Mitochondrial Ca2+ Dynamics in MCU Knockout C. elegans Worms
Abstract
:1. Introduction
2. Results
2.1. Mitochondrial [Ca2+] Oscillations in Control and MCU-Defective Worms
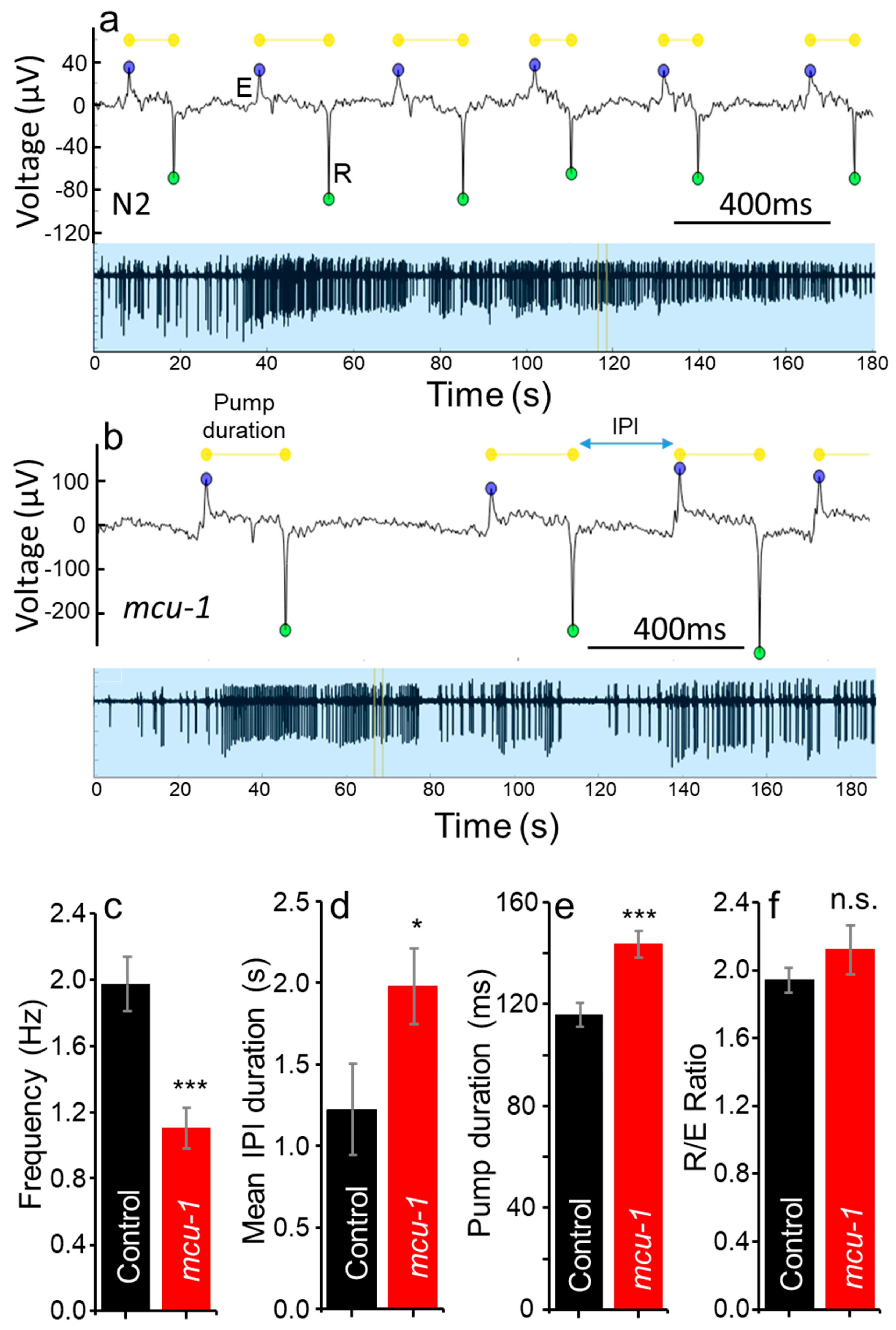
2.2. Electropharyngeograms of Control and MCU-Defective Worms
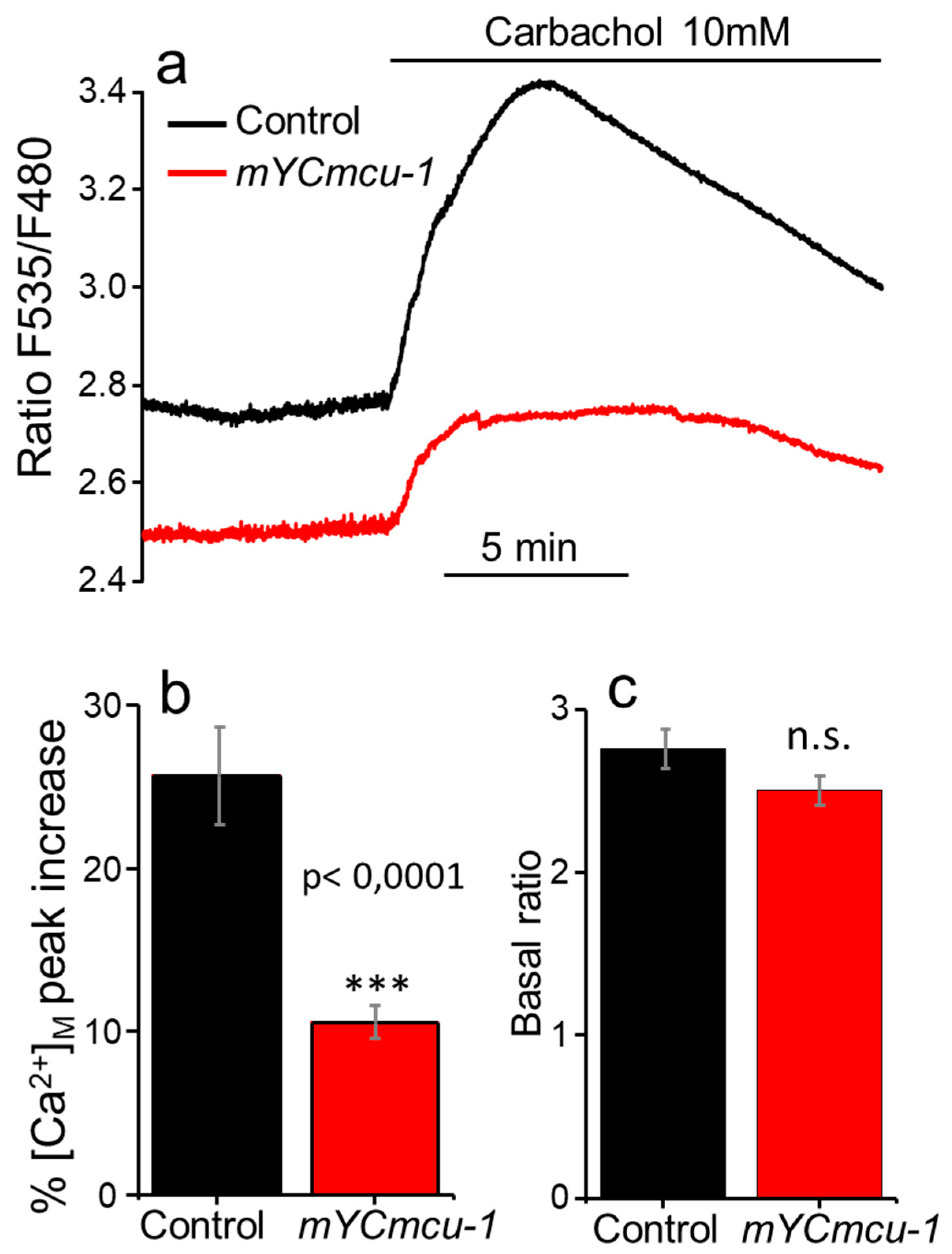
2.3. Mitochondrial [Ca2+] in Control and MCU-Defective Worms under Strong Stimulation
3. Discussion
4. Materials and Methods
4.1. C. elegans Strains and Maintenance
4.2. Genotyping and RT-qPCR of the mcu-1 and mYCmcu-1 Mutants
4.3. Mitochondrial Distribution of the YC3.60 Ca2+ Sensor Studied by Confocal Microscopy
4.4. Mitochondrial Calcium Imaging
4.5. Electropharyngeogram
4.6. Materials
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MCU | Mitochondrial Calcium Uniporter |
| ER | Endoplasmic Reticulum |
References
- Theurey, P.; Rieusset, J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 32–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Filadi, R.; Pozzan, T. Generation and functions of second messengers microdomains. Cell Calcium 2015, 58, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Alonso, M.T.; Carnicero, E.; Cuchillo-Ibáẽz, I.; Albillos, A.; García, A.G.; García-Sancho, J.; Alvarez, J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell Biol. 2000, 2, 57–61. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Rasola, A.; Bernardi, P. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium 2011, 50, 222–233. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [Green Version]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Pendin, D.; Greotti, E.; Pozzan, T. The elusive importance of being a mitochondrial Ca2+ uniporter. Cell Calcium 2014, 55, 139–145. [Google Scholar] [CrossRef]
