Soc. 1988, 110, 7870–7872. [Google Scholar] [CrossRef]
- Kay, L.E.; Keifer, P.; Saarinen, T. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Am. Chem. Soc. 1992, 114, 10663–10665. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis: Basic Principles and Applications, 3rd ed.; Umetrics Academy: Malmö, Sweden, 2013. [Google Scholar]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]





| PCD (n = 24) | nCF/nPCD (n = 17) | HS (n = 17) | PCD Test Set (n = 17) | |
|---|---|---|---|---|
| Anthropometric data | ||||
| Age, yr b | 17.2 ± 0.9 (7.0–33.5) | 14.1 ± 1.1 (7.2–25.3) | 16.8 ± 0.9 (8.1–26.9) | 17.4 ± 0.9 (11.2–31.8) |
| Gender, M (%)/F (%) | 16 (67)/8 (33) | 5 (29)/12 (71) | 8 (47)/9 (53) | 12 (70)/5 (30) |
| Chest HRCT abnormalities c,d | ||||
| Bronchiectasis, n (%) | 24 (100) | 17 (100) | - | 17 (100) |
| Sputum culture e | ||||
| P. aeruginosa, n (%) | 1 (4) | 2 (12) | - | 2 (10) |
| S. aureus, n (%) | 1 (4) | 1 (6) | - | 4 (20) |
| H. influenzae, n (%) | 7 (29) | 3 (18) | - | 12 (60) |
| S. pneumoniae, n (%) | 3 (12.5) | - | - | 12 (60) |
| Spirometry f | ||||
| FEV1, % pred | 81.0 ± 20.9 | 83.2 ± 25.8 | - | 80.3 ± 2.5 |
| FVC, % pred | 90.1 ± 25.9 | 88.2 ± 20.4 | - | 92.6 ± 3.9 |
| FEV1/FVC, (%) | 79.0 ± 12.7 | 84.0 ± 17.2 | - | 79.9 ± 13.8 |
| FEF25–75, % pred | 52.3 ± 25.0 | 69.1 ± 39.0 | - | 54.1 ± 25.9 |
| nCF/nPCD versus HS | PCD versus HS | |
|---|---|---|
| Methanol |  | Methanol |
| Acetone/Acetoin | ‒ | |
| Ethanol | Ethanol | |
| 2-Propanol | 2-Propanol | |
| Propionate | ‒ | |
| ‒ | Lactate | |
| Formate |  | Formate |
| Acetate | Acetate | |
| Lactate | ‒ | |
| SFAs | SFAs |
| nCF/nPCD | PCD | HS |
|---|---|---|
| Methanol | Lactate | Formate |
| Acetone/Acetoin | Ethanol | Acetate |
| 2-Propanol | SFAs |
| nCF/nPCD | PCD |
|---|---|
| Methanol | Formate |
| Acetone/Acetoin | Ethanol |
| 2-Propanol | Acetate |
| Isobutyrate | Lactate |
| Propionate | SFAs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paris, D.; Palomba, L.; Mirra, V.; Borrelli, M.; Corcione, A.; Santamaria, F.; Maniscalco, M.; Motta, A. NMR Profiling of Exhaled Breath Condensate Defines Different Metabolic Phenotypes of Non-Cystic Fibrosis Bronchiectasis. Int. J. Mol. Sci. 2020, 21, 8600. https://doi.org/10.3390/ijms21228600
Paris D, Palomba L, Mirra V, Borrelli M, Corcione A, Santamaria F, Maniscalco M, Motta A. NMR Profiling of Exhaled Breath Condensate Defines Different Metabolic Phenotypes of Non-Cystic Fibrosis Bronchiectasis. International Journal of Molecular Sciences. 2020; 21(22):8600. https://doi.org/10.3390/ijms21228600
Chicago/Turabian StyleParis, Debora, Letizia Palomba, Virginia Mirra, Melissa Borrelli, Adele Corcione, Francesca Santamaria, Mauro Maniscalco, and Andrea Motta. 2020. "NMR Profiling of Exhaled Breath Condensate Defines Different Metabolic Phenotypes of Non-Cystic Fibrosis Bronchiectasis" International Journal of Molecular Sciences 21, no. 22: 8600. https://doi.org/10.3390/ijms21228600