- Harrington, J.L.; Murphy, E. The mitochondrial calcium uniporter: Mice can live and die without it. J. Mol. Cell. Cardiol. 2015, 78, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallafacchina, G.; Zanin, S.; Rizzuto, R. Recent advances in the molecular mechanism of mitochondrial calcium uptake. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, J.; Nguyen, T.; Liu, C.; Sun, J.; Teng, Y.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. The Physiological Role of Mitochondrial Calcium Revealed by Mice Lacking the Mitochondrial Calcium Uniporter. Nat. Cell Biol. 2013, 15, 1464–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmström, K.M.; Pan, X.; Liu, J.C.; Menazza, S.; Liu, J.; Nguyen, T.T.; Pan, H.; Parks, R.J.; Anderson, S.; Noguchi, A.; et al. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J. Mol. Cell. Cardiol. 2015, 85, 178–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Chisholm, A.D. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev. Cell 2014, 31, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Illera, P.; Sanchez-Blanco, A.; Lopez-Burillo, S.; Fonteriz, R.I.; Alvarez, J.; Montero, M. Long-term monitoring of Ca2+ dynamics in C. elegans pharynx: An in vivo energy balance sensor. Oncotarget 2016, 7, 67732–67747. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Illera, P.; García-Casas, P.; Arias-Del-Val, J.; Fonteriz, R.I.; Alvarez, J.; Montero, M. Pharynx mitochondrial [Ca2+] dynamics in live C. elegans worms during aging. Oncotarget 2017, 8, 55889–55900. [Google Scholar] [CrossRef] [Green Version]
- Trojanowski, N.F.; Raizen, D.M.; Fang-Yen, C. Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Sci. Rep. 2016, 6, 22940. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Li, H.; Tai, Y.; Huang, J.; Su, Y.; Abramowitz, J.; Zhu, M.X.; Birnbaumer, L.; Wang, Y. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 11011–11016. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T.; Menazza, S.; Holmström, K.M.; Parks, R.J.; Liu, J.; Sun, J.; Liu, J.; Pan, X.; Murphy, E. The ins and outs of mitochondrial calcium. Circ. Res. 2015, 116, 1810–1819. [Google Scholar] [CrossRef] [Green Version]
- Elustondo, P.A.; Nichols, M.; Robertson, G.S.; Pavlov, E.V. Mitochondrial Ca2+ uptake pathways. J. Bioenergy Biomembr. 2017, 49, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamada, S.; Tominaga, T.; Ichikawa, M.; Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 10554–10559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006 Feb 11:1-11; PMCID:PMC4781397. Available online: https://pubmed.ncbi.nlm.nih.gov/18050451/ (accessed on 1 October 2020). [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Illera, P.; García-Casas, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J. Mitochondrial Ca2+ Dynamics in MCU Knockout C. elegans Worms. Int. J. Mol. Sci. 2020, 21, 8622. https://doi.org/10.3390/ijms21228622
Álvarez-Illera P, García-Casas P, Fonteriz RI, Montero M, Alvarez J. Mitochondrial Ca2+ Dynamics in MCU Knockout C. elegans Worms. International Journal of Molecular Sciences. 2020; 21(22):8622. https://doi.org/10.3390/ijms21228622
Chicago/Turabian StyleÁlvarez-Illera, Pilar, Paloma García-Casas, Rosalba I Fonteriz, Mayte Montero, and Javier Alvarez. 2020. "Mitochondrial Ca2+ Dynamics in MCU Knockout C. elegans Worms" International Journal of Molecular Sciences 21, no. 22: 8622. https://doi.org/10.3390/ijms21228622






